Oxygen–Ozone Therapy Associated with Alpha Lipoic Acid Plus Palmitoylethanolamide and Myrrh versus Ozone Therapy in the Combined Treatment of Sciatic Pain Due to Herniated Discs: Observational Study on 318 Patients
Abstract
:1. Introduction
2. Materials and Methods
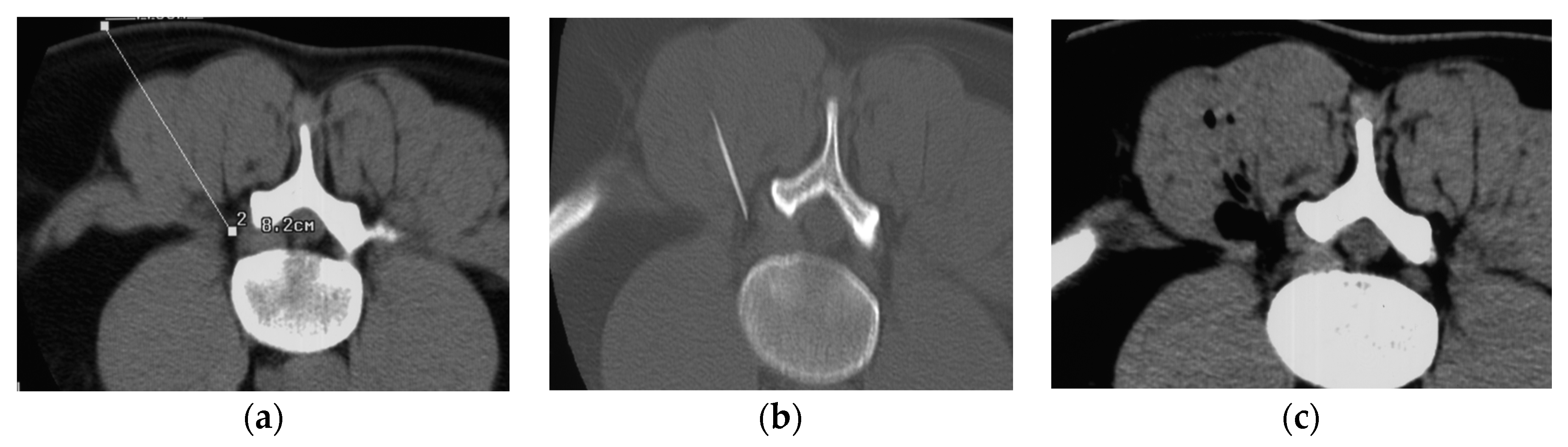
3. Infiltration Technique
- Excellent: resolution of pain and return to normal activity carried out prior to pain onset.
- Good or satisfactory: more than 50% reduction of pain.
- Poor: partial reduction of pain below 70%.
4. Statistical Analysis
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andreula, C.F.; Simonetti, L.; De Santis, F.; Agati, R.; Ricci, R.; Leonardi, M. Minimally Invasive Oxygen-Ozone Therapy for Lumbar Disk Herniation. AJNR 2003, 24, 996–1000. [Google Scholar] [PubMed]
- Andreula, C.F.; Muto, M.; Leonardi, M. Interventional spinal procedures. Eur. J. Radiol. 2004, 50, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, M.; Fontana, A.; Cotticelli, B.; Dalla Volta, G.; Guindani, M.; Leonardi, M. Intraforaminal O2-O3 versus periradicular steroidal infiltrations in lower back pain: Randomized controlled study. Am. J. Neuroradiol. 2005, 26, 996–1000. [Google Scholar] [PubMed]
- Bonetti, M.; Cotticelli, B.; Raimondi, D.; Valdenassi, L.; Richelmi, P.; Bertè, F.A. Ossigeno-ozono terapia vs infiltrazioni epidurali cortisoniche. Riv. Neuroradiol. 2000, 13, 203–206. [Google Scholar] [CrossRef]
- Bonetti, M.; Zambello, A.; Leonardi, M.; Princiotta, C. Herniated disks unchanged over time: Size reduced after oxygen-ozone theapy. Interv. Neuroradiol. 2016, 22, 466–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonetti, M.; Zambello, A.; Princiotta, C.; Pellicanò, G.; Della Gatta, L.; Muto, M. Non-discogenic low back pain treated with oxygen-ozone: Outcome in selected applications. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. 1), 21–30. [Google Scholar] [PubMed]
- Dall’Olio, M.; Princiotta, C.; Cirillo, L.; Budai, C.; de Santis, F.; Bartolini, S.; Serchi, E.; Leonardi, M. Oxygen-Ozone Therapy for Herniated Lumbar Disc in Patients with Subacute Partial motor Weakness Due to Nerve Root Compression Interventional. Neuroradiology 2014, 20, 547–554. [Google Scholar]
- Gallucci, M.; Limbucci, N.; Zugaro, L.; Barile, A.; Stavroulis, E.; Ricci, A.; Galzio, R.; Masciocchi, C. Sciatica: Treatment with Intradiscal and Intraforaminal Injections of Steroid and Oxygen-Ozone versus Steroid Only. Radiology 2007, 242, 907–913. [Google Scholar] [CrossRef]
