Perceiving “Complex Autonomous Systems” in Symmetry Dynamics: Elementary Coordination Embedding in Circadian Cycles
Abstract
1. Introduction
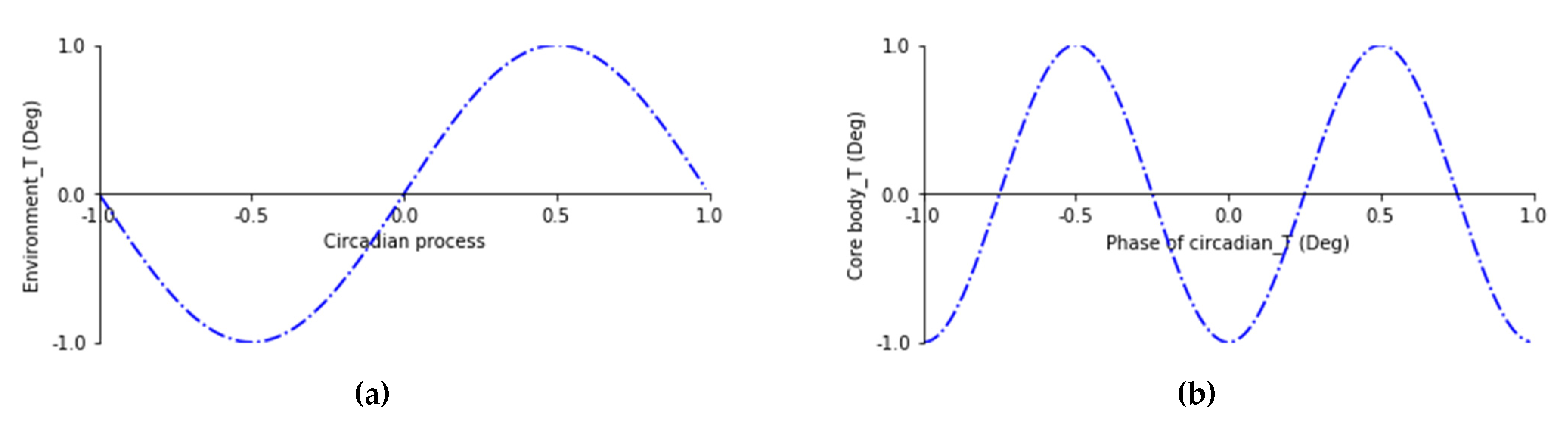
1.1. Modeling Thermoregulatory Symmetry Breaking
1.2. Purpose and Hypotheses
2. Experiment I
2.1. Experiment I Apparatus and Procedure
2.2. Experiment I Proposed Analyses
2.3. Experiment I Statistical Data Testing for the Hypotheses
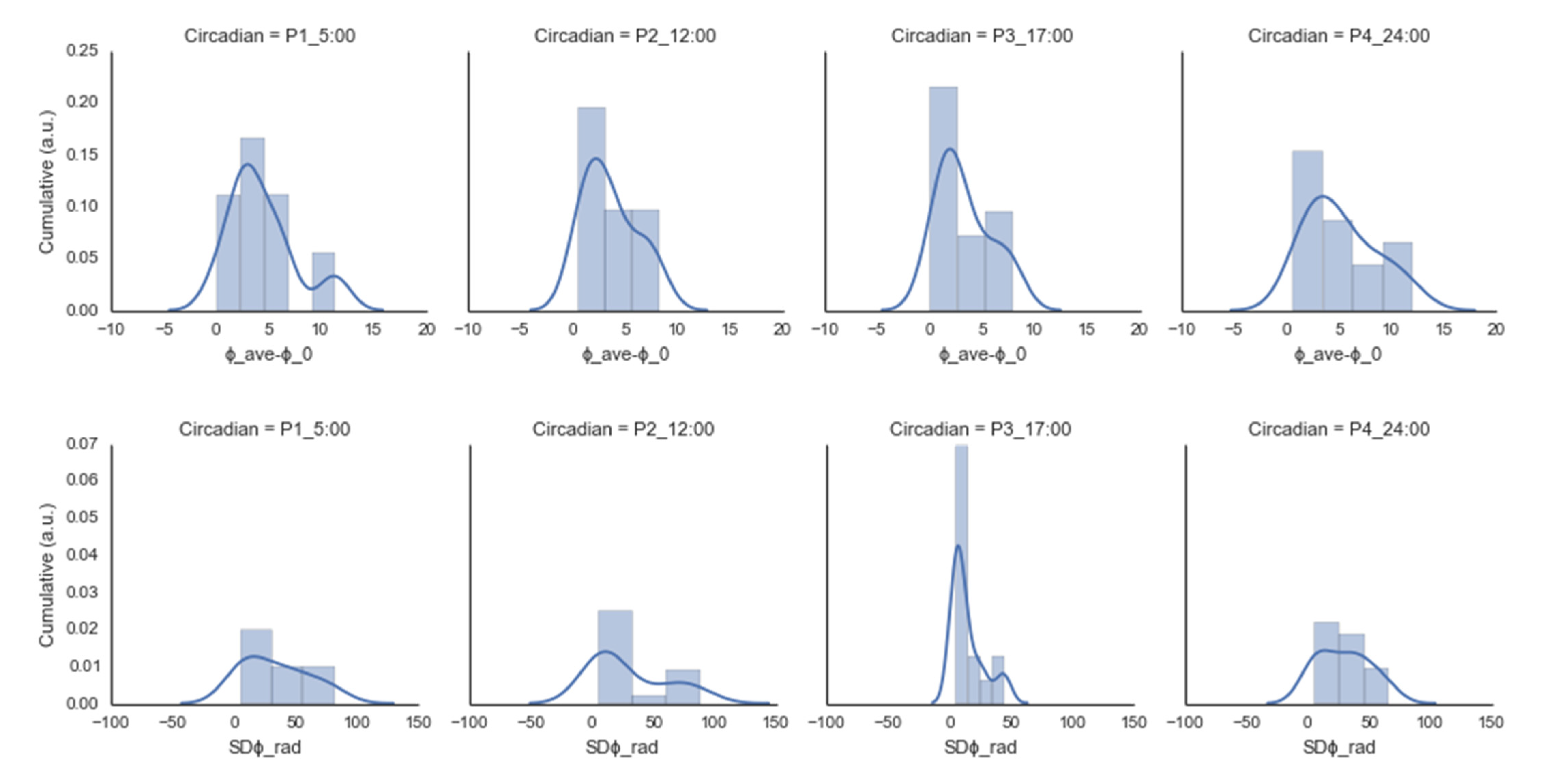
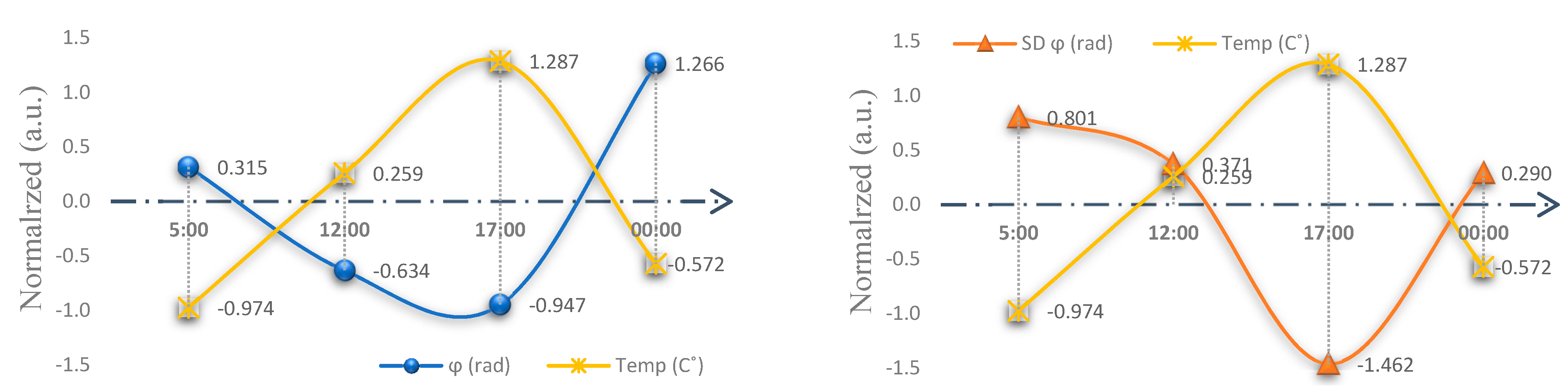
2.4. Experiment I Results
3. Experiment II
3.1. Experiment II Apparatus and Procedure
3.2. Experiment II Proposed Analyses
3.3. Experiment II Results
4. Experiment III
4.1. Experiment III Apparatus and Procedure
4.2. Experiment III Proposed Analyses
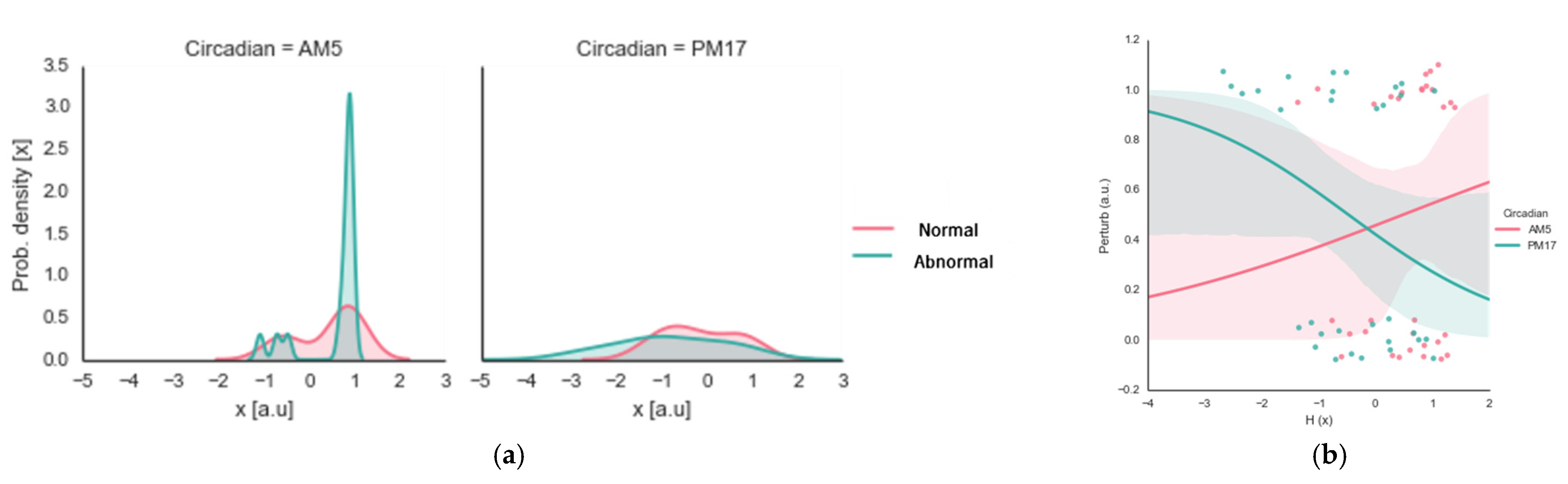
4.3. Experiment III Results
5. Discussion
5.1. Practical Implications
5.2. Limitations and Suggestions for Future Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maury, E.; Ramsey, K.M.; Bass, J. Circadian rhythms and metabolic syndrome: From experimental genetics to human disease. Circ. Res. 2010, 106, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R.; Menaker, M. The circadian rhythm of body temperature. Physiol. Behav. 1992, 51, 613–637. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y. Organization of the mammalian circadian system. In Ciba Foundation Symposium 183: Circadian Clocks and Their Adjustment; Chadwick, D.J., Ackrill, K., Eds.; John Wiley & Sons: Chichester, UK, 2007; p. 88. [Google Scholar]
- Aschoff, J. Circadian control of body temperature. J. Therm. Biol. 1983, 8, 143–147. [Google Scholar] [CrossRef]
- Krauchi, K.; Wirz-Justice, A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 267, R819–R829. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, S.; Cabanac, M. The influence of temporary semi-supine and supine postures on temperature regulation in humans. J. Therm. Biol. 2002, 27, 109–114. [Google Scholar] [CrossRef]
