Differences in Carbon and Nitrogen Migration and Transformation Driven by Cyanobacteria and Macrophyte Activities in Taihu Lake
Abstract
1. Introduction
2. Method
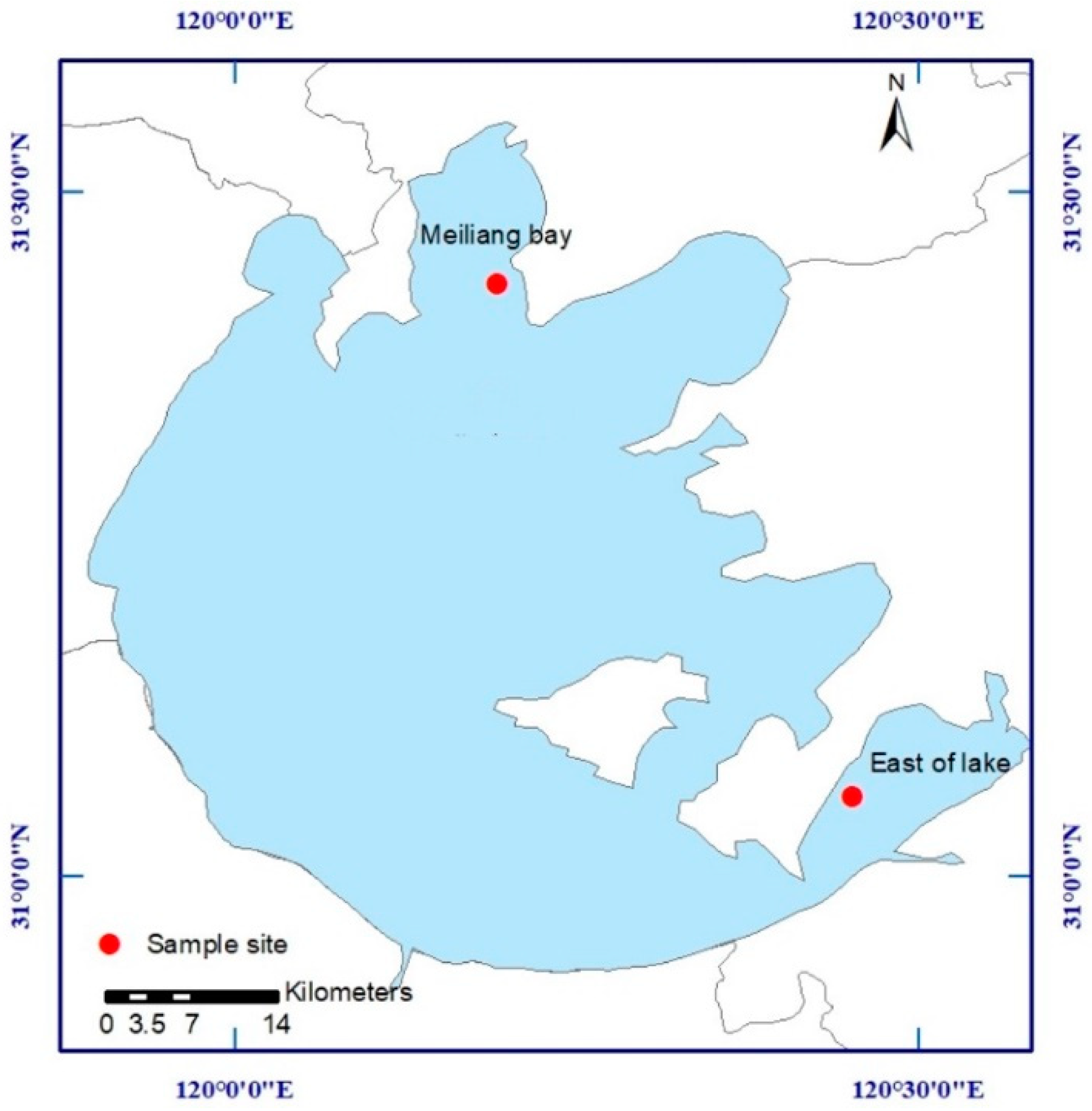
2.1. Study Area and Sampling
2.2. Measurement Methods
2.3. DNA Extraction and 16S rRNA Gene High-Throughput Sequencing
2.4. Data Analysis
3. Results
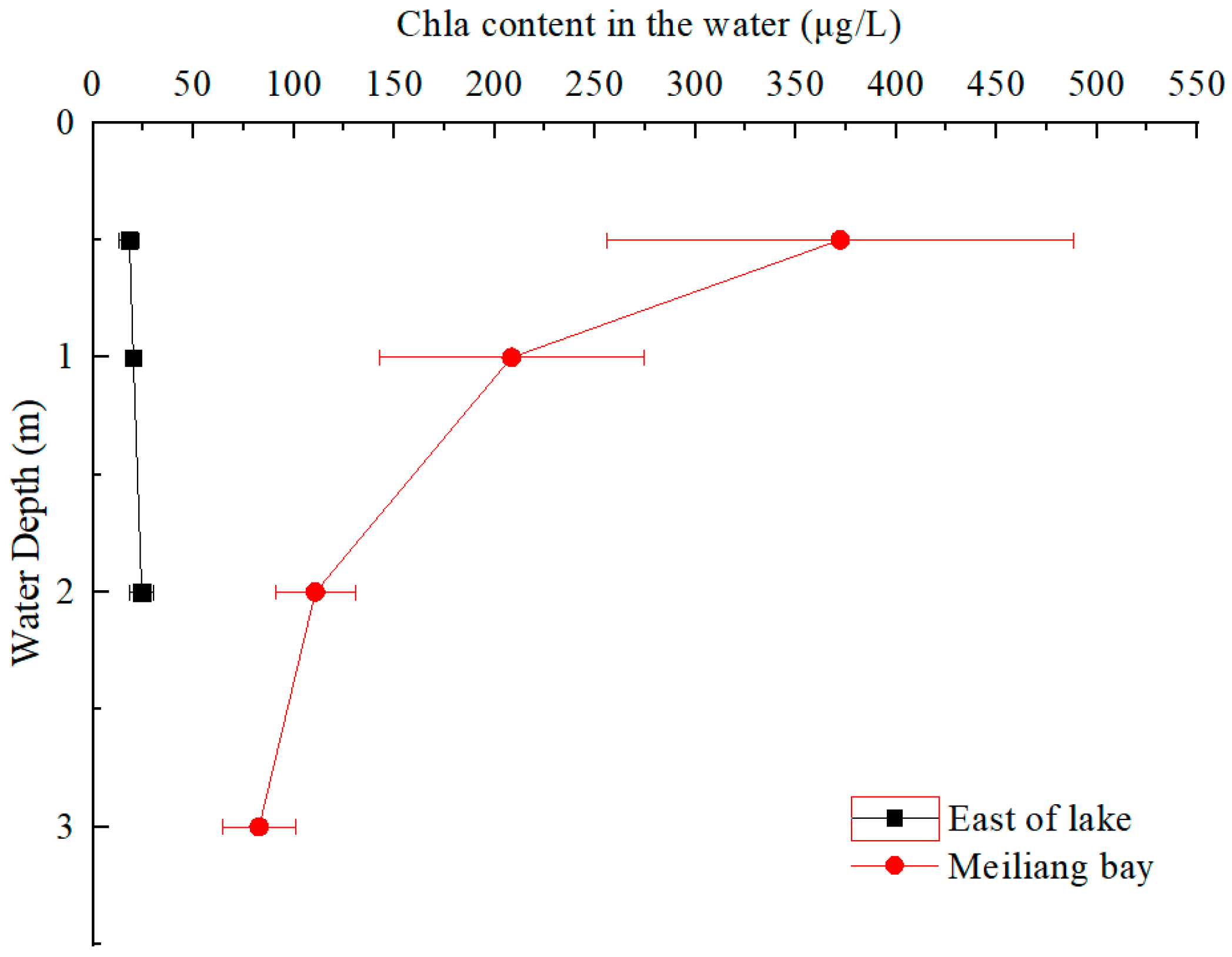
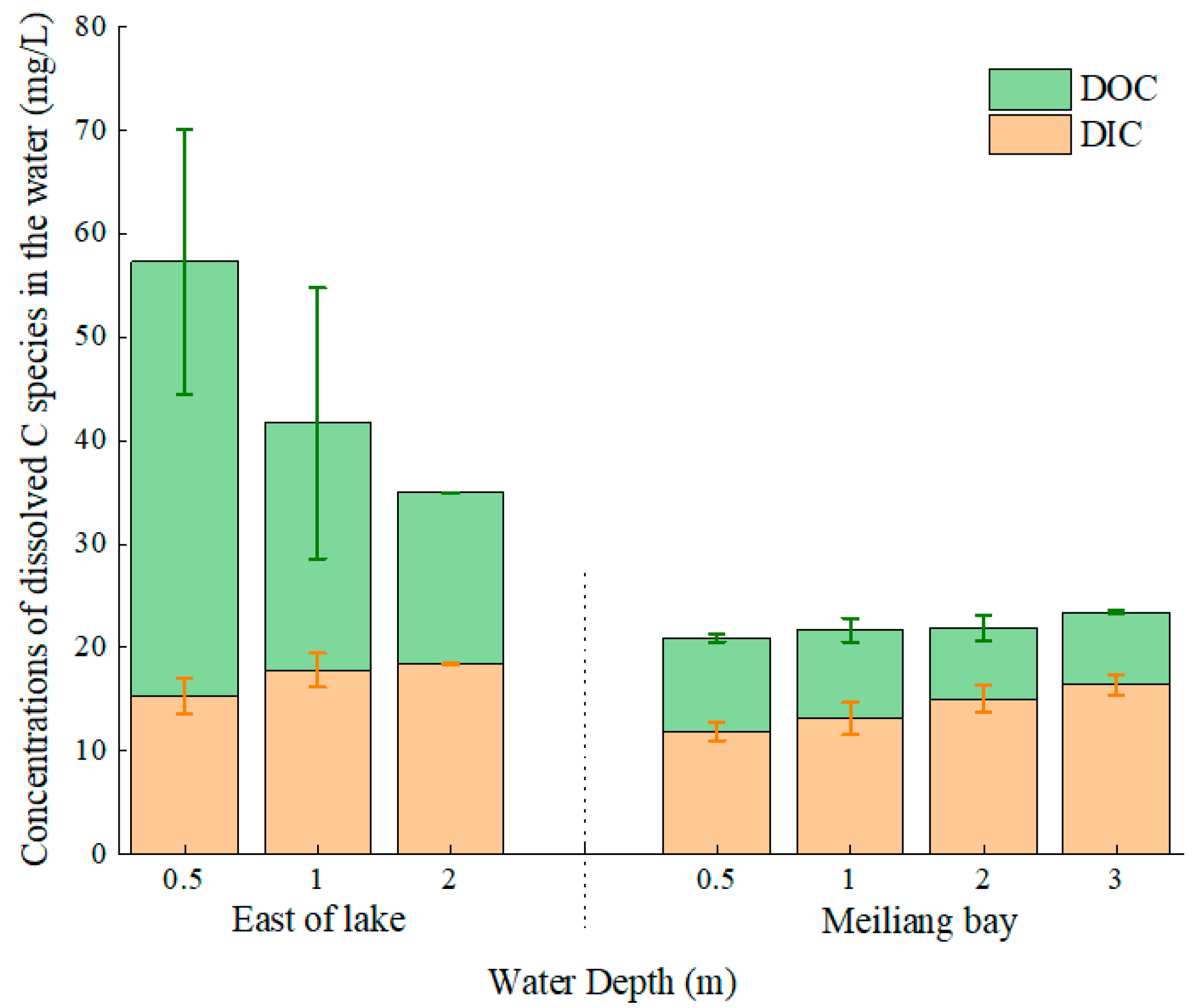
3.1. Chla and Dissolved C Species in the Water
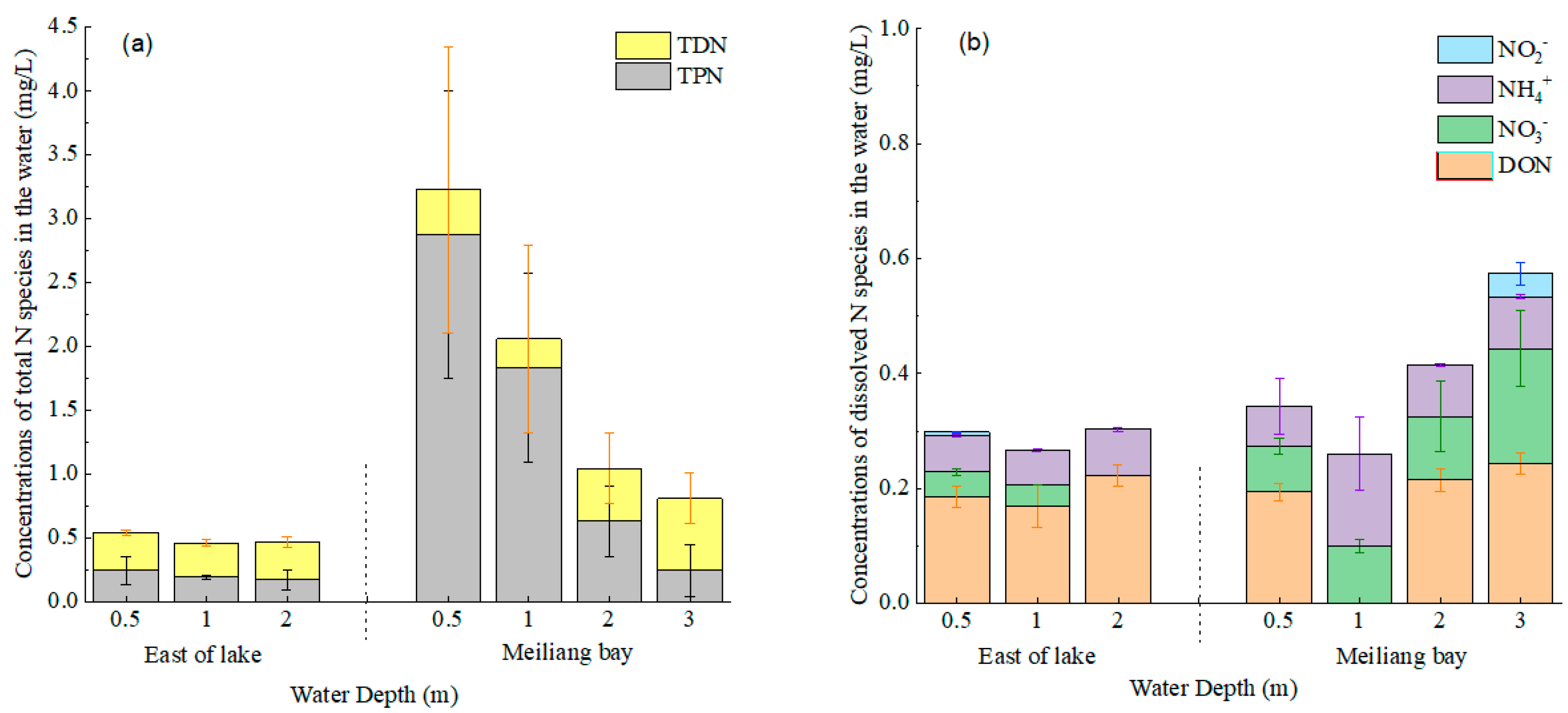
3.2. Water N Species
3.3. Sediment N Species and Organic Matter
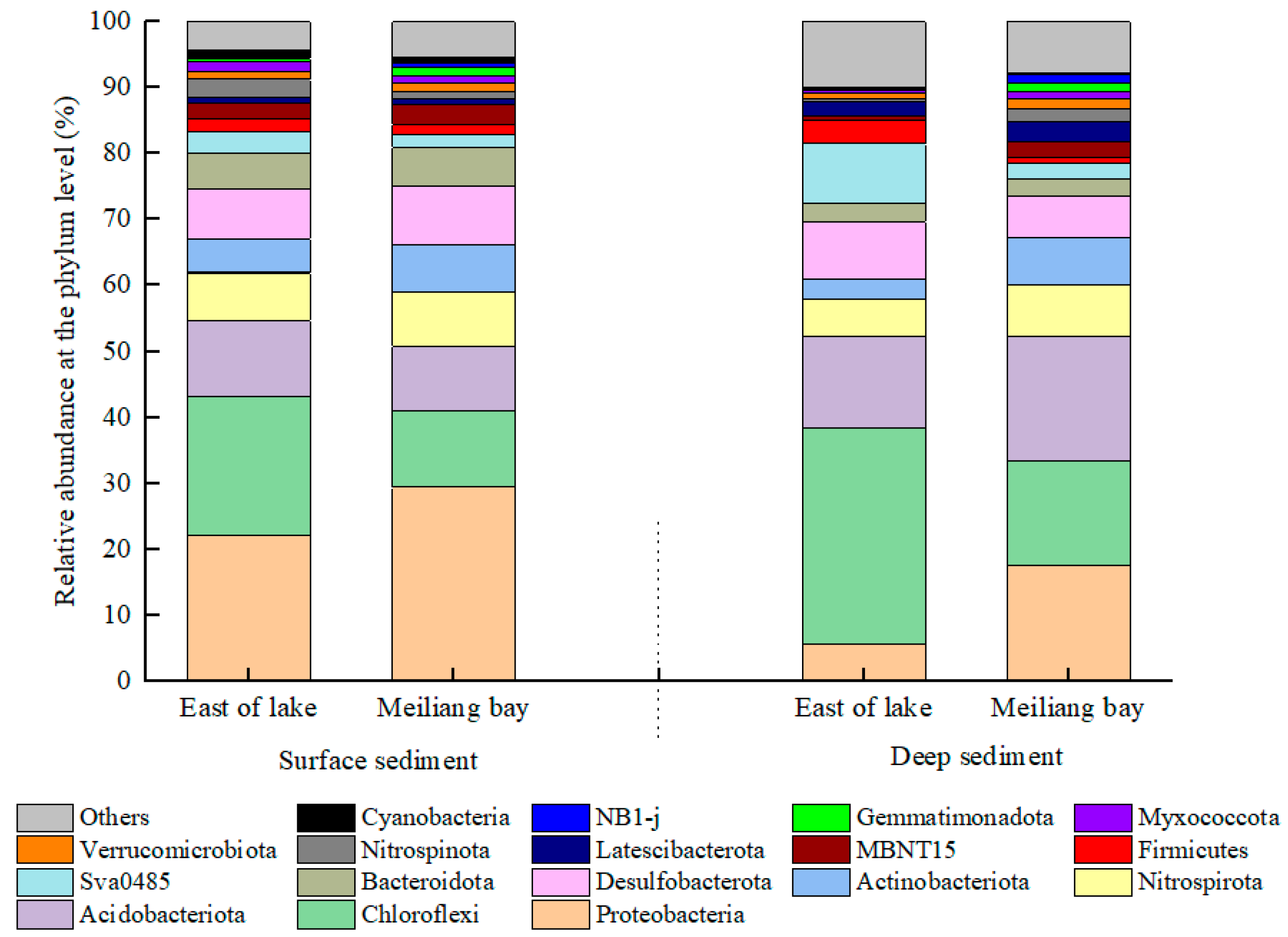
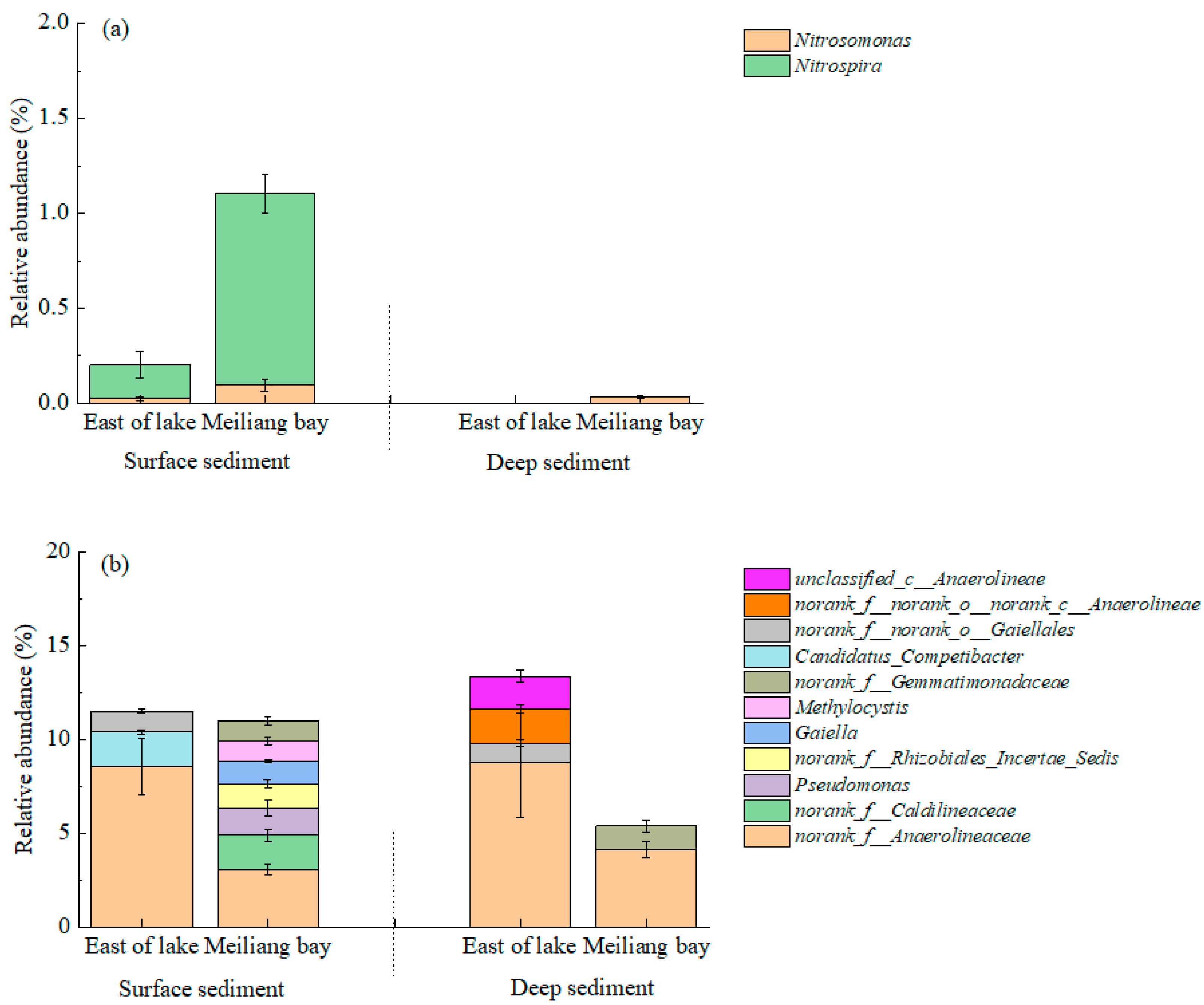
3.4. Bacterial Community Structure
3.5. Relationship between Environmental Factors and Bacterial Community Structure
4. Discussion
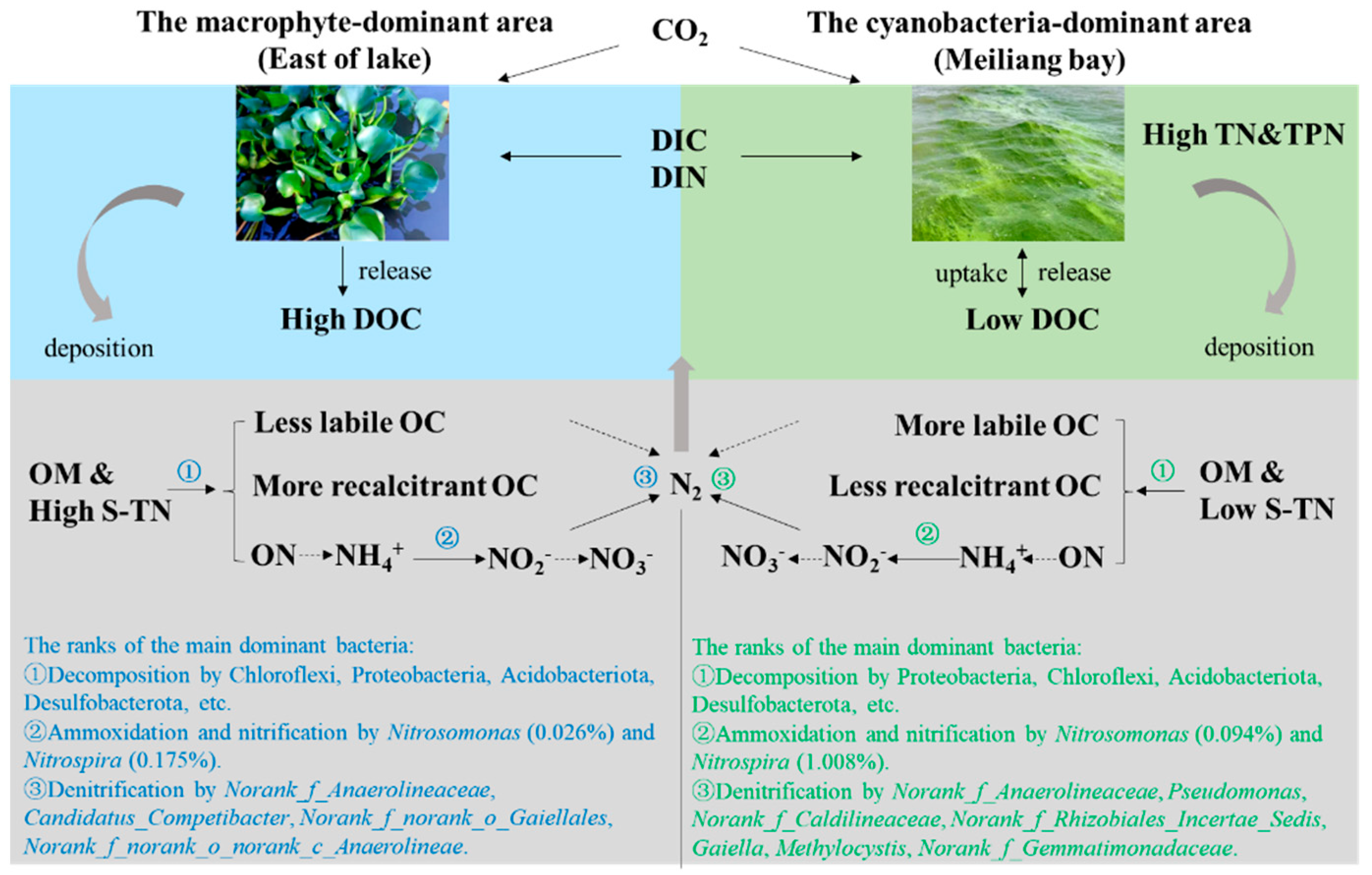
4.1. Vertical Migration and Transformation of C and N Driven by Cyanobacteria and Macrophyte Activities
4.2. Heterogeneity of Bacterial Communities in the Sediments and Their Potential Influences on C and N Transformation
4.3. Environmental Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, T.; Qin, B.; Brookes, J.D.; Yan, W.; Ji, X.; Feng, J. Spatial distribution of sediment nitrogen and phosphorus in Lake Taihu from a hydrodynamics-induced transport perspective. Sci. Total Environ. 2019, 650, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.J.; Wollheim, W.M.; Mulholland, P.J.; Webster, J.R.; Meyer, J.L.; Tank, J.L.; Marti, E.; Bowden, W.B.; Valett, H.M.; Hershey, A.E.; et al. Control of nitrogen export from watersheds by headwater streams. Science 2001, 292, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Review of Inorganic Nitrogen Transformations and Effect of Global Climate Change on Inorganic Nitrogen Cycling in Ocean Ecosystems. Ocean Sci. J. 2016, 51, 159–167. [Google Scholar] [CrossRef]
