Diabetic Foot Complications: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Statistical Analysis
3. Results
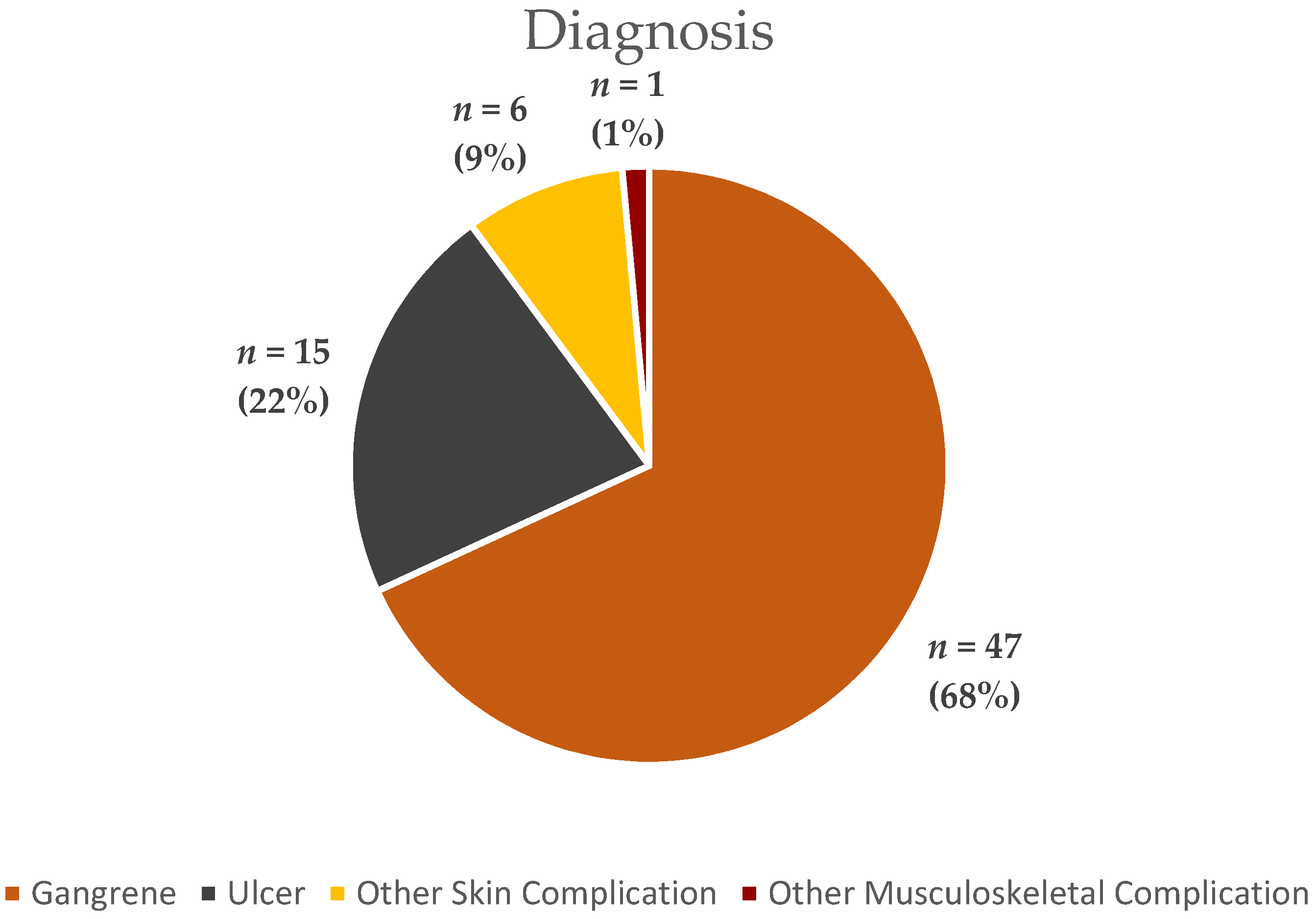
3.1. Descriptive Statistics
3.2. Study Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Rockman, C.B.; Guo, Y.; Chesner, J.; Schwartzbard, A.Z.; Weintraub, H.S.; Adelman, M.A.; Riles, T.S.; Berger, J.S. Diabetes and vascular disease in different arterial territories. Diabetes Care 2014, 37, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, A.; La Muraglia, G.M.; Henke, P.; Meissner, M.H.; Loretz, L.; Zinszer, K.M.; Driver, V.R.; Frykberg, R.; Carman, T.L.; Marston, W.; et al. The management of diabetic foot: A clinical practice guideline by the society for vascular surgery in collaboration with the American podiatric medical association and the society for vascular medicine. J. Vasc. Surg. 2016, 63, 3S–21S. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Stern, J.R.; Wong, C.K.; Yerovinkina, M.; Spindler, S.J.; See, A.S.; Panjaki, S.; Loven, S.L.; D’Andrea, R.F.; Nowygrod, R. A meta-analysis of long-term mortality and associated risk factors following lower extremity amputation. Ann. Vasc. Surg. 2017, 42, 322–327. [Google Scholar] [CrossRef]
- Walsh, J.W.; Hoffstad, O.J.; Sullivan, M.O.; Margolis, D.J. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet. Med. 2016, 33, 1493–1498. [Google Scholar] [CrossRef]
- Goodridge, D.; Trepman, E.; Embil, J.M. Health-related quality of life in diabetic patients with foot ulcers: Literature review. J. Wound Ostomy Cont. Nurs. 2005, 32, 368–377. [Google Scholar] [CrossRef]
- De Smet, G.H.J.; Kroese, L.F.; Menon, A.G.; Jeekel, J.; Van Pelt, A.W.J.; Kleinrensink, G.-J.; Lange, J.F. Oxygen therapies and their effects on wound healing. Wound Repair Regen. 2017, 25, 591–608. [Google Scholar] [CrossRef]
- Prompers, L.; Schaper, N.; Apelqvist, J.; Edmonds, M.; Jude, E.; Mauricio, D.; Uccioli, L.; Urbancic, V.; Bakker, K.; Holstein, P.; et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE study. Diabetologia 2008, 51, 747–755. [Google Scholar] [CrossRef]
- Stancu, B.; Rednic, G.; Ovidiu, G.; Mironiuc, A.; Gherman, C. Medical, social and christian aspects in patients with major lower limb amputations. J. Study Relig. Ideol. 2016, 15, 82–101. [Google Scholar]
- Sima, D.I.; Bondor, C.I.; Vereşiu, I.A.; Gâvan, N.A.; Borzan, C.M. Hospitalization costs of lower limb ulcerations and amputations in patients with diabetes in Romania. Int. J. Environ. Res. Public Health 2021, 18, 2230. [Google Scholar] [CrossRef] [PubMed]
- Spoden, M.; Nimptsch, U.; Mansky, T. Amputation rates of the lower limb by amputation level—Observational study using german national hospital discharge data from 2005 to 2015. BMC Health Serv. Res. 2019, 19, 8. [Google Scholar] [CrossRef]
- McGuire, J.; Thomson, A.; Kennedy, P.G. The Biomechanics of Diabetic Foot Amputation. Wounds 2021, WNDS20210414-2. Available online: https://pubmed.ncbi.nlm.nih.gov/33872198/ (accessed on 30 September 2022). [CrossRef]