- Cagnacci, A.; Kräuchi, K.; Wirz-Justice, A.; Volpe, A. Homeostatic versus circadian effects of melatonin on core body temperature in humans. J. Biol. Rhythms 1997, 12, 509–517. [Google Scholar] [CrossRef]
- Borb, A.A.; Achermann, P. Sleep homeostasis and models of sleep regulation. J. Biol. Rhythms 1999, 14, 559–570. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Edholm, O.G.; Fox, R.H.; Wolf, H.S. Body temperature during exercise and rest in cold and hot climates. Arch. Sci. Physiol. 1973, 27, A339–A355. [Google Scholar]
- Aschoff, J.; Heise, A.; Itoh, S.; Ogata, K.; Yoshimura, H. Thermal Conductance in Man: Its Dependence on Time of Day and on Ambient Temperature; Igaku Shoin Limited: Tokyo, Japan, 1972; pp. 334–348. [Google Scholar]
- Waterhouse, J.; Drust, B.; Weinert, D.; Edwards, B.; Gregson, W.; Atkinson, G.; Kao, S.; Aizawa, S.; Reilly, T. The circadian rhythm of core temperature: Origin and some implications for exercise performance. Chronobiol. Int. 2005, 22, 207–225. [Google Scholar] [CrossRef]
- Aldemir, H.; Atkinson, G.; Cable, T.; Edwards, B.; Waterhouse, J.; Reilly, T. A comparison of the immediate effects of moderate exercise in the early morning and late afternoon on core temperature and cutaneous thermoregulatory mechanisms. Chronobiol. Int. 2000, 17, 197–207. [Google Scholar] [CrossRef]
- Kay, B.A.; Kelso, J.A.; Saltzman, E.L.; Schöner, G. Space–time behavior of single and bimanual rhythmical movements: Data and limit cycle model. J. Exp. Psychol. 1987, 13, 178–192. [Google Scholar] [CrossRef]
- Turvey, M.T. Coordination. Am. Psychol. 1990, 45, 938–953. [Google Scholar] [CrossRef]
- Kugler, P.N.; Turvey, M.T. Information, Natural Law, and the Self-Assembly of Rhythmic Movement. Routledge: London, UK, 2015. [Google Scholar]
- Collins, J.J.; Stewart, I.N. Coupled nonlinear oscillators and the symmetries of animal gaits. J. Nonlinear Sci. 1993, 3, 349–392. [Google Scholar] [CrossRef]
- Mottet, D.; Bootsma, R.J. The dynamics of goal-directed rhythmical aiming. Biol. Cybern. 1999, 80, 235–245. [Google Scholar] [CrossRef]