- Ryther, J.H.; Dunstan, W.M. Nitrogen, Phosphorus, and Eutrophication in the Coastal Marine Environment. Science 1971, 171, 1008–1013. [Google Scholar] [CrossRef]
- Jiang, H.Y. Study on Nitrogen and Carbon Cycles in Lakes in the Middle and Late Period of Algal Bloom Outbreak. Master’s Thesis, Yangzhou University, Yangzhou, China, 2017. [Google Scholar]
- Zeng, S.B.; Liu, H.; Liu, Z.H.; Kaufmann, G.; Zeng, Q.R.; Chen, B. Seasonal and diurnal variations in DIC, NO3- and TOC concentrations in Check for spring-pond ecosystems under different land-uses at the Shawan Karst Test Site, SW China: Carbon limitation of aquatic photosynthesis. J. Hydrol. 2019, 574, 811–821. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, M.; Shi, X.; Kong, F.; Ma, R.; Yu, Y.J.W.R. Nutrient reduction magnifies the impact of extreme weather on cyanobacterial bloom formation in large shallow Lake Taihu (China). Water Res. 2016, 103, 302–310. [Google Scholar] [CrossRef]
- Zhao, H.J.; Jiang, Y.J.; Xiao, Q.; Zhang, C.; Behzad, H.M. Coupled carbon-nitrogen cycling controls the transformation of dissolved inorganic carbon into dissolved organic carbon in karst aquatic systems. J. Hydrol. 2021, 592, 125764. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, H.Z.; Jeppesen, E.; Xu, C.; Wang, H.J. Reply to Cao et al.’s comment on “Does the responses of Vallisneria natans (Lour.) Hara to high nitrogen loading differ between the summer high-growth season and the low-growth season? Science of the Total Environment 2017, 601–602, 1513–1521”. Sci. Total Environ. 2018, 615, 1093–1094. [Google Scholar] [CrossRef]
- Setien, I.; Fuertes-Mendizabal, T.; Gonzalez, A.; Aparicio-Tejo, P.M.; Gonzalez-Murua, C.; Gonzalez-Moro, M.B.; Estavillo, J.M. High irradiance improves ammonium tolerance in wheat plants by increasing N assimilation. J. Plant Physiol. 2013, 170, 758–771. [Google Scholar] [CrossRef]
