Health Risk of Heavy Metals Related to Consumption of Vegetables in Areas of Industrial Impact in the Republic of Kazakhstan—Case Study for Oskemen
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery from Various Environments—A review. Front. Environ. Sci. 2019, 7, 66. [Google Scholar] [CrossRef]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: A Review. Water Air Soil Poll. 2019, 230, 164. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity and amelioration-A review. Sci. Total Environ. 2019, 651, 2927–2942. [Google Scholar] [CrossRef]
- Sandeep, G.; Vijayalatha, K.R.; Anitha, T. Heavy metals and its impact in vegetable crops. Int. J. Chem. Stud. 2019, 7, 1612–1621. [Google Scholar]
- Nag, R.; O’Rourke, S.M.; Cummins, E. Risk factors and assessment strategies for the evaluation of human or environmental risk from metal(loid)s—A focus on Ireland. Sci. Total Environ. 2022, 802, 149839. [Google Scholar] [CrossRef]
- Vanisree, C.R.; Sankhla, M.S.; Singh, P.; Jadhav, E.B.; Verma, R.K.; Awasthi, K.K.; Awasthi, G.; Naga, V. Heavy metal contamination of food crops: Transportation via food chain, human consumption, toxicity and management strategies. In Environmental Impact and Remediation of Heavy Metals; Salek, H., Hassam, A., Eds.; IntechOpen: London, UK, 2022. [Google Scholar]
- Manzoor, J.; Sharma, M.; Wani, K.A. Heavy metal in vegetables and their impact on the nutrient quality of vegetables: A review. J. Plant Nutr. 2018, 41, 1744–1763. [Google Scholar] [CrossRef]
- Ćwieląg-Drabek, M.; Piekut, A.; Gut, K.; Grabowski, M. Risk of cadmium, lead and zinc exposure from consumption of vegetables produced in areas with mining and smelting past. Sci. Rep. 2020, 10, 3363. [Google Scholar] [CrossRef]
- Jakubus, M.; Graczyk, M. The Effect of Compost and Fly Ash Treatment of Contaminated Soil on the Immobilisation and Bioavailability of Lead. J. Agronomy 2021, 11, 1188. [Google Scholar] [CrossRef]
- Jakubus, M.; Bakinowska, E. The effect of immobilizing agents on Zn and Cu availability for plants in relation to their potential health risk. Appl. Sci. 2022, 12, 6538. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Sharma, A.; Bakshi, P.; Sharna, P.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. Ecological and human health risks appraisal of metal(loid)s in agricultural soils: A review. Geol. Ecol. Landsc. 2021, 5, 173–185. [Google Scholar] [CrossRef]
- Gupta, N.; Yadaw, K.K.; Kumar, V.; Krishnan, S.; Kumar, S.; Nejad, Z.D.; Khan, M.A.; Alam, J. Evaluating heavy metals contamination in soil and vegetables in the region of North India: Levels, transfer and potential human health risk analysis. Environ. Toxicol. Pharmacol. 2021, 82, 103563. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Bilal, M.; Asghar, W.; Azeem, M.; Ahmad, M.I.; Abbas, A.; Ahmad, M.Z.; Shahzad, T. Heavy metal accumulation in vegetables and assessment of their potential health risk. J. Environ. Anal. Chem. 2018, 5, 234. [Google Scholar] [CrossRef]
- Guo, G.; Zhang, D.; Wang, Y. Probabilistic human health risk assessment of heavy metal intake via vegetable consumption around Pb/Zn smelters in southwest China. Int. J. Environ. Res. Public Health 2019, 16, 3267. [Google Scholar] [CrossRef]
- Leblebeci, Z.; Kar, M. Heavy metals accumulation in vegetables irrigated with different water sources and their human daily intake in Nevsehir. J. Agric. Sci. Technol. 2018, 20, 401–415. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Boluspaeva, L.; Panin, M.; Mocek, A.; Spychalski, W. Heavy metals in soils of the town of Ust-Kamenogorsk in the Republic of Kazakhstan. Soil Sci. Annu. 2011, 62, 40–47. [Google Scholar]
- ISO 11466; Soil Quality-Extraction of Trace Elements Soluble in Aqua Regia. International Organization of Standardization: Genève, Switzerland, 1995.
- Ostrowska, A.; Gawliński, S.; Szczubialka, Z. Methods for Analysis and Evaluation of Soil and Plant Properties, 1st ed.; IOŚ Warszawa: Warszawa, Poland, 1991; pp. 158–167. (In Polish) [Google Scholar]
- WHO. Food Safety Issue. GEMS/FOOD. Regional Diets. Food Safety Department WHO. 2003. Available online: https://apps.who.int/iris/handle/10665/42833 (accessed on 22 November 2022).
- Jan, F.A.; Ishaq, M.; Khan, S.; Ihsanullah, I.; Ahmad, I.; Schakirullah, H. A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir). J. Hazard. Mater. 2010, 179, 612–621. [Google Scholar] [CrossRef]
- US-EPA. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures. EPA/630/R-00/002. 2000. Available online: https://ordspub.epa.gov/ords/eims/eimscomm.getfile?p_download_id=4486 (accessed on 18 November 2022).
- Crispo, M.; Dosbon, M.C.; Blevins, R.S.; Meredith, W.; Lake, J.A.; Edmonson, J.L. Heavy metals and metalloids concentrations across UK urban horticultural soils and the factors infuencing their bioavailability to food crops. Environ. Pollut. 2021, 288, 117960. [Google Scholar] [CrossRef]
- Tobarra, M.A.; López, L.A.; Cadarso, M.A.; Gómez, N.; Cazcarro, I. Is seasonal households’ consumption good for the nexus carbon/water footprint? Te Spanish fruits and vegetables case. Environ. Sci. Technol. 2018, 52, 12066–12077. [Google Scholar] [CrossRef]
- Proshad, R.; Kormoker, T.; Islam, S.M.; Chandra, K. Potential health risk of heavy metals via consumption of rice and vegetables grown in the industrial areas of Bangladesh. Hum. Ecol. Risk Assess. 2019, 26, 921–943. [Google Scholar] [CrossRef]
- Woszczyk, M.; Spychalski, W.; Boluspaeva, L. Trace metal (Cd, Cu, Pb, Zn) fractionation in urban-industrial soils of Ust-Kamenogorsk (Oskemen), Kazakhstan—Implications for the assessment of environmental quality. Environ. Monit. Assess. 2018, 190, 362. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, D.; Proshad, R.; Uwiringiyimana, E.; Wang, Z. Assessment of the pollution levels of potential toxic elements in urban vegetable gardens in southwest China. Sci. Rep. 2021, 11, 22824. [Google Scholar] [CrossRef] [PubMed]
- Laaouidi, Y.; Bahmed, A.; Naylo, A.; El Khalil, H.; Ouvrard, S.; Schwartz, C.; Boularbah, A. Trace Elements in Soils and Vegetables from Market Gardens of Urban Areas in Marrakech City. Biol. Trace Elem. Res. 2020, 195, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Order of the Minister of Health of the Republic of Kazakhstan dated 21 April 2021. On Approval of the Hygienic Standards for the Safety of the Environment. Available online: https://adilet.zan.kz/rus/docs/V2100022595 (accessed on 19 December 2022).
- Standards for Maximum Permissible Concentrations of Harmful Substances, Harmful Organisms and Other Biological Substances that Pollute the Soil, Approved by the Joint Order of the Ministry of Health of the Republic of Kazakhstan Dated 30 January 2004 № 99 and the Ministry of Environmental Protection of the Republic of Kazakhstan dated January 27, 2004. Available online: https://online.zakon.kz/Document/?doc_id=1046570&pos=32;-47#pos=32;-47 (accessed on 19 December 2022).
- Directive 86/278/EWG. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31986L0278 (accessed on 22 November 2022).