- Boulton, A.J.M.; Armstrong, D.G.; Albert, S.F.; Frykberg, R.G.; Hellman, R.; Kirkman, M.S.; Lavery, L.A.; Lemaster, J.W.; Mills, J.L.; Mueller, M.J.; et al. Comprehensive foot examination and risk assessment: A report of the task force of the foot care interest group of the American diabetes association, with endorsement by the American association of clinical endocrinologists. Diabetes Care 2008, 31, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Biz, C.; Belluzzi, E.; Crimì, A.; Bragazzi, N.L.; Nicoletti, P.; Mori, F.; Ruggieri, P. Minimally invasive metatarsal osteotomies (MIMOs) for the treatment of plantar diabetic forefoot ulcers (PDFUs): A systematic review and meta-analysis with metaregressions. Appl. Sci. 2021, 11, 9628. [Google Scholar] [CrossRef]
- Shahi, S.; Kumar, A.; Kumar, S.; Surya, M.S.; Gupta, S.; Singh, T. Open access publishing the journal of diabetic foot complications prevalence of diabetic foot ulcer and associated risk factors in diabetic patients from North India. J. Diabet. Foot Complicat. 2012, 4, 83–91. [Google Scholar]
- Cerqueira, L.D.O.; Júnior, E.G.D.; Barros, A.L.D.S.; Cerqueira, J.R.; de Araújo, W.J.B. WIFI classification: The Society for vascular surgery lower extremity threatened limb classification system, a literature review. J. Vasc. Bras. 2020, 19, e20190070. [Google Scholar] [CrossRef]
- Huang, E.S.; Laiteerapong, N.; Liu, J.Y.; John, P.M.; Moffet, H.H.; Karter, A.J. Rates of complications and mortality in older patients with diabetes mellitus: The diabetes and aging study. JAMA Intern. Med. 2014, 174, 251–258. [Google Scholar] [CrossRef]
- Shamshirgaran, S.M.; Mamaghanian, A.; Aliasgarzadeh, A.; Aiminisani, N.; Iranparvar-Alamdari, M.; Ataie, J. Age differences in diabetes-related complications and glycemic control. BMC Endocr. Disord. 2017, 17, 25. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Cheema, A.; Adeloye, D.; Sidhu, S.; Sridhar, D.; Chan, K.Y. Urbanization and prevalence of type 2 diabetes in Southern Asia: A systematic analysis. J. Glob. Health 2014, 4, 010404. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Astell-Burt, T.; Bi, Y.; Feng, X.; Jiang, Y.; Li, Y.; Page, A.; Wang, L.; Xu, Y.; Wang, L.; et al. Geographical variation in diabetes prevalence and detection in China: Multilevel spatial analysis of 98,058 adults. Diabetes Care 2015, 38, 72–81. [Google Scholar] [CrossRef]
- Al-Mahroos, F.; Al-Roomi, K. Diabetic neuropathy, foot ulceration, peripheral vascular disease and potential risk factors among patients with diabetes in Bahrain: A nationwide primary care diabetes clinic-based study. Ann. Saudi Med. 2007, 27, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bruun, C.; Siersma, V.; Guassora, A.D.; Holstein, P.; De Fine Olivarius, N. Amputations and foot ulcers in patients newly diagnosed with type 2 diabetes mellitus and observed for 19 years. The role of age, gender and co-morbidity. Diabet. Med. 2013, 30, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubeaan, K.; Al Derwish, M.; Ouizi, S.; Youssef, A.M.; Subhani, S.N.; Ibrahim, H.M.; Alamri, B.N. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS ONE 2015, 10, e0124446. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Gregg, E.W.; Kahn, H.S.; Williams, D.E.; De Rekeneire, N.; Narayan, K.M.V. Peripheral insensate neuropathy—A tall problem for US adults? Am. J. Epidemiol. 2006, 164, 873–880. [Google Scholar] [CrossRef]
- Magliano, D.J.; Islam, R.M.; Barr, E.L.M.; Gregg, E.; Pavkov, M.E.; Harding, J.L.; Tabesh, M.; Koye, D.N.; Shaw, J.E. Trends in incidence of total or type 2 diabetes: Systematic review. BMJ 2019, 366, l5003. [Google Scholar] [CrossRef]
- Hjelm, K.; Nyberg, P.; Apelqvist, J. Gender influences beliefs about health and illness in diabetic subjects with severe foot lesions. J. Adv. Nurs. 2002, 40, 673–684. [Google Scholar] [CrossRef]
- Ansari, S.; Akhdar, F.; Mandoorah, M.; Moutaery, K. Causes and effects of road traffic accidents in Saudi Arabia. Public Health 2000, 114, 37–39. [Google Scholar] [CrossRef]
- Al-Wahbi, A.M. The diabetic foot. In the Arab world. Saudi Med. J. 2006, 27, 147–153. [Google Scholar]
- Neil, H.A.; Thompson, A.V.; Thorogood, M.; Fowler, G.H.; Mann, J.I. Diabetes in the elderly: The oxford community diabetes study. Diabet. Med. 1989, 6, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.A.; Carrington, A.L.; Ashe, H.; Bath, S.; Every, L.C.; Griffiths, J.; Hann, A.W.; Hussein, A.; Jackson, N.; Johnson, K.E.; et al. The north-west diabetes foot care study: Incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet. Med. 2002, 19, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.M.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.S.; Martin, B.D.; Duerksen, F.; Nicolle, L.E.; Garrett, M.; Simonsen, J.N.; Trepman, E.; Embil, J.M. Diabetic foot complications in a northern Canadian aboriginal community. Foot Ankle Int. 2006, 27, 1065–1073. [Google Scholar] [CrossRef]
- Schaper, N.C.; Apelqvist, J.; Bakker, K. The international consensus and practical guidelines on the management and prevention of the diabetic foot. Curr. Diabetes Rep. 2003, 3, 475–479. [Google Scholar] [CrossRef]
- Kim, P.J.; Steinberg, J.S. Complications of the diabetic foot. Endocrinol. Metab. Clin. N. Am. 2013, 42, 833–847. [Google Scholar] [CrossRef]
- Hinchliffe, R.J.; Brownrigg, J.R.W.; Andros, G.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Mills, J.L.; Reekers, J.; Shearman, C.P.; Zierler, R.E.; et al. Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: A systematic review. Diabetes Metab. Res. Rev. 2016, 32, 136–144. [Google Scholar] [CrossRef]
- Bus, S.A.; Armstrong, D.G.; Van Deursen, R.W.; Lewis, J.E.A.; Caravaggi, C.F.; Cavanagh, P.R.; International working group on the diabetic foot. IWGDF guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes. Diabetes Metab. Res. Rev. 2016, 32, 25–36. [Google Scholar] [CrossRef]