- Su, H.J.; Wu, Y.; Xie, P.; Chen, J.; Cao, T.; Xia, W.L. Effects of taxonomy, sediment, and water column on C:N:P stoichiometry of submerged macrophytes in Yangtze floodplain shallow lakes, China. Environ. Sci. Pollut. Res. 2016, 23, 22577–22585. [Google Scholar] [CrossRef]
- Xiao, L.W.; Zhu, B.; Kumwimba, M.N.; Jiang, S.W. Plant soaking decomposition as well as nitrogen and phosphorous release in the water-level fluctuation zone of the Three Gorges Reservoir. Sci. Total Environ. 2017, 592, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, S.; Peng, X.; Liu, B.; Zhang, X.; Ge, F.; Zhou, Q.; Wu, Z. Potential ecological implication of Cladophora oligoclora decomposition: Characteristics of nutrient migration, transformation, and response of bacterial community structure. Water Res. 2021, 190, 116741. [Google Scholar] [CrossRef] [PubMed]
- Sima, W.; Hu, M.; He, Q.; Qiu, Y.; Lv, Y.; Dai, L.; Shao, Q.; Zhou, T.; Li, H.; Zhou, M. Regulation of nitrogen dynamics at the sediment–water interface during HAB degradation and subsequent reoccurrence. RSC Adv. 2020, 10, 13480–13488. [Google Scholar] [CrossRef] [PubMed]
- Liorens-Mares, T.; Yooseph, S.; Goll, J.; Hoffman, J.; Vila-Costa, M.; Borrego, C.M.; Dupont, C.L.; Casamayor, E.O. Connecting biodiversity and potential functional role in modern euxinic environments by microbial metagenomics. ISME J. 2015, 9, 1648–1661. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Wu, J.P.; Hong, Y.G.; Jiao, L.J.; Li, Y.B.; Wang, L.M.; Wang, Y.; Chang, X.Y. Nitrogen loss by nirS-type denitrifying bacterial communities in eutrophic coastal sediments. Int. Biodeterior. Biodegrad. 2020, 150, 1–9. [Google Scholar] [CrossRef]
- Dai, Z.M.; Liu, G.F.; Chen, H.H.; Chen, C.R.; Wang, J.K.; Ai, S.Y.; Wei, D.; Li, D.M.; Ma, B.; Tang, C.X.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Cai, W.; Li, Y.; Shen, Y.; Wang, C.; Wang, P.F.; Wang, L.F.; Niu, L.H.; Zhang, W.L. Vertical distribution and assemblages of microbial communities and their potential effects on sulfur metabolism in a black-odor urban river. J. Environ. Manag. 2019, 235, 368–376. [Google Scholar] [CrossRef]