- Ashraf, I.; Ahmad, F.; Sharif, A.; Altaf, A.R.; Teng, H. Heavy Metals Assessment in Water, Soil, Vegetables and Their Associated Health Risks via Consumption of Vegetables, District Kasur, Pakistan. SN Appl. Sci. 2021, 3, 552. [Google Scholar] [CrossRef]
- Moghtaderi, T.; Mahmoudi, S.; Shakeri, A.; Masihabadi, H.M. Heavy Metals Contamination and Human Health Risk Assessment in Soils of an Industrial Area, Bandar Abbas—South Central Iran. Hum. Ecol. Risk Assess. 2018, 24, 1058–1073. [Google Scholar] [CrossRef]
- Chowdhury, M.A.H.; Chowdhury, T.; Rahman, M.A. Heavy metal accumulation in tomato and cabbage grown in some industrially contaminated soils of Bangladesh: Heavy metals in contaminated soils and vegetables. J. Bangladesh Agric. Univ. 2019, 17, 288–294. [Google Scholar] [CrossRef]
- Haque, M.M.; Niloy, M.N.; Khirul, A.M.; Alam, F.M.; Tareq, M.S. Appraisal of Probabilistic Human Health Risks of Heavy Metals in Vegetables from Industrial, Non-industrial and Arsenic Contaminated Areas of Bangladesh. Heliyon 2021, 7, e06309. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, J. Risk Assessment of Potentially Toxic Elements (PTEs) Pollution at a Rural Industrial Wasteland in an Abandoned Metallurgy Factory in North China. Int. J. Environ. Res. Public Health 2018, 15, 85. [Google Scholar] [CrossRef]
