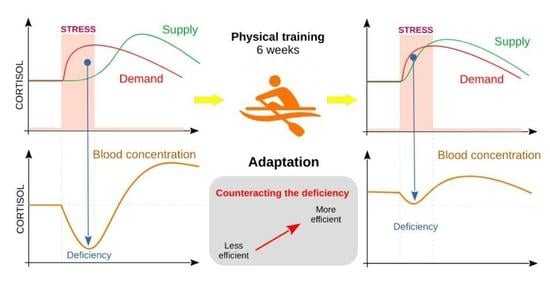
Stressor-Induced Temporal Cortisol Deficiency as a Primary Trigger for Adaptation to Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Experiment Conditions
2.3. Experiment Protocol
- The first measurement of cortisol concentration at its initial level (I) at 7:00 a.m., before breakfast;
- an exertion stress test of 2000 m rowing at maximum intensity;
- the second measurement of cortisol concentration immediately after the exertion (E); and
- the third measurement of cortisol concentration after restitution (R) on the second day at 7:00 a.m., before breakfast.
2.4. Measurements
2.5. Statistical Analysis
- Cortisol concentration levels:
- ○
- I—initial (1st day, morning);
- ○
- E—after the exertion test;
- ○
- R—after restitution (2nd day, morning).
- Exertion-induced changes in cortisol levels:
- ○
- IE = RC(I, E)—immediate reaction to exertion;
- ○
- IR = RC(I, R)—the expression of this reaction after recovery.
- The training camp effects:
- ○
- ∆IE = (IEafter − IEbefore)—change in immediate reaction to exertion;
- ○
- ∆IR = (IRafter − IRbefore)—change in the after-recovery expression of the reaction;
- ○
- ∆I = RC(Ibefore, Iafter)—change in initial concentration levels;
- ○
- ∆E = RC(Ebefore, Eafter)—change in concentration levels just after exertion;
- ○
- ∆R = RC(Rbefore, Rafter)—change in concentration levels after recovery.
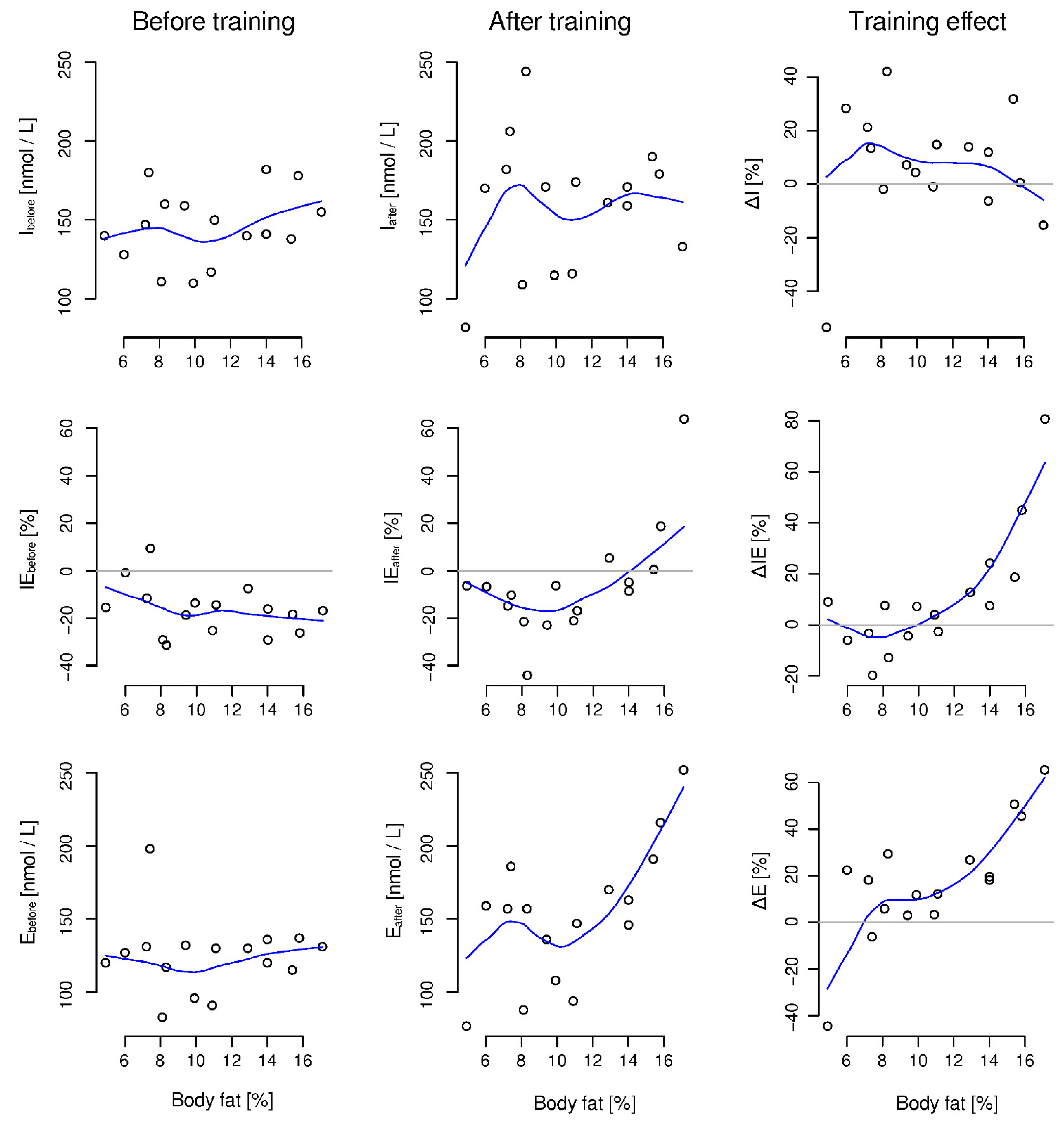
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spencer, R.L.; Deak, T. A users guide to HPA axis research. Physiol. Behav. 2017, 178, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Danan, D.; Todder, D.; Zohar, J.; Cohen, H. Is PTSD-Phenotype Associated with HPA-Axis Sensitivity? Feedback Inhibition and Other Modulating Factors of Glucocorticoid Signaling Dynamics. Int. J. Mol. Sci. 2021, 22, 6050. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; Piacentini, M.F.; Busschaert, B.; Buyse, L.; De Schutter, G.; Stray-Gundersen, J. Hormonal responses in athletes: The use of a two bout exercise protocol to detect subtle differences in (over)training status. Eur. J. Appl. Physiol. 2004, 91, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Vedhara, K.; Hyde, J.; Gilchrist, I.D.; Tytherleigh, M.; Plummer, S. Acute stress, memory, attention and cortisol. Psychoneuroendocrinology 2000, 25, 535–549. [Google Scholar] [CrossRef]