- Abell, G.C.J.; Revill, A.T.; Smith, C.; Bissett, A.P.; Volkman, J.K.; Robert, S.S. Archaeal ammonia oxidizers and nirS-type denitrifiers dominate sediment nitrifying and denitrifying populations in a subtropical macrotidal estuary. ISME J. 2010, 4, 286–300. [Google Scholar] [CrossRef]
- Wu, D.X.; Cheng, M.L.; Zhao, S.M.; Peng, N.; Hu, R.G.; Hu, J.L.; Liang, Y.X. Algal Growth Enhances Light-Mediated Limitation of Bacterial Nitrification in an Aquaculture System. Water Air Soil Pollut. 2020, 231, 73. [Google Scholar] [CrossRef]
- Fan, X.F.; Ding, S.M.; Gong, M.D.; Chen, M.S.; Gao, S.S.; Jin, Z.F.; Tsang, D.C.W. Different Influences of Bacterial Communities on Fe (III) Reduction and Phosphorus Availability in Sediments of the Cyanobacteria- and Macrophyte-Dominated Zones. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Qin, B.; Zhu, G.; Gao, G.; Zhang, Y.; Li, W.; Paerl, H.W.; Carmichael, W.W. A Drinking Water Crisis in Lake Taihu, China: Linkage to Climatic Variability and Lake Management. Environ. Manag. 2010, 45, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.J.; Li, J.; Chen, G.X. The current situation of cyanobacteria outbreak in Lake Taihu and innovative control measures. World Trop. Agric. Inf. 2021, 4, 58–59. (In Chinese) [Google Scholar]
- Editorial Board of Water and Wastewater Monitoring and Analysis Methods. Water and Wastewater Monitoring and Analysis Methods; China Environmental Science Press: Beijing, China, 2002; pp. 670–671. (In Chinese) [Google Scholar]
- Qian, B.; Liu, L.; Xiao, X. Comparative tests on different methods for content of soil organic matter. J. Hohai Univ. 2011, 39, 34–38. (In Chinese) [Google Scholar]