- Holscher, C.M.; Hicks, C.W.; Canner, J.K.; Sherman, R.L.; Malas, M.B.; Black, J.H.; Mathioudakis, N.; Abularrage, C.J. Unplanned 30-day readmission in patients with diabetic foot wounds treated in a multidisciplinary setting. J. Vasc. Surg. 2018, 67, 876–886. [Google Scholar] [CrossRef]
- Mota, M.; Popa, S.G.; Mota, E.; Mitrea, A.; Catrinoiu, D.; Cheta, D.M.; Guja, C.; Hancu, N.; Ionescu-Tirgoviste, C.; Lichiardopol, R.; et al. Prevalence of diabetes mellitus and prediabetes in the adult Romanian population: PREDATORR study. J. Diabetes 2016, 8, 336–344. [Google Scholar] [CrossRef]
- Rastogi, A.; Goyal, G.; Kesavan, R.; Bal, A.; Kumar, H.; Mangalanadanam, K.P.; Jude, E.B.; Armstrong, D.G.; Bhansali, A. Long term outcomes after incident diabetic foot ulcer: Multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study. Diabetes Res. Clin. Pract. 2020, 162, 108113. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.W.; Stuck, R.M.; Pinzur, M.; Lee, T.A.; Budiman-Mak, E. Lower-extremity amputation risk after charcot arthropathy and diabetic foot ulcer. Diabetes Care 2010, 33, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula, W.V.; Bus, S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable with cancer. J. Foot Ankle Res. 2020, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Atlaw, A.; Kebede, H.B.; Abdela, A.A.; Woldeamanuel, Y. Bacterial isolates from diabetic foot ulcers and their antimicrobial resistance profile from selected hospitals in Addis Ababa, Ethiopia. Front. Endocrinol. 2022, 13, 987487. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Nash, F.; Baker, N.; Fowler, D.; Rayman, G. Reduction in diabetic amputations over 11 years in a defined, U.K. population: Benefits of multidisciplinary team work and continuous prospective audit. Diabetes Care 2008, 31, 99–101. [Google Scholar] [CrossRef]
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228. [Google Scholar] [CrossRef]
| Variable | Number of Records | Mean ± SD Median (Min–Max) |
|---|---|---|
| Age (years) | n = 69 | 66.20 ± 1.33 |
| Hospitalization (days) | n = 69 | 9 (1–49) |
| Variable | Number of Records (Percent of Total) | |
|---|---|---|
| Gender | Male | n = 51 (74%) |
| Female | n = 18 (26%) | |
| Environment | Urban | n = 34 (52%) |
| Rural | n = 32 (48%) | |
| Complications | Yes | n = 16 (27%) |
| No | n = 44 (73%) | |
| Bacteriology | Positive | n = 30 (46%) |
| Negative | n = 35 (54%) | |
| Reintervention | Yes | n = 24 (24%) |
| No | n = 45 (65%) | |
| ICU admission | Yes | n = 9 (13%) |
| No | n = 60 (87%) | |
| Group | Median Hospitalization Duration (Days) | p | |
|---|---|---|---|
| Age (years) | <68 | 12 | <0.01 * |
| >68 | 8 | ||
| Post-operative complications | Yes | 17.5 | <0.01 * |
| No | 8.5 | ||
| Bacteriology | Positive | 10.5 | 0.291 |
| Negative | 9 | ||