- Pikovsky, A.; Rosenblum, M.; Kurths, J. Synchronization: A universal concept in nonlinear sciences. Am. J. Phys. 2002, 70, 655. [Google Scholar] [CrossRef]
- Park, C. Eigenproperties of perception (dynamic touch) and action (phase dynamic) out of diversities. Hum. Mov. Sci. 2022, 85, 102999. [Google Scholar] [CrossRef]
- Kelso, J.S.; Tuller, B.; Vatikiotis-Bateson, E.; Fowler, C.A. Functionally specific articulatory cooperation following jaw perturbations during speech: Evidence for coordinative structures. J. Exp. Psychol. 1984, 10, 812–832. [Google Scholar] [CrossRef]
- Zanone, P.-G.; Kelso, J.A.S. The coordination dynamics of learning: Theoretical structure and experimental agenda. In Interlimb Coordination; Swinnen, S.P., Heuer, H., Massion, J., Casaer, P., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 461–490. [Google Scholar]
- Turvey, M.T.; Schmidt, R.C. A low-dimensional nonlinear dynamic governing interlimb rhythmic coordination. In Interlimb Coordination; Swinnen, S.P., Heuer, H., Massion, J., Casaer, P., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 277–300. [Google Scholar]
- Weinert, D.; Waterhouse, J. Diurnally changing effects of locomotor activity on body temperature in laboratory mice. Physiol. Behav. 1998, 63, 837–843. [Google Scholar] [CrossRef]
- Kräuchi, K.; Wirz-Justice, A. Circadian clues to sleep onset mechanisms. Neuropsychopharmacology 2001, 25, S92–S96. [Google Scholar] [CrossRef]
- Frank, T.D. Collective behavior of biophysical systems with thermodynamic feedback loops: A case study for a nonlinear Markov model—The Takatsuji system. Mod. Phys. Lett. B 2011, 25, 551–568. [Google Scholar] [CrossRef]
- Gibson, J.J. The perception of visual surfaces. Am. J. Psychol. 1950, 63, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Reed, E.S. Encountering the World: Toward an Ecological Psychology; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Shaw, R.; Kinsella-Shaw, J. Ecological mechanics: A physical geometry for intentional constraints. Hum. Mov. Sci. 1988, 7, 155–200. [Google Scholar] [CrossRef]