- Liu, B.; Zhou, F.; Wang, G.; Xu, K.; Xia, J. Research progress on forms of nitrogen and determination in the sediments. Acta Ecol. Sin. 2011, 31, 6947–6958. (In Chinese) [Google Scholar]
- Raymond, P.A.; Oh, N.H.; Turner, R.E.; Broussard, W. Anthropogenically enhanced fluxes of water and carbon from the Mississippi River. Nature 2008, 451, 449–452. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Lei, J.Q.; Hu, L.C.; Xiao, Q.; Wang, J.L.; Zhang, C.; Ali, H. Biogeochemical and physical controls on the evolution of dissolved inorganic carbon (DIC) and delta C-13(DIC) in karst spring-waters exposed to atmospheric CO2(g): Insights from laboratory experiments. J. Hydrol. 2020, 583, 124294. [Google Scholar] [CrossRef]
- Trimmer, M.; Grey, J.; Heppell, C.M.; Hildrew, A.G.; Lansdown, K.; Stahl, H.; Yvon-Durocher, G. River bed carbon and nitrogen cycling: State of play and some new directions. Sci. Total Environ. 2012, 434, 143–158. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Westerhoff, P.; Baker, L.; Hu, Q.; Esparza-Soto, M.; Sommerfeld, M. Characteristics and reactivity of algae-produced dissolved organic carbon. J. Environ. Eng. 2005, 131, 1574–1582. [Google Scholar] [CrossRef]
- Mueller, B.; den Haan, J.; Visser, P.M.; Vermeij, M.J.A.; van Duyl, F.C. Effect of light and nutrient availability on the release of dissolved organic carbon (DOC) by Caribbean turf algae. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Jimenez-Ramos, R.; Tomas, F.; Reynes, X.; Romera-Castillo, C.; Perez-Llorens, J.L.; Egea, L.G. Carbon metabolism and bioavailability of dissolved organic carbon (DOC) fluxes in seagrass communities are altered under the presence of the tropical invasive alga Halimeda incrassata. Sci. Total Environ. 2022, 839, 156325. [Google Scholar] [CrossRef]