- Murray, H.; Pinchin, T.A.; Macfie, S.M. Compost application affects metal uptake in plants grown in urban garden soils and potential human health risk. J. Soils Sediments 2011, 11, 815–829. [Google Scholar] [CrossRef]
- Sung, Y.C.; Park, C.B. The effect of site- and landscape-scale factors on lead contamination of leafy vegetables grown in urban gardens. Lands. Urban Plan. 2018, 177, 38–46. [Google Scholar] [CrossRef]
- ALINORM 01/12A. 24; Food additives and contaminants, Joint Codex Alimentarius Commission, FAO/WHO. Food standards Programme, FAO/WHO: Geneve, Switzerland, 2001.
- Yang, Y.; Zhang, F.S.; Li, H.F.; Jiang, R.F. Accumulation of cadmium in the edible parts of six vegetable species grown in Cd-contaminated soils. J. Environ. Manag. 2009, 90, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Prasad, S.; Cabral-Pinto, M.M.S.; Jeon, B.-H.; Kumar, S.; Abdellattif, M.H.; Alsukaibia, A.K.D. Investigation of Heavy Metal Accumulation in Vegetables and Health Risk to Humans from Their Consumption. Front. Environ. Sci. 2022, 10, 791052. [Google Scholar] [CrossRef]
- Moyo, B.; Matodzi, V.; Legodi, A.M.; Pakade, V.E.; Tavengwa, N.T. Determination of Cd, Mn and Ni accumulated in fruits, vegetables and soil in the Thohoyandou town area, South Africa. Water S.A. 2020, 46, 285–290. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, W.; Zhou, X.; Liu, L.; Gu, J.; Wang, W.; Zou, J.; Tian, T.; Peng, P.; Liao, B. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef]