- Yadlapalli, S.; Jiang, C.; Bahle, A.; Reddy, P.; Meyhofer, E.; Shafer, O.T. Circadian clock neurons constantly monitor environmental temperature to set sleep timing. Nature 2018, 555, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Iberall, A.S.; Soodak, H. A physics for complex systems. In Self-Organizing Systems: The Emergence of Order; Yates, F.E., Ed.; Plenum Press: New York, NY, USA, 1987; pp. 499–520. [Google Scholar]
- Turvey, M.T.; Carello, C. On intelligence from first principles: Guidelines for inquiry into the hypothesis of physical intelligence (PI). Ecol. Psychol. 2012, 24, 3–32. [Google Scholar] [CrossRef]
- Folkard, S. Diurnal variation. In Stress and Fatigue in Human Performance; Hockey, R., Ed.; Wiley: New York, NY, USA, 1983; pp. 245–272. [Google Scholar]
- Rutenfranz, J.; Hellbrügge, T. Über Tagesschwankungen der Rechengeschwindigkeit bei 11jÄhrigen Kindern. Z. Kinderheilk. 1957, 80, 65–82. [Google Scholar] [CrossRef]
- Blake, M.J.F. Time of day effects on performance in a range of tasks. Psychon. Sci. 1967, 9, 349–350. [Google Scholar] [CrossRef]
- Folkard, S.; Knauth, P.; Monk, T.H.; Rutenfranz, J. The effect of memory load on the circadian variation in performance efficiency under a rapidly rotating shift system. Ergonomics 1976, 19, 479–488. [Google Scholar] [CrossRef]
- Monk, T.H.; Weitzman, E.D.; Fookson, J.E.; Moline, M.L.; Kronauer, R.E.; Gander, P.H. Task variables determine which biological clock controls circadian rhythms in human performance. Nature 1983, 304, 543–545. [Google Scholar] [CrossRef]
- Soodak, H.; Iberall, A. Homeokinetics: A physical science for complex systems. Science 1978, 201, 579–582. [Google Scholar] [CrossRef]
- Iberall, A.S. The physics, chemical physics, and biological physics of the origin of life on earth. Ecol. Psychol. 2001, 13, 315–327. [Google Scholar] [CrossRef]