- Kamjunke, N.; Tittel, J. Mixotrophic Algae Constrain the Loss of Organic Carbon by Exudation. J. Phycol. 2009, 45, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, O.; Colmer, T.D.; Sand-Jensen, K. Underwater photosynthesis of submerged plants—Recent advances and methods. Front. Plant Sci. 2013, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Noges, P.; Cremona, F.; Laas, A.; Martma, T.; Room, E.I.; Toming, K.; Viik, M.; Vilbaste, S.; Noges, T. Role of a productive lake in carbon sequestration within a calcareous catchment. Sci. Total Environ. 2016, 550, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.L.; Zhu, W.Q.; Pan, M.; Zhou, Z.M.; Li, S.Y. Characteristics and evaluation for nitrogen pollution in water and surface sediments of Xixi Wetland. In Proceedings of the 2013 Third International Conference on Intelligent System Design and Engineering Applications (ISDEA), Hong Kong, China, 16–18 January 2013; pp. 419–423. [Google Scholar]
- Wang, Y.G.; Chen, X.H.; Guo, W.B.; Zhou, H.B. Distinct bacterial and archaeal diversities and spatial distributions in surface sediments of the Arctic Ocean. FEMS Microbiol. Lett. 2018, 365, 273. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.M.; Lu, P.L.; Lu, P.Z.; Zhang, H. Effects of river sediment addition to the beach microbial communities. Toxicol. Environ. Chem. 2014, 96, 68–83. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, Y.Y.; Wu, Z.; Feng, Q.Y.; Xie, S.G.; Liu, Y. Spatiotemporal variation of planktonic and sediment bacterial assemblages in two plateau freshwater lakes at different trophic status. Appl. Microbiol. Biotechnol. 2016, 100, 4161–4175. [Google Scholar] [CrossRef]
- Bennett, A.C.; Murugapiran, S.K.; Kees, E.D.; Sauer, H.M.; Hamilton, T.L. Temperature and Geographic Location Impact the Distribution and Diversity of Photoautotrophic Gene Variants in Alkaline Yellowstone Hot Springs. Microbiol. Spectr. 2022, 10, e0146521. [Google Scholar] [CrossRef]