- Jakubus, M.; Bakinowska, E.; Tatuśko-Krygier, N. Compost utilisation in a heavy metal immobilisation process evaluated by bioconcentration factors. J. Elem. 2019, 24, 1291–1307. [Google Scholar] [CrossRef]
- Rai, K.P.; Lee, S.S.; Zhan, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health, risk, fate, mechanism and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; He, M.; Zeng, F.; Li, X. Accumulation of heavy metals in native plants growing on mining-influenced sites in Jinchang: A typical industrial city (China). Environ. Earth Sci. 2017, 76, 446–461. [Google Scholar] [CrossRef]
- Hu, B.; Jia, X.; Hu, J.; Xu, D.; Xia, F.; Li, Y. Assessment of heavy metal pollution and health risks in the soil—plant—human system in the Yangtze River Delta, China. Int. J. Environ. Res. Public Health 2017, 14, 1042. [Google Scholar] [CrossRef]
- Bounar, A.; Boukaka, K.; Leghouchi, E. Determination of Heavy Metals in Tomatoes Cultivated under green Houses and Human Health Risk Assessment. Qual. Assur. Saf. Crops Foods 2020, 12, 76–86. [Google Scholar] [CrossRef]
- Al-Qahtani, K. Assessment of Heavy Metals Accumulation in Native Plant Species from Soils Contaminated in Riyadh City, Saudi Arabia. Life Sci. 2012, 9, 384–392. [Google Scholar]