- Iliakis, E. Ozone treatment in low back pain. Orthopaedics 1995, 1, 29–33. [Google Scholar]
- Iliakis, E.; Valadakis, V.; Vynios, D.H.; Tsiganos, C.P.; Agapitos, E. Rationalization of the activity of medical ozone on intervertebral disc and histological and biochemical study. Riv. Neuroradiol. 2001, 14 (Suppl. 1), 25–30. [Google Scholar] [CrossRef]
- Leonardi, M.; Simonetti, L.; Barbara, C. Effetti dell’ozono sul nucleo polposo: Reperti anatomo-patologici su un caso operato. Riv. Neuroradiol. 2001, 14 (Suppl. 1), 57–61. [Google Scholar] [CrossRef]
- Leonardi, M.; Albini Riccioli, L.; Battaglia, S.; de Santis, F.; Cenni, P.; Raffi, L.; Simonetti, L. Oxygen-ozone chemonucleolysis for herniated disc with sciatica. A comparison of treatments in patients with subacute and chronic symptoms. Riv. Ital. Di Ossigeno-Ozonoterapia 2006, 5, 33–36. [Google Scholar]
- Leonardi, M.; Simonetti, L.; Raffi, L.; Cenni, P.; Barbara, C. Mini-invasive treatment of herniated disc by oxygen-ozone injection. Interv. Neuroradiol. 2003, 9 (Suppl. 2), 75. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, F.N.; Dotta, L.; Sasse, A.; Teixera, M.J.; Fonoff, E.T. Ozone therapy as a treatment for low back pain secondary to herniated disc: A systematic review and meta-analysis of randomized controlled trials. Pain Physician 2012, 2, 15. [Google Scholar]
- Muto, M.; Andreula, C.; Leonardi, M. Treatment of herniated lumbar disc by intradiscal and intraforaminal oxygen-ozone (O2-O3) injection. J. Neuroradiol. 2004, 31, 183–189. [Google Scholar] [CrossRef]
- Pellicanò, F.; Martinetti, F.; Tavanti, V.; Bonetti, M.; Leonardi, M.; Muto, M.; Andreula, C.; Villari, N. The Italian Oxygen-Ozone Therapy Federation (FIO) study on oxygen-ozone treatment of herniated disc. Int. J. Ozone Ther. 2007, 6, 7–15. [Google Scholar]
- Perri, M.; Grattacaso, G.; Di Tunno, V.; Marsecano, C.; Di Cesare, E.; Splendiani, A.; Gallucci, M. MRI DWI/ADC signal predicts shrinkage of lumbar disc herniation after O2-O3 discolysis. Neuroradiol. J. 2015, 28, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Rahimi-Movaghar, V.; Eslami, V. The major efficient mechanisms of ozone therapy are obtained in intradiscal procedures. Pain Physician 2012, 15, E1007-8. [Google Scholar]
- Rimeika, G.; Saba, L.; Arthimulam, G.; Della Gatta, L.; Davidovic, K.; Bonetti, M.; Franco, D.; Russo, C.; Muto, M. Metanalysis on the effectiveness of low back pain treatment with oxygen-ozone mixture: Comparison between image-guided and non-image-guided injection techniques. Eur. J. Radiol. Open. 2021, 6, 8. [Google Scholar] [CrossRef]