- Atashgahi, S.; Aydin, R.; Dimitrov, M.R.; Sipkema, D.; Hamonts, K.; Lahti, L.; Maphosa, F.; Kruse, T.; Saccenti, E.; Springael, D.; et al. Impact of a wastewater treatment plant on microbial community composition and function in a hyporheic zone of a eutrophic river. Sci. Rep. 2015, 5, 17284. [Google Scholar] [CrossRef]
- Bian, X.; Gong, H.; Wang, K.J. Pilot-Scale Hydrolysis-Aerobic Treatment for Actual Municipal Wastewater: Performance and Microbial Community Analysis. Int. J. Environ. Res. Public Health 2018, 15, 477. [Google Scholar] [CrossRef]
- Kandasamy, S.; Weerasuriya, N.; White, J.F.; Patterson, G.; Lazarovits, G. Chapter 23—Soil’s Physical and Nutritional Balance Is Essential for Establishing a Healthy Microbiome. In Microbiome Stimulants for Crops; Woodhead Publishing: Sawston, UK, 2021; pp. 381–404. [Google Scholar]
- Cheng, W.; Zhang, J.X.; Wang, Z.; Wang, M.; Xie, S.G. Bacterial communities in sediments of a drinking water reservoir. Ann. Microbiol. 2014, 64, 875–878. [Google Scholar] [CrossRef]
- Langwig, M.V.; De Anda, V.; Dombrowski, N.; Seitz, K.W.; Rambo, I.M.; Greening, C.; Teske, A.P.; Baker, B.J. Large-scale protein level comparison of Deltaproteobacteria reveals cohesive metabolic groups. ISME J. 2022, 16, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Wymore, A.S.; Liu, C.M.; Hungate, B.A.; Schwartz, E.; Price, L.B.; Whitham, T.G.; Marks, J.C. The Influence of Time and Plant Species on the Composition of the Decomposing Bacterial Community in a Stream Ecosystem. Microb. Ecol. 2016, 71, 825–834. [Google Scholar] [CrossRef]
- Du, J.J.; Zhao, G.Y.; Wang, F.Y.; Zhao, D.; Chen, X.X.; Zhang, S.R.; Jia, Y.; Tian, X.J. Growth stimulation of Microcystis aeruginosa by a bacterium from hyper-eutrophic water (Taihu Lake, China). Aquat. Ecol. 2013, 47, 303–313. [Google Scholar] [CrossRef]