- Zhong, T.; Xue, D.; Zhao, L.; Zhang, X. Concentration of heavy metals in vegetables and potential health risk assessment in China. Environ. Geochem. Health 2018, 40, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, Z.; Wang, J.; Guo, J.; Chen, X.; Zhuang, J. Health risk assessment of heavy metals via dietary intake of wheat grown in Tianjin sewage irrigation area. Ecotoxicology 2015, 24, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Malik, N.R. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab. J. Chem. 2014, 7, 91–99. [Google Scholar] [CrossRef]
- Alsafran, M.; Usman, K.; Rizwan, M.; Ahmed, T.; Al Jabri, H. The Carcinogenic and Non-Carcinogenic Health Risks of Metal(oid)s Bioaccumulation in Leafy Vegetables: A Consumption Advisory. Front. Environ. Sci. 2021, 9, 742269. [Google Scholar] [CrossRef]
- US-EPA. Reference dose (RfD): Description and use in Health Risk Assessments. Background Document 1A, Integrated Risk Information System (IRIS); United States Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Hu, J.; Wu, F.; Wu, S.; Cao, Z.; Lin, X.; Wong, M. Bioaccessibility, dietary exposure and human risk assessment of heavy metals from market vegetables in Hong Kong revealed with an in vitro gastrointestinal model. Chemosphere 2013, 91, 455–461. [Google Scholar] [CrossRef]
- Li, Z.Y.; Ma, Z.W.; Kuijp, T.J.; Yuan, Z.W.; Huang, L.S. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef]