- Johnson, M.P.; Duffy, J.F.; Dijk, D.J.; Ronda, J.M.; Dyal, C.M.; Czeisler, C.A. Short-term memory, alertness, and performance: A reappraisal of their relationship to body temperature. J. Sleep Res. 1992, 1, 24–29. [Google Scholar] [CrossRef]
- Shaw, R. Processes, acts, and experiences: Three stances on the problem of intentionality. Ecol. Psychol. 2001, 13, 275–314. [Google Scholar] [CrossRef]
- Mahner, M.; Bunge, M. Foundations of Biophilosophy; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Gottlieb, G. Environmental and behavioral influences on gene activity. Curr. Dir. Psychol. Sci. 2000, 9, 93–97. [Google Scholar] [CrossRef]

| Condition | Participants (N) | Circadian Points | Trials at Each Circadian | Task/Rest (min) |
|---|---|---|---|---|
| Normal | 8 | 5:00 12:00 17:00 00:00 | 6 | 1/5 |
| Participants (Index) | Circadian 5:00 | Circadian 12:00 | Circadian 17:00 | Circadian 00:00 | ||||
|---|---|---|---|---|---|---|---|---|
| P1_IW | 1.16 | 0.604 | 0.89 | 0.529 | 1.58 | 0.676 | 6.02 | 4.314 |
| P2_IW | 4.08 | 4.318 | 2.88 | 1.080 | 5.01 | 0.625 | 3.82 | 1.604 |
| P3_IW | 1.22 | 0.67 | 4.04 | 0.604 | 1.55 | 0.540 | 3.91 | 0.770 |
| P4_IW | 2.67 | 7.739 | 1.76 | 8.378 | 6.67 | 1.01 | 7.67 | 0.679 |
| P5_IW | 6.18 | 4.079 | 3.06 | 1.367 | 2.72 | 1.134 | 4.68 | 2.061 |
| P6_IW | 1.95 | 3.801 | 5.68 | 5.971 | 5.81 | 3.331 | 4.625 | 4.114 |
| P7_IW | 3.52 | 4.013 | 4.47 | 2.096 | 0.84 | 0.617 | 3.87 | 5.525 |
| P8_IW | 4.25 | 1.738 | 5.57 | 4.004 | 1.96 | 3.598 | 7.16 | 4.413 |
| Circadian 5:00 | Circadian 12:00 | Circadian 17:00 | Circadian 00:00 | |||||
|---|---|---|---|---|---|---|---|---|
| N(I) | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| AVER | 4.382 | 3.370 | 3.546 | 3.003 | 3.269 | 1.441 | 5.221 | 2.935 |
| STDEV | 3.165 | 2.351 | 1.717 | 2.872 | 2.229 | 1.267 | 1.536 | 1.870 |
| SES | 1.119 | 0.831 | 0.607 | 1.015 | 0.788 | 0.448 | 0.543 | 0.661 |
| Temp (°C) | 36.607 | 36.834 | 37.023 | 36.681 | ||||
| Condition | Participants (N) | Circadian Points | Trials at Each Circadian Point | Task/Rest (min) |
|---|---|---|---|---|