- Splendiani, A.; Perri, M.; Conchiglia, A.; Fasano, F.; Di Egidio, G.; Masciocchi, C.; Gallucci, M. MR assessment of lumbar disk herniation treated with oxygen-ozone diskolysis: The role of DWI and related ADC versus intervertebral disk volumetric analysis for detecting treatment response. Neuroradiol. J. 2013, 26, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Steppan, J.; Meaders, T.; Muto, M.; Murphy, K.J. A metanalysis of the effectiveness and safety of ozone treatments for herniated lumbar discs. J. Vasc. Interv. Radiol. 2010, 21, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Y.; Jiang, J.; Ding, T.; Wang, J. Treatment of the lumbar disc herniation with intradiscal and intraforaminal injection of oxygen-ozone. J. Back Musculoskelet. Rehabil. 2013, 26, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Sizer, P.S., Jr.; Phelps, V.; Matthijs, O. Pain generators of the lumbar spine. Pain Pract. 2001, 1, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V. Ozone: A New Medical Drug; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Bocci, V. Scientific and medical aspects of ozone therapy. State of the art. Arch. Med. Res. 2006, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Bilska, A.; Wlodek, L. Lipoic acid: The drug of the future? Pharm. Rep. 2005, 57, 570–577. [Google Scholar]
- Costantino, M.; Guaraldi, C.; Costantino, D.; De Grazia, S.; Unfer, V. Peripheral neuropathy in obstetrics: Efficacy and safety of alpha lipoic acid supplementation. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2766–2771. [Google Scholar]
- Han, T.; Bai, J.; Liu, W.; Hu, Y. A systematic review and metanalysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur. J. Endocrinol. 2012, 167, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Matsugo, S.; Yan, L.J.; Han, D.; Trischler, H.J.; Packer, L. Elucidation of antioxidant activity of alpha-lipoic acid towards hydroxyl radical. Biochem. Biophys. Res. Commun. 1995, 208, 161–167. [Google Scholar] [CrossRef]
- Mijnhout, G.S.; Kollen, B.J.; Alkhalaf, A.; Kleefstra, N.; Bilo, H.J.G. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: A meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2012, 201, 456279. [Google Scholar] [CrossRef]
- Nagamatsu, M.; Nickander, K.K.; Schmelzer, J.D.; Raya, A.; Wittrock, D.A.; Tritschler, H.; Low, P.A. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care 1995, 18, 1160–1167. [Google Scholar] [CrossRef]
- Park, S.; Karunakaran, U.; Jeoung, N.H.; Jeon, J.H.; Lee, I.K. Physiological effect and therapeutic application of alpha lipoic acid. Curr. Med. Chem. 2014, 21, 3636–3645. [Google Scholar] [CrossRef]
- Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Sharifi-Rad Insights on the Use of alpha-Lipoic Acid for Therapeutic Purposes. J. Biomol. 2019, 9, 356. [Google Scholar]
- Ziegler, D.; Ametov, A.S.; Barinov, A.; Dyck, P.J.; Gurieva, I.; Low, P.A.; Munzel, U.; Yakhno, N.; Raz, I.; Novosadova, M.; et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy. Diabetes Care 2006, 29, 2365–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler, D.; Hanefeld, M.; Ruhnau, K.-J.; Lobisch, M.; Schütte, K.; Gries, F.A. Treatment of symptomatic diabetic peripheral neuropathy with the antioxidant alpha-lipoic acid: A 3-week multicentre randomized controlled trial (ALADIN study). Diabetologia 1995, 38, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- El Ashry, E.S.; Rashed, N.; Salama, O.M.; Saleh, A. Components, therapeutic value, and uses of myrrh. Pharmazie 2003, 58, 163–168. [Google Scholar] [PubMed]
- Nomicos, E.Y. Myrrh: Medical marvel or myth of the Magi? Holist Nurs. Pract. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Paladini, A.; Fusco, M.; Cenacchi, T.; Schievano, P.C.; Piroli, A.; Varrassi, G. Palmitoylethanolamide, a special food for medical purposes, in the treatment of chronic pain: A pooled data meta-analysis. Pain Physician 2016, 19, 11–24. [Google Scholar]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Fernandez-Cuadros, M.E.; Albaladejo-Florin, M.J.; Martin-Martin, L.M.; Alava-Rabasa, S.; Pérez-Moro, O.S. Effectiveness of Physical Therapy Plus TIOBEC®(α-Lipoic Acid Vitamin B, C, and E) on Complex Regional Pain Syndrome (CRPS): A Small Case Series/Pilot Study. Middle East J. Rehabil. Health Stud. 2020, 7, e100636. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Cuadros, M.E.; Albaladejo-Florín, M.J.; Álava-Rabasa, S.; Casique-Bocanegra, L.O.; López-Muñoz, M.J.; Rodríguez-de-Cía, J.; Pérez-Moro, O.S. A nutraceutical combination of Alpha-lipoic acid, Palmitoylethanolamide and Myrrha might be effective in COVID-19 prevention. Lett. Ed./Med. Health Sci. 2020. [Google Scholar] [CrossRef]
- Le, H.; Sandhu, F.A.; Fessler, R.G. Clinical outcomes after minimal-access surgery for recurrent lumbar disc herniation. Neurosurg. Focus 2003, 15, E12. [Google Scholar] [CrossRef] [PubMed]
- Lidar, Z.; Lifshutz, J.; Bhattacharjee, S.; Kurpad, S.N.; Maiman, D.J. Minimally invasive, extracavitary approach for thoracic disc herniation: Technical report and preliminary results. Spine J. 2006, 6, 157–163. [Google Scholar] [CrossRef] [PubMed]