- Galal, T.M.; Hassan, L.M.; Ahmed, D.A.; Alamri, S.A.M.; Alrumman, S.A.; Eid, E.M. Heavy metals uptake by the global economic crop (Pisum sativum L.) grown in contaminated soils and its associated health risks. PLoS ONE 2021, 16, e0252229. [Google Scholar] [CrossRef]
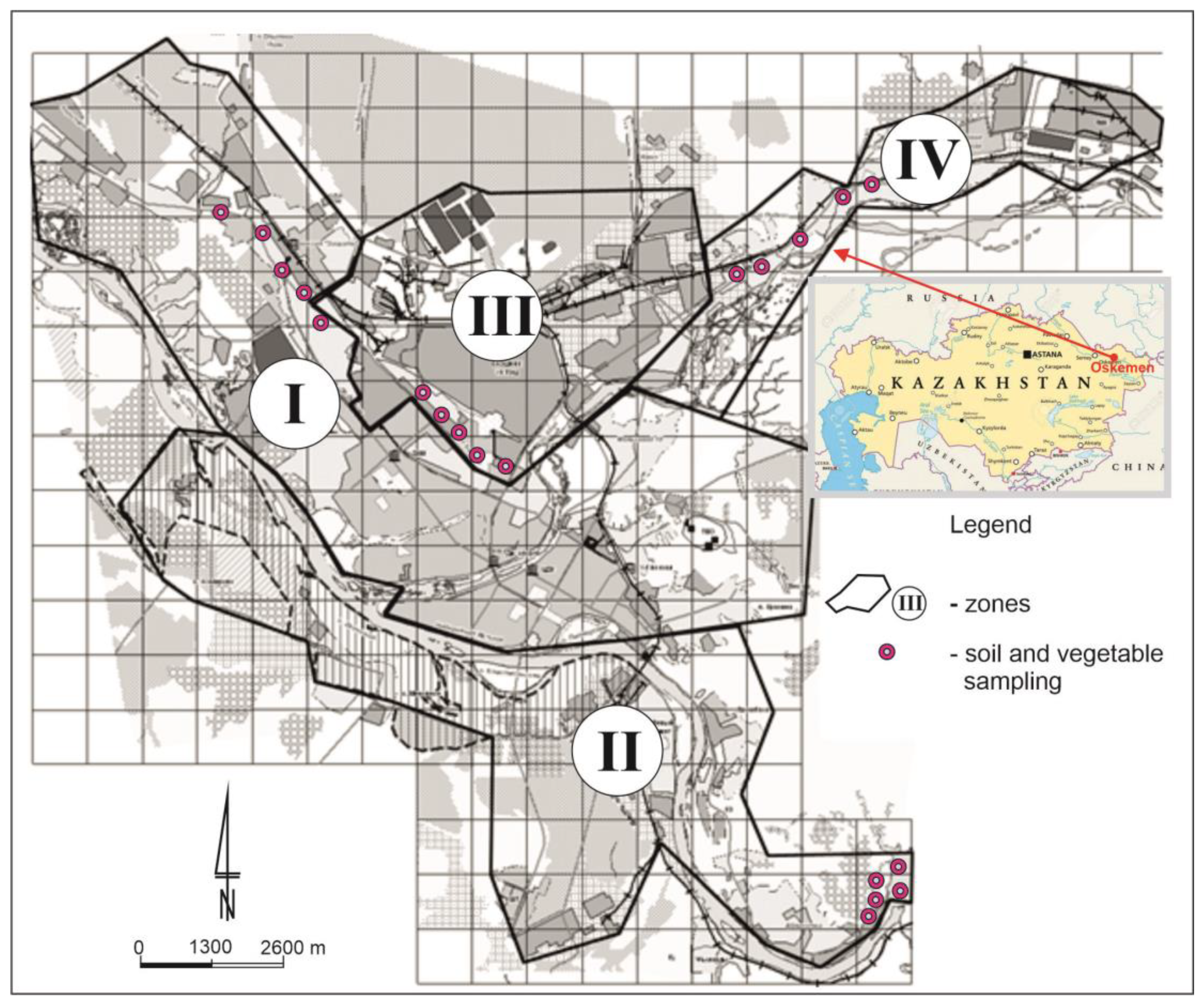
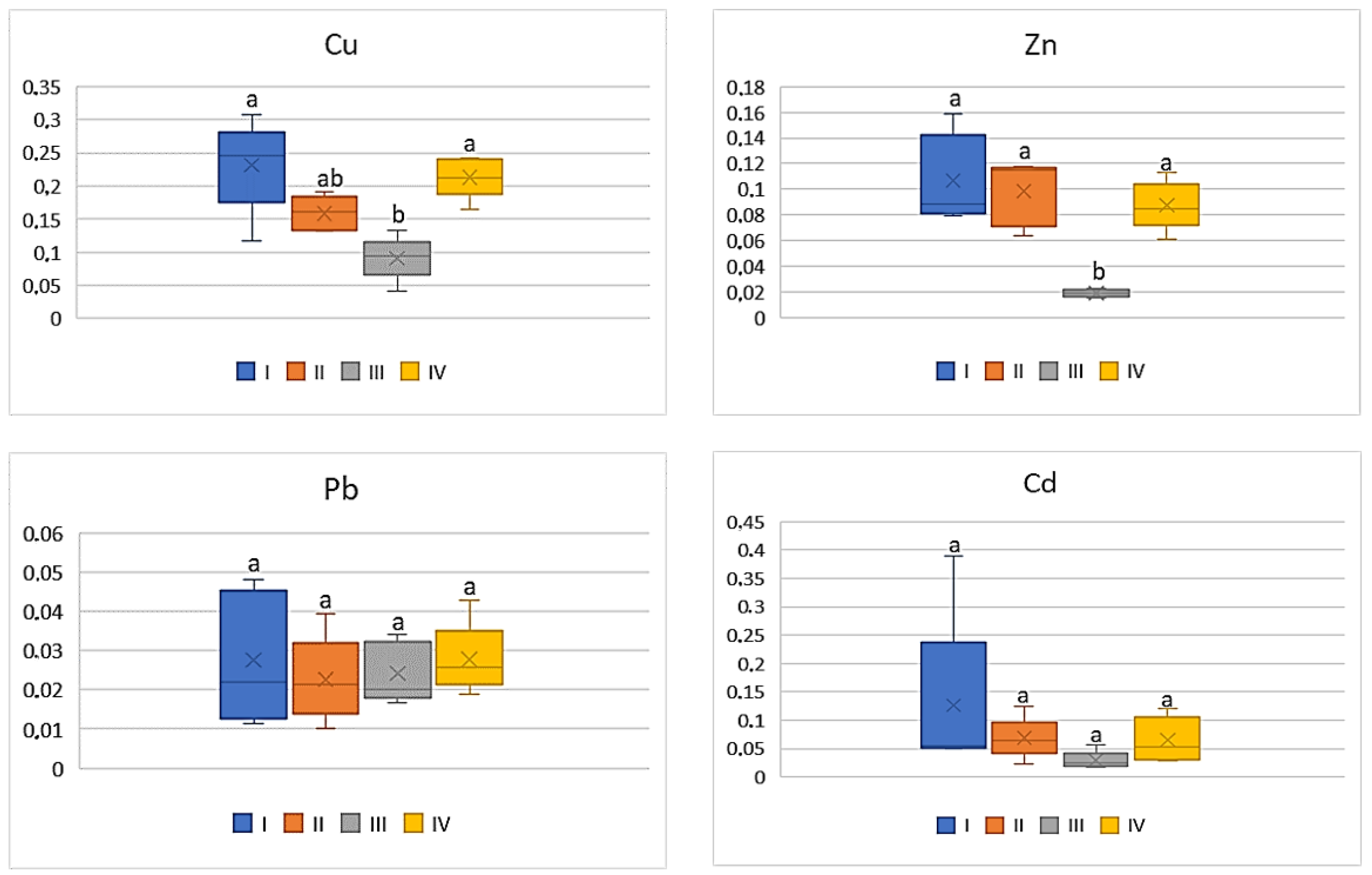
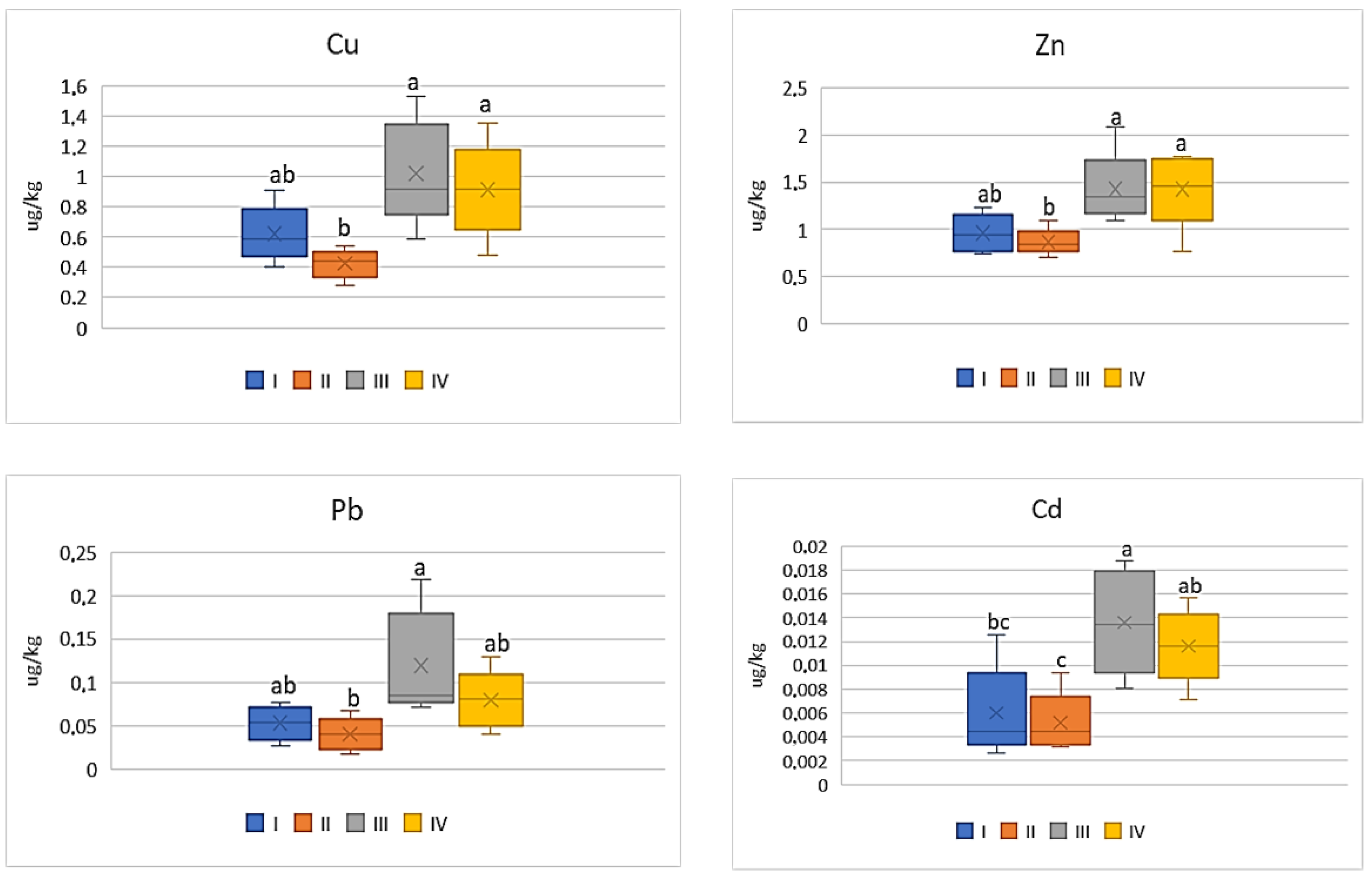

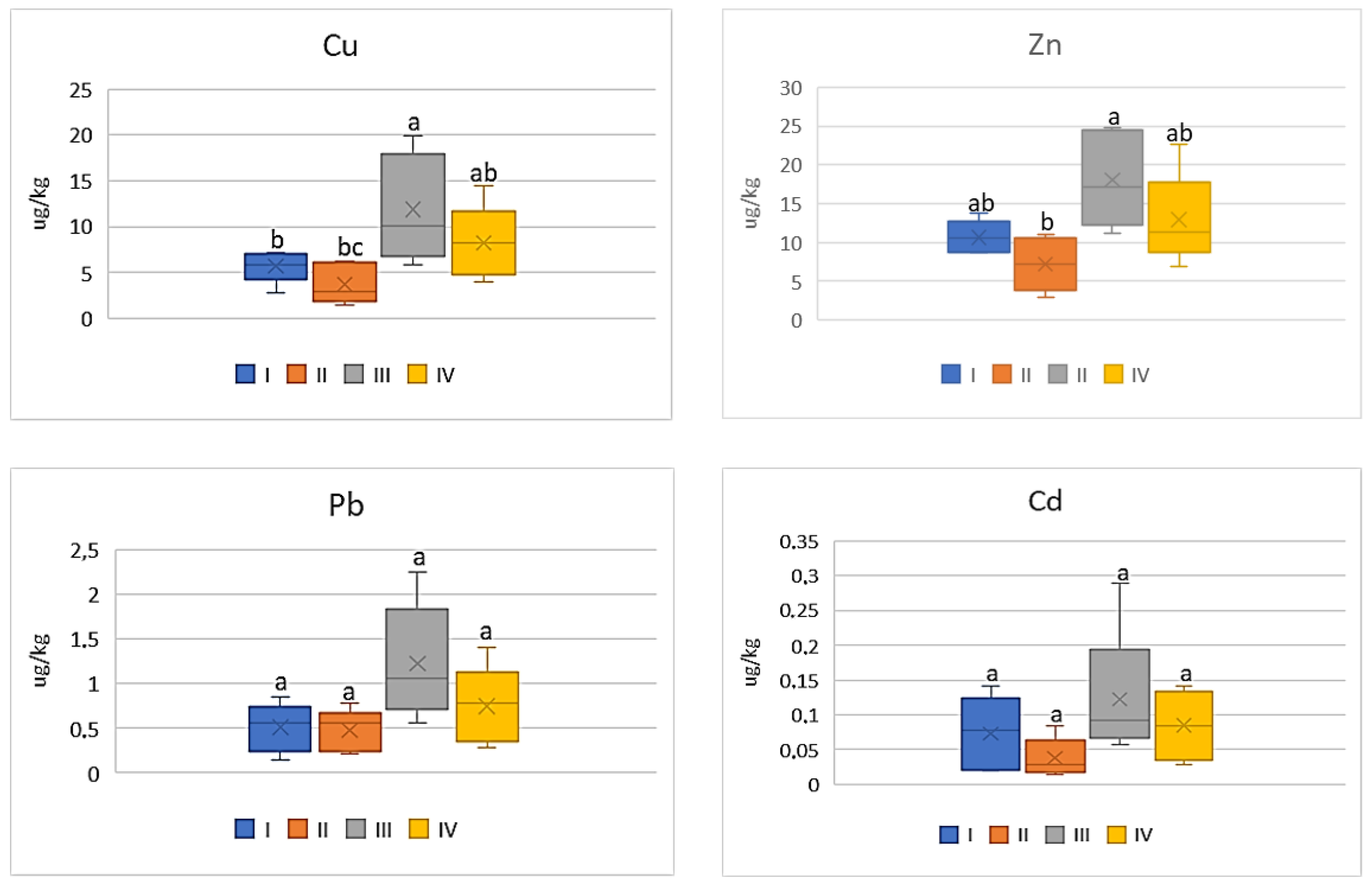
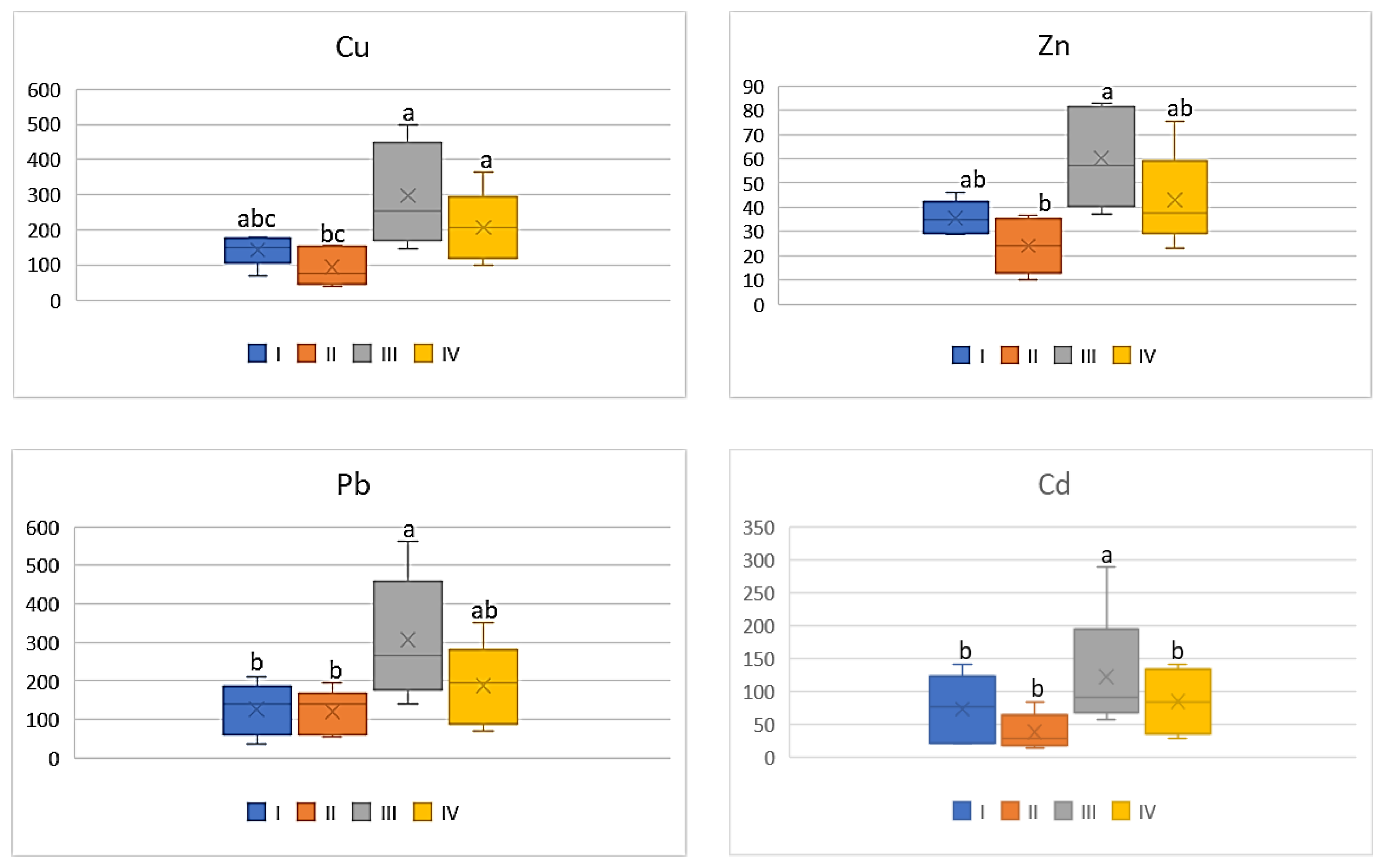
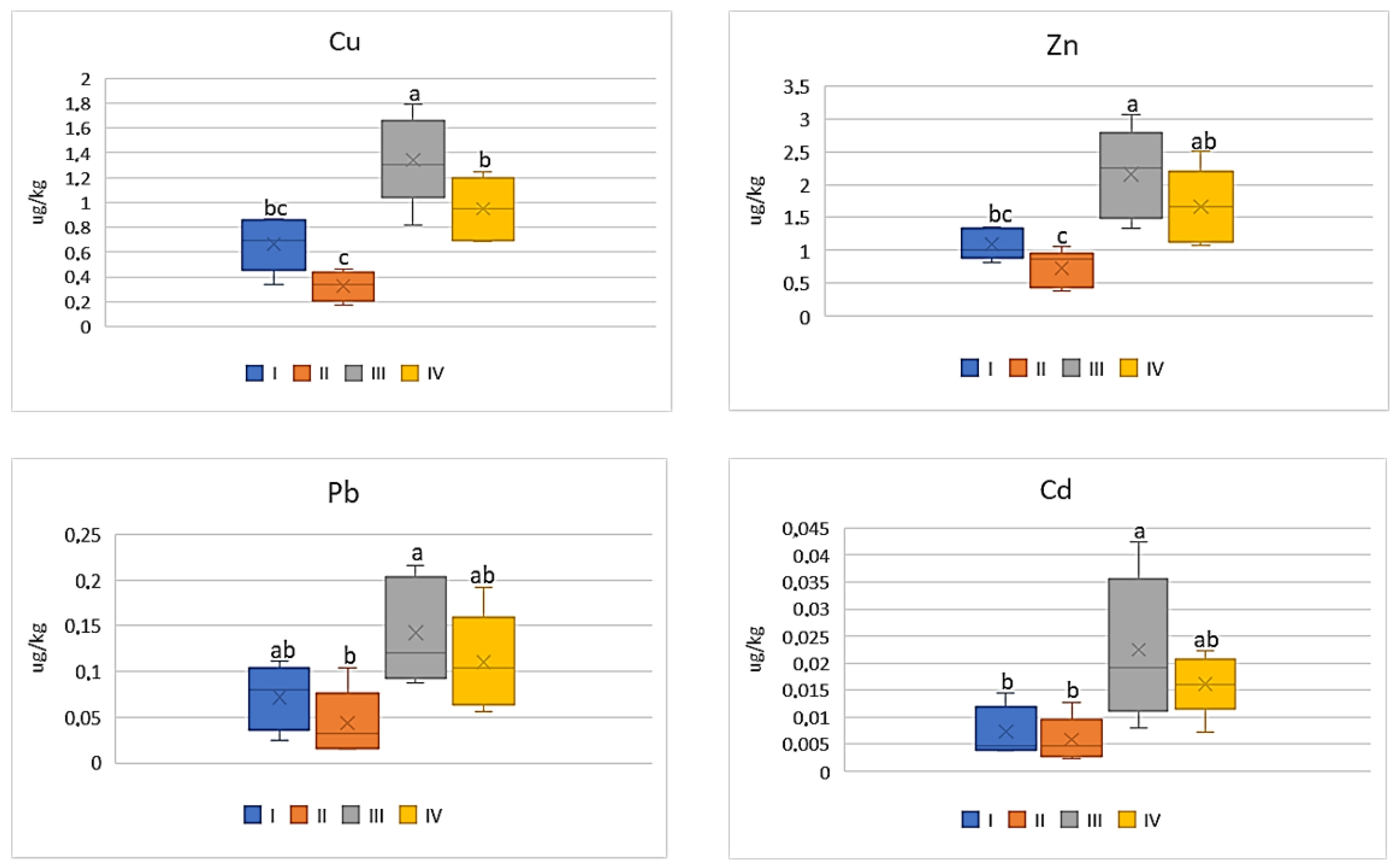
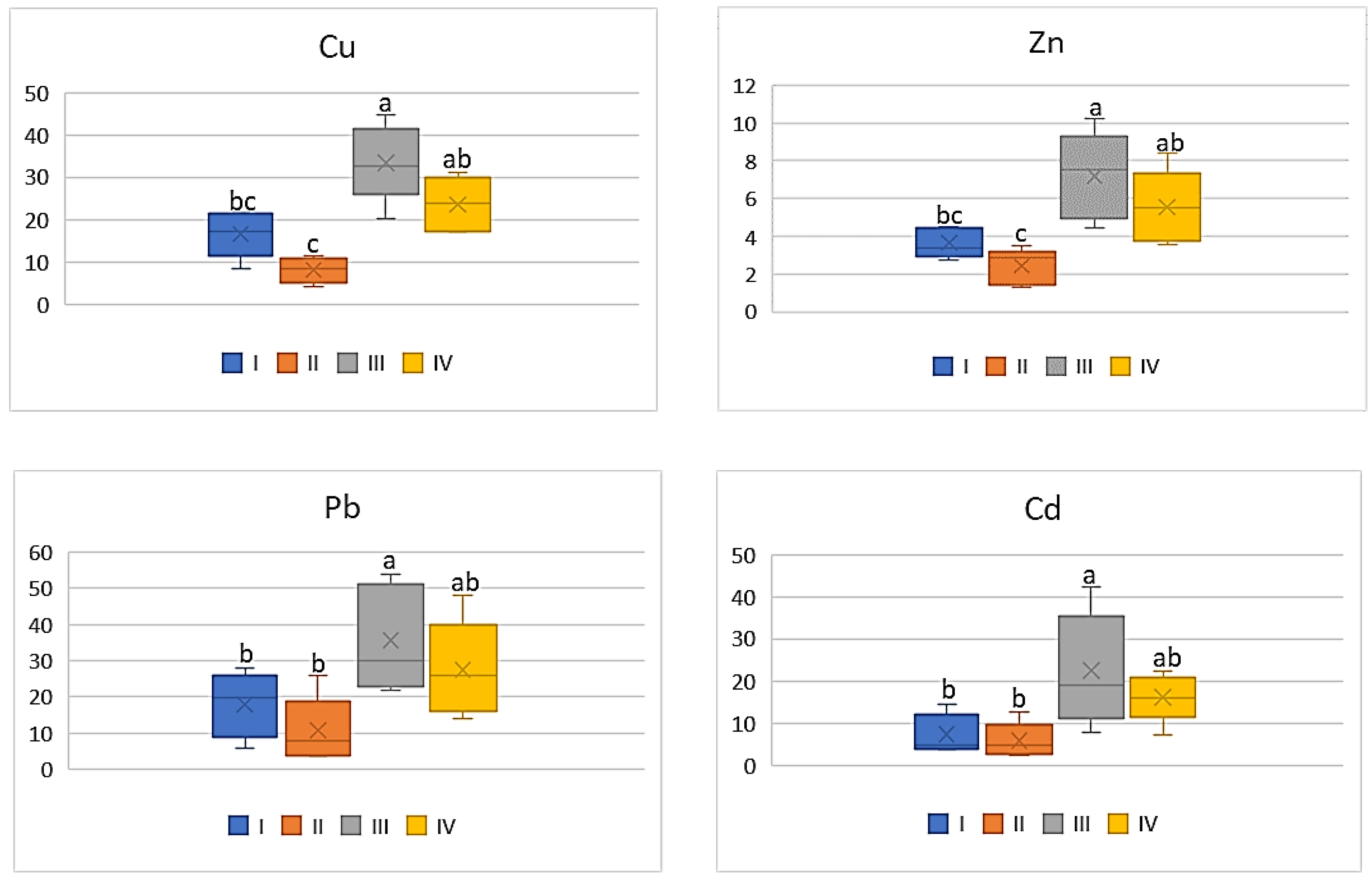
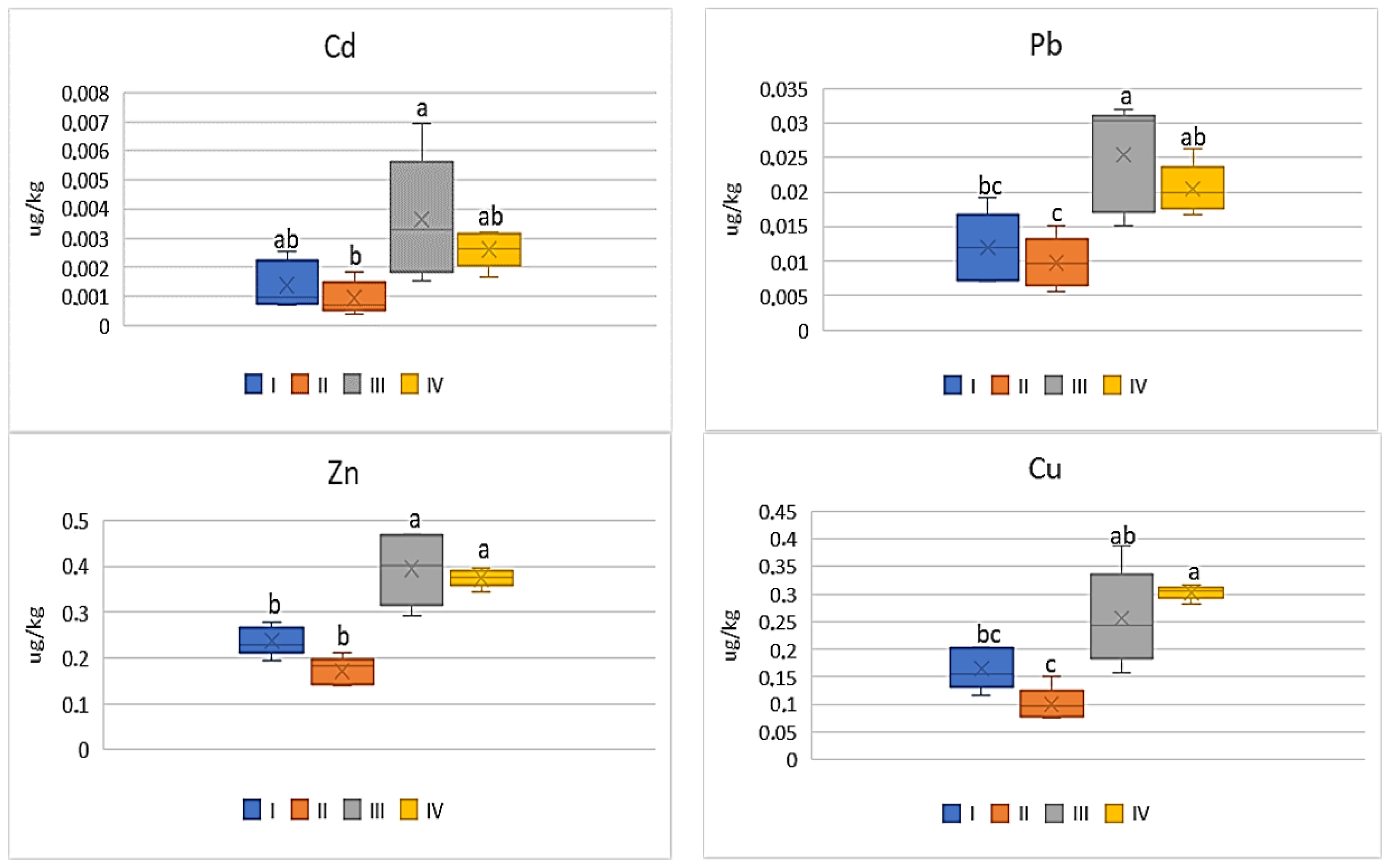
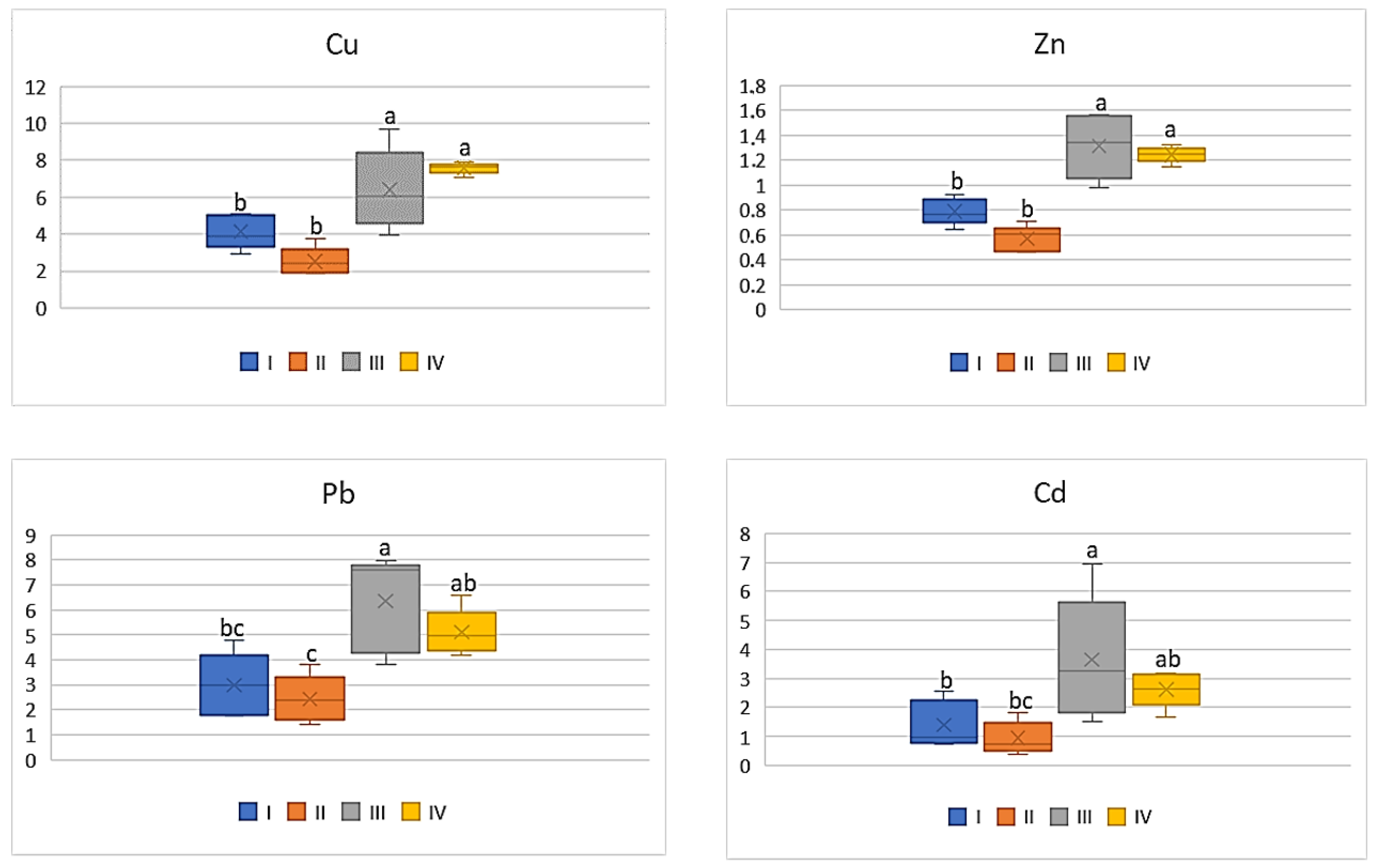
| Zone | Distance from the Emission Centre (m) | % Clay | pH-CaCl2 | CEC Cmol (+)·kg−1 | TC % | TN % | C:N |
|---|---|---|---|---|---|---|---|
| I * | 4500 | 3.8–6.1 ** 4.8 ± 0.9 *** | 7.23–7.33 7.28 ± 0.05 | 12.8–17.0 14.7 ± 1.7 | 2.13–3.47 2.60 ± 0.63 | 0.15–0.24 0.20 ± 0.04 | 12.0–15.1 12.9 ± 1.2 |
| II | 13,000 | 4.1–5.3 4.4 ± 0.6 | 7.19–7.61 7.44 ± 0.18 | 8.1–16.5 12.8 ± 3.4 | 0.98–3.26 2.17 ± 0.91 | 0.09–0.27 0.17 ± 0.06 | 10.9–14.2 12.6 ± 1.2 |
| III | 2200 | 2.9–4.1 3.9 ± 0.7 | 6.42–7.54 7.40 ± 0.34 | 12.0–25.3 16.0 ± 5.4 | 2.12–5.55; 3.18 ± 1.38 | 0.17–0.36 0.23 ± 0.08 | 11.8–15.4 13.3 ± 1.5 |