| Normal | 8 | 5:00 17:00 | 6 | 1/5 |
| Abnormal (heat-based) | 8 | 5:00 17:00 | 6 | 1/5 |
| Circadian 5:00 | Circadian 12:00 | Circadian 17:00 | Circadian 00:00 | |
|---|---|---|---|---|
| N(I) | 8 | 8 | 8 | 8 |
| Norm (H) | 0.410 | −0.165 | 0.564 | −0.809 |
| Vari (H) | 0.651 | 0.664 | 0.627 | 0.745 |
| SES | 0.230 | 0.235 | 0.222 | 0.264 |
| Condition | Participants (N) | Circadian Points | Trials at Each Circadian Point | Task/Rest (min) |
|---|---|---|---|---|
| Normal | 8 | 5:00 17:00 | 6 | 1/5 |
| Abnormal (ice-based) | 8 | 5:00 17:00 | 6 | 1/5 |
| Circadian 5:00 | Circadian 12:00 | Circadian 17:00 | Circadian 00:00 | |
|---|---|---|---|---|
| N(I) | 8 | 8 | 8 | 8 |
| Norm (H) | 0.404 | −0.172 | 0.608 | −0.840 |
| Vari (H) | 0.446 | 1.031 | 0.518 | 0.993 |
| SES | 0.158 | 0.365 | 0.183 | 0.351 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Hwang, J.; Ahn, J.W.; Park, Y.J. Perceiving “Complex Autonomous Systems” in Symmetry Dynamics: Elementary Coordination Embedding in Circadian Cycles. Int. J. Environ. Res. Public Health 2023, 20, 166. https://doi.org/10.3390/ijerph20010166
Park C, Hwang J, Ahn JW, Park YJ. Perceiving “Complex Autonomous Systems” in Symmetry Dynamics: Elementary Coordination Embedding in Circadian Cycles. International Journal of Environmental Research and Public Health. 2023; 20(1):166. https://doi.org/10.3390/ijerph20010166
Chicago/Turabian StylePark, Chulwook, Jean Hwang, Jae Woong Ahn, and Yu Jin Park. 2023. "Perceiving “Complex Autonomous Systems” in Symmetry Dynamics: Elementary Coordination Embedding in Circadian Cycles" International Journal of Environmental Research and Public Health 20, no. 1: 166. https://doi.org/10.3390/ijerph20010166
APA StylePark, C., Hwang, J., Ahn, J. W., & Park, Y. J. (2023). Perceiving “Complex Autonomous Systems” in Symmetry Dynamics: Elementary Coordination Embedding in Circadian Cycles. International Journal of Environmental Research and Public Health, 20(1), 166. https://doi.org/10.3390/ijerph20010166