- Juretschko, S.; Timmermann, G.; Schmid, M.; Schleifer, K.H.; Pommerening-Roser, A.; Koops, H.P.; Wagner, M. Combined molecular and conventional analyses of nitrifying bacterium diversity in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl. Environ. Microbiol. 1998, 64, 3042–3051. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.; Jayadradhan, R.K.V.; Pillai, D.; Geetha, P.P.; Joseph, V.; Sarojini, B.S.I. Nitrospira as versatile nitrifiers: Taxonomy, ecophysiology, genome characteristics, growth, and metabolic diversity. J. Basic Microbiol. 2021, 61, 88–109. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, G.; Zhao, L.; Yao, X.; Zhang, Y.; Gao, G.; Qin, B. Influence of algal bloom degradation on nutrient release at the sediment–water interface in Lake Taihu, China. Environ. Sci. Pollut. Res. 2013, 20, 1803–1811. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Wu, H.; Sun, N.; Tang, Y.; Dai, Y.; Dai, T. Differences in Carbon and Nitrogen Migration and Transformation Driven by Cyanobacteria and Macrophyte Activities in Taihu Lake. Int. J. Environ. Res. Public Health 2023, 20, 371. https://doi.org/10.3390/ijerph20010371
Han C, Wu H, Sun N, Tang Y, Dai Y, Dai T. Differences in Carbon and Nitrogen Migration and Transformation Driven by Cyanobacteria and Macrophyte Activities in Taihu Lake. International Journal of Environmental Research and Public Health. 2023; 20(1):371. https://doi.org/10.3390/ijerph20010371
Chicago/Turabian StyleHan, Chaonan, Hao Wu, Ningning Sun, Yu Tang, Yan Dai, and Tianhao Dai. 2023. "Differences in Carbon and Nitrogen Migration and Transformation Driven by Cyanobacteria and Macrophyte Activities in Taihu Lake" International Journal of Environmental Research and Public Health 20, no. 1: 371. https://doi.org/10.3390/ijerph20010371
APA StyleHan, C., Wu, H., Sun, N., Tang, Y., Dai, Y., & Dai, T. (2023). Differences in Carbon and Nitrogen Migration and Transformation Driven by Cyanobacteria and Macrophyte Activities in Taihu Lake. International Journal of Environmental Research and Public Health, 20(1), 371. https://doi.org/10.3390/ijerph20010371