| IV | 6000 | 3.9–5.1 4.5 ± 0.5 | 7.14–7.70 7.44 ± 0.26 | 10.6–18.4 14.7 ± 2.8 | 1.54–3.67 2.68 ± 0.78 | 0.12–0.26 0.20 ± 0.05 | 12.8–14.1 13.3 ± 0.6 |
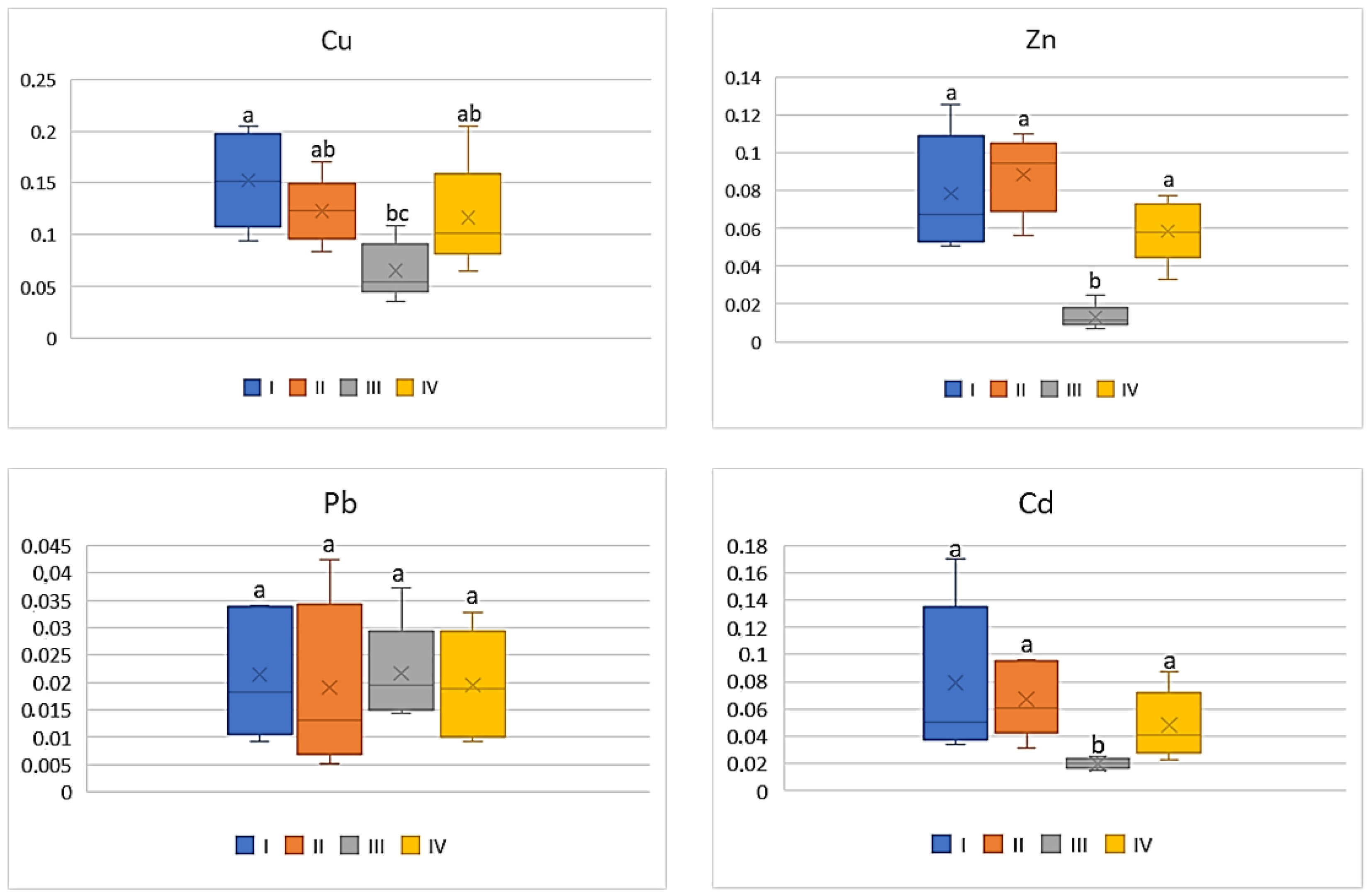
| Study Zones | Soil | Carrot | Tomato | Cabbage | Beetroot |
|---|---|---|---|---|---|
| Cu | |||||
| I * | 98.0 bc ** | 9.54 b | 8.12 ab | 8.30 bc | 20.68 bc |
| II | 79.38 c | 9.54 b | 5.34 b | 4.10 c | 16.62 c |
| III | 366.2 a | 22.92 a | 16.86 a | 16.78 a | 37.90 a |
| IV | 181.1 b | 20.50 a | 11.70 ab | 11.86 ab | 32.02 c |
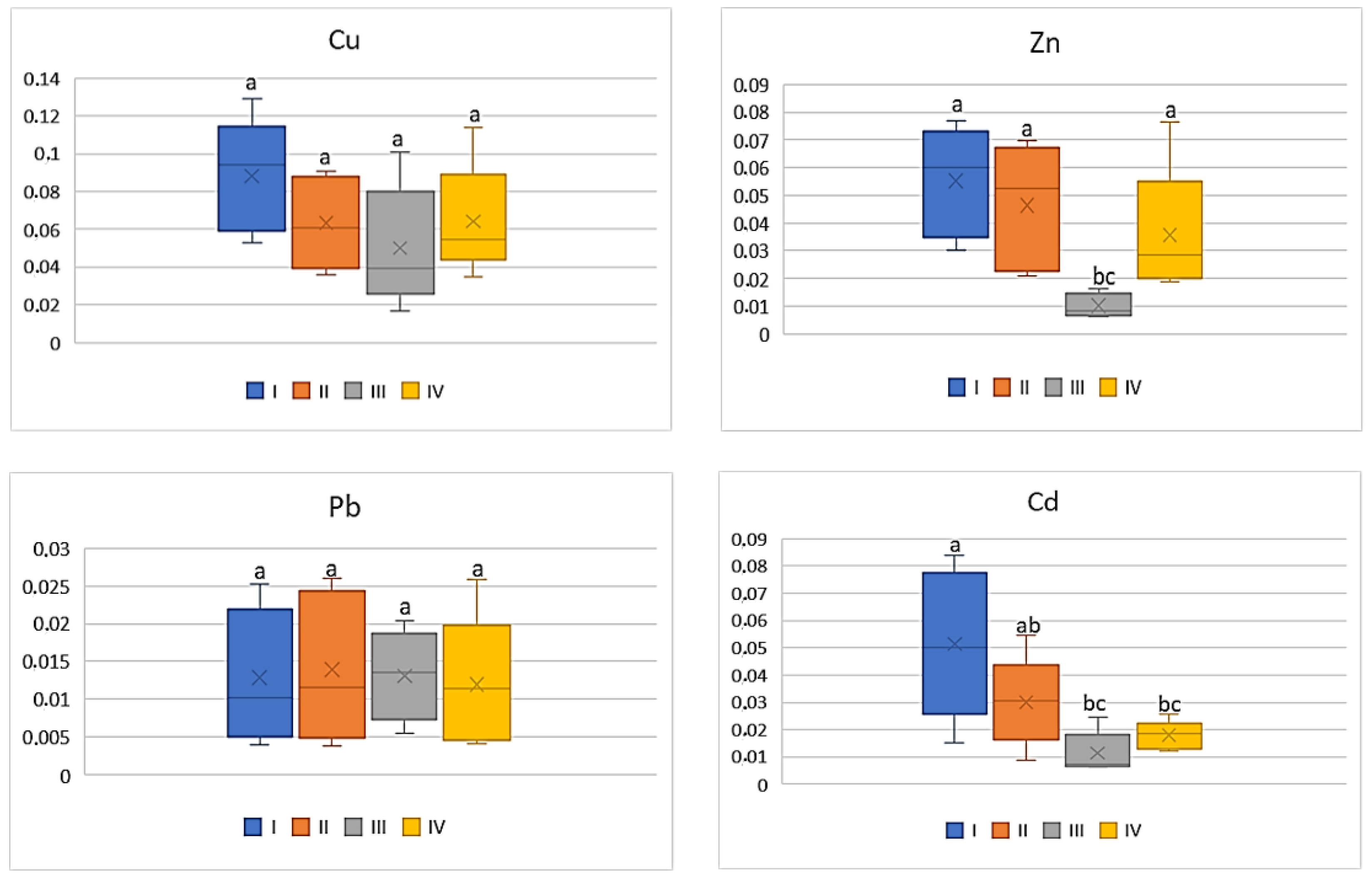
| Zn | |||||
| I | 304.58 bc | 21.5 ab | 15.12 ab | 13.72 bc | 29.70 b |
| II | 223.94 c | 19.34 b | 10.24 b | 9.14 c | 21.48 b |
| III | 2645.13 a | 31.88 a | 25.66 a | 27 a | 49.28 a |
| IV | 552.02 b | 21.92 a | 18.32 ab | 20.82 ab | 46.78 a |
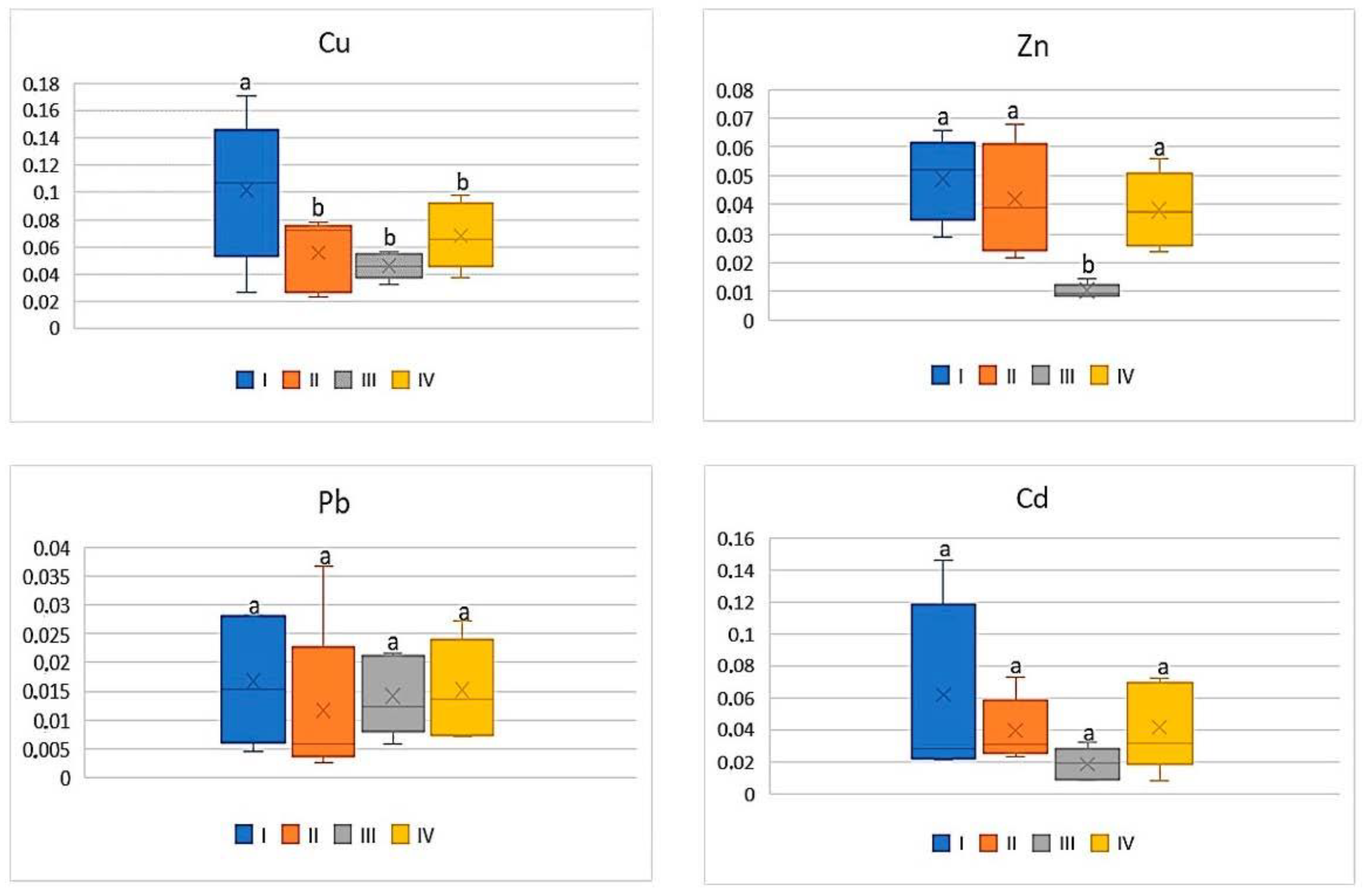
| Pb | |||||
| I | 62.10 c | 1.18 ab | 0.72 a | 0.90 ab | 1.50 bc |
| II | 58.66 d | 0.90 bc | 0.68 a | 0.54 b | 1.22 c |
| III | 134.88 a | 2.66 a | 1.74 a | 1.78 a | 3.18 a |
| IV | 58.66 b | 1.78 ab | 1.06 a | 1.38 ab | 2.56 ab |
| Cd | |||||
| I | 1.92 bc | 0.13 bc | 0.10 a | 0.09 b | 0.17 b |
| II | 1.77 bc | 0.12 c | 0.05 a | 0.07 b | 0.12 b |
| III | 15.2 a | 0.31 a | 0.17 a | 0.28 a | 0.46 a |