| Group A | O2-O3 Treatment | ||
|---|---|---|---|
| Outcome | Excellent | Good | Poor |
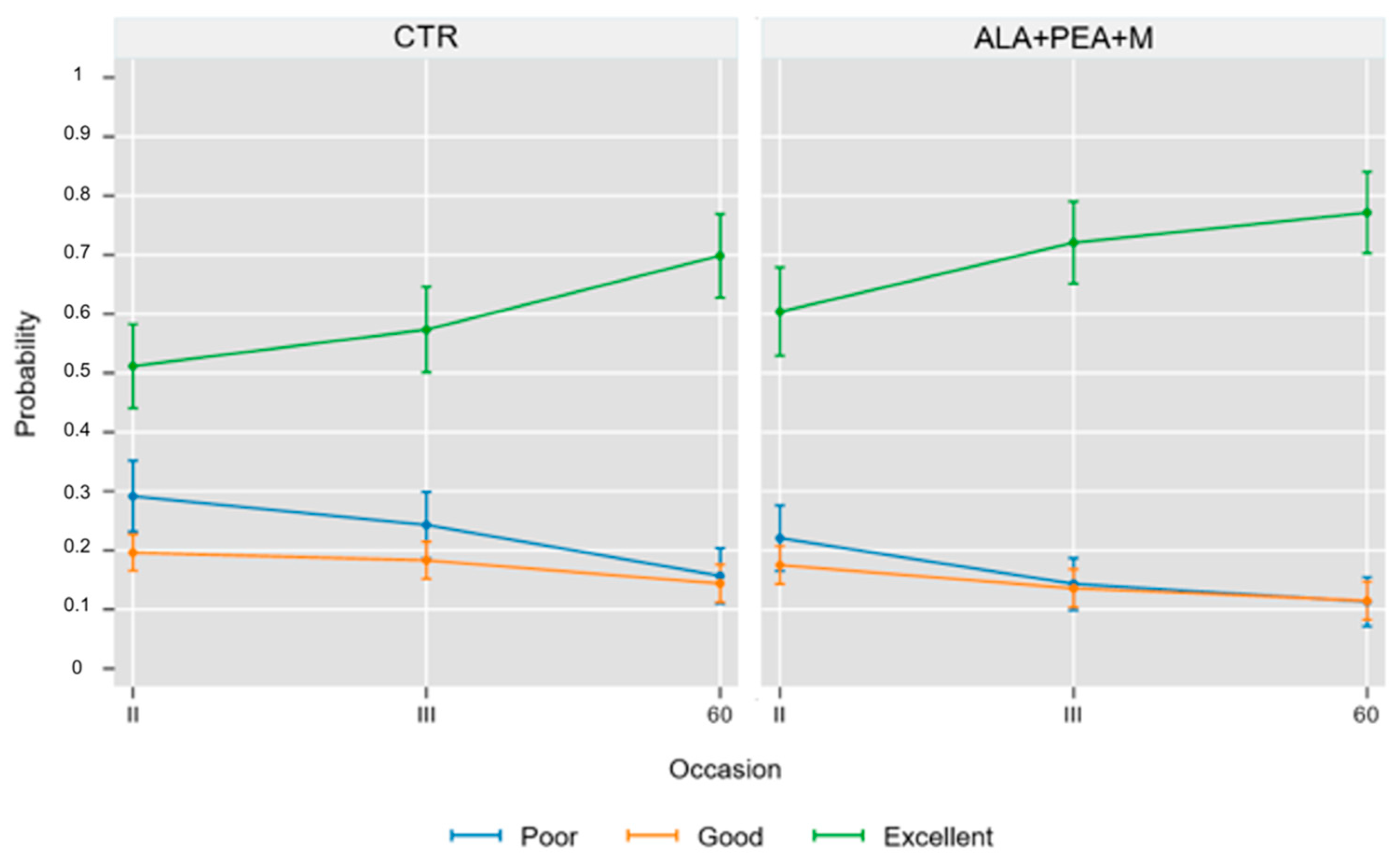
| 2nd treatment (9 ± 2 days) | 83 (50.3%) | 37 (22.4%) | 45 (27.3%) |
| 3rd treatment (18 ± 2 days) | 94 (57%) | 32 (19.4%) | 39 (23.6%) |
| 60 ± 8 days | 116 (70.3%) | 21 (12.7%) | 28 (17.0%) |
| Group B | O2-O3 Treatment + ALA, PEA, and Myrrh | ||
|---|---|---|---|
| Outcome | Excellent | Good | Poor |
| 2nd treatment (9 ± 2 days) | 92 (60.1%) | 28 (18.3%) | 33 (21.6%) |
| 3rd treatment (18 ± 2 days) | 106 (69.3%) | 22 (14.4%) | 25 (16.3%) |
| 60 ± 8 days | 119 (77.8%) | 13 (8.5%) | 21 (13.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonetti, M.; Lauritano, D.; Ottaviani, G.M.; Fontana, A.; Zambello, A.; Della Gatta, L.; Muto, M.; Carinci, F. Oxygen–Ozone Therapy Associated with Alpha Lipoic Acid Plus Palmitoylethanolamide and Myrrh versus Ozone Therapy in the Combined Treatment of Sciatic Pain Due to Herniated Discs: Observational Study on 318 Patients. Int. J. Environ. Res. Public Health 2022, 19, 5716. https://doi.org/10.3390/ijerph19095716
Bonetti M, Lauritano D, Ottaviani GM, Fontana A, Zambello A, Della Gatta L, Muto M, Carinci F. Oxygen–Ozone Therapy Associated with Alpha Lipoic Acid Plus Palmitoylethanolamide and Myrrh versus Ozone Therapy in the Combined Treatment of Sciatic Pain Due to Herniated Discs: Observational Study on 318 Patients. International Journal of Environmental Research and Public Health. 2022; 19(9):5716. https://doi.org/10.3390/ijerph19095716
Chicago/Turabian StyleBonetti, Matteo, Dorina Lauritano, Gian Maria Ottaviani, Alessandro Fontana, Alessio Zambello, Luigi Della Gatta, Mario Muto, and Francesco Carinci. 2022. "Oxygen–Ozone Therapy Associated with Alpha Lipoic Acid Plus Palmitoylethanolamide and Myrrh versus Ozone Therapy in the Combined Treatment of Sciatic Pain Due to Herniated Discs: Observational Study on 318 Patients" International Journal of Environmental Research and Public Health 19, no. 9: 5716. https://doi.org/10.3390/ijerph19095716
APA StyleBonetti, M., Lauritano, D., Ottaviani, G. M., Fontana, A., Zambello, A., Della Gatta, L., Muto, M., & Carinci, F. (2022). Oxygen–Ozone Therapy Associated with Alpha Lipoic Acid Plus Palmitoylethanolamide and Myrrh versus Ozone Therapy in the Combined Treatment of Sciatic Pain Due to Herniated Discs: Observational Study on 318 Patients. International Journal of Environmental Research and Public Health, 19(9), 5716. https://doi.org/10.3390/ijerph19095716