| Reintervention | Yes | 15 | <0.01 * |
| No | 8 | ||
| ICU admission | Yes | 11 | 0.555 |
| No | 9 | ||
| Parameter | Age (Years) | OR | RR | p | ||
|---|---|---|---|---|---|---|
| <68 | >68 | |||||
| Post-operative complications | Yes | n =11 | n = 5 | 2.895 (95%CI, 0.860–9.745) | 2.2 (95%CI, 0.809–6.718) | 0.143 |
| No | n = 19 | n = 25 | ||||
| Bacteriology | Positive | n = 15 | n = 15 | 1.059 (95%CI, 0.399–2.808) | 1.031 (95%CI, 0.571–1.855) | 1 |
| Negative | n = 17 | n = 18 | ||||
| Reintervention | Yes | n = 17 | n = 7 | 4.402 (95%CI, 1.508–12.847) | 2.649 (95%CI, 1.212–6.286) | <0.01 * |
| No | n = 16 | n = 29 | ||||
| ICU admission | Yes | n = 5 | n = 4 | 1.429 (95%CI, 0.349–5.847) | 1.364 (95%CI, 0.338–5.764) | 0.728 |
| No | n = 28 | n = 32 | ||||
| Parameter | Environment | OR | RR | p | ||
|---|---|---|---|---|---|---|
| Urban | Rural | |||||
| Post-operative complications | Yes | n =10 | n = 5 | 2.421 (95%CI, 0.705–8.312) | 1.931 (95%CI, 0.695–5.984) | 0.23 |
| No | n = 19 | n = 23 | ||||
| Bacteriology | Positive | n = 20 | n = 10 | 3.333 (95%CI, 1.174–9.462) | 1.875 (95%CI, 1.022–3.558) | <0.05 * |
| Negative | n = 12 | n = 20 | ||||
| Reintervention | Yes | n = 10 | n = 13 | 1.642 (95%CI, 0.592–4.557) | 1.381 (95%CI, 0.656–2.965) | 0.44 |
| No | n = 24 | n = 19 | ||||
| ICU admission | Yes | n = 3 | n = 5 | 1.914 (95%CI, 0.418–8.762) | 1.771 (95%CI, 0.394–9.031) | 0.469 |
| No | n = 31 | n = 27 | ||||
| Parameter | Post-Operative Complications | OR | RR | p | ||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| Bacteriology | Positive | n = 8 | n = 19 | 1.444 (95%CI, 0.444–4.696) | 1.207 (95%CI, 0.566–2.087) | 0.564 |
| Negative | n = 7 | n = 24 | ||||
| Reintervention | Yes | n = 10 | n = 13 | 3.974 (95%CI, 1.195–13.216) | 2.115 (95%CI, 1.022–3.659) | <0.05 * |
| No | n = 6 | n = 31 | ||||
| ICU admission | Yes | n = 4 | n = 3 | 4.556 (95%CI, 0.893–23.235) | 3.667 (95%CI, 0.742–19.449) | 0.074 |
| No | n = 12 | n = 41 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stancu, B.; Ilyés, T.; Farcas, M.; Coman, H.F.; Chiș, B.A.; Andercou, O.A. Diabetic Foot Complications: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 187. https://doi.org/10.3390/ijerph20010187
Stancu B, Ilyés T, Farcas M, Coman HF, Chiș BA, Andercou OA. Diabetic Foot Complications: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2023; 20(1):187. https://doi.org/10.3390/ijerph20010187
Chicago/Turabian StyleStancu, Bogdan, Tamás Ilyés, Marius Farcas, Horațiu Flaviu Coman, Bogdan Augustin Chiș, and Octavian Aurel Andercou. 2023. "Diabetic Foot Complications: A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 20, no. 1: 187. https://doi.org/10.3390/ijerph20010187
APA StyleStancu, B., Ilyés, T., Farcas, M., Coman, H. F., Chiș, B. A., & Andercou, O. A. (2023). Diabetic Foot Complications: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 20(1), 187. https://doi.org/10.3390/ijerph20010187