| IV | 6.33 b | 0.26 ab | 0.12 a | 0.20 ab | 0.33 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boluspayeva, L.; Jakubus, M.; Spychalski, W.; Abzhalelov, A.; Bitmanov, Y. Health Risk of Heavy Metals Related to Consumption of Vegetables in Areas of Industrial Impact in the Republic of Kazakhstan—Case Study for Oskemen. Int. J. Environ. Res. Public Health 2023, 20, 275. https://doi.org/10.3390/ijerph20010275
Boluspayeva L, Jakubus M, Spychalski W, Abzhalelov A, Bitmanov Y. Health Risk of Heavy Metals Related to Consumption of Vegetables in Areas of Industrial Impact in the Republic of Kazakhstan—Case Study for Oskemen. International Journal of Environmental Research and Public Health. 2023; 20(1):275. https://doi.org/10.3390/ijerph20010275
Chicago/Turabian StyleBoluspayeva, Laura, Monika Jakubus, Waldemar Spychalski, Akhan Abzhalelov, and Yertas Bitmanov. 2023. "Health Risk of Heavy Metals Related to Consumption of Vegetables in Areas of Industrial Impact in the Republic of Kazakhstan—Case Study for Oskemen" International Journal of Environmental Research and Public Health 20, no. 1: 275. https://doi.org/10.3390/ijerph20010275
APA StyleBoluspayeva, L., Jakubus, M., Spychalski, W., Abzhalelov, A., & Bitmanov, Y. (2023). Health Risk of Heavy Metals Related to Consumption of Vegetables in Areas of Industrial Impact in the Republic of Kazakhstan—Case Study for Oskemen. International Journal of Environmental Research and Public Health, 20(1), 275. https://doi.org/10.3390/ijerph20010275