- Tsai, C.L.; Wang, C.H.; Pan, C.Y.; Chen, F.C.; Huang, T.H.; Chou, F.Y. Executive function and endocrinological responses to acute resistance exercise. Front. Behav. Neurosci. 2014, 8, 262. [Google Scholar] [CrossRef]
- Kemmler, W.; Wildt, L.; Engelke, K.; Pintag, R.; Pavel, M.; Bracher, B.; Weineck, J.; Kalender, W. Acute hormonal responses of a high impact physical exercise session in early postmenopausal women. Eur. J. Appl. Physiol. 2003, 90, 199–209. [Google Scholar] [CrossRef]
- Heaney, J.L.; Carroll, D.; Phillips, A.C. DHEA, DHEA-S and cortisol responses to acute exercise in older adults in relation to exercise training status and sex. Age 2013, 35, 395–405. [Google Scholar] [CrossRef]
- Rimmele, U.; Seiler, R.; Marti, B.; Wirtz, P.H.; Ehlert, U.; Heinrichs, M. The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology 2009, 34, 190–198. [Google Scholar] [CrossRef]
- Adam, E.K.; Quinn, M.E.; Tavernier, R.; McQuillan, M.T.; Dahlke, K.A.; Gilbert, K.E. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 83, 25–41. [Google Scholar] [CrossRef]
- Gerber, M.; Imboden, C.; Beck, J.; Brand, S.; Colledge, F.; Eckert, A.; Holsboer-Trachsler, E.; Pühse, U.; Hatzinger, M. Effects of Aerobic Exercise on Cortisol Stress Reactivity in Response to the Trier Social Stress Test in Inpatients with Major Depressive Disorders: A Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1419. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.A.; Wand, G. Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol Res. 2012, 34, 468–483. [Google Scholar] [PubMed]
- Tops, M.; van Peer, J.M.; Wijers, A.A.; Korf, J. Acute cortisol administration reduces subjective fatigue in healthy women. Psychophysiology 2006, 43, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, E.; Dahlman, A.S.; Börjesson, M.; Gullstrand, L.; Jonsdottir, I.H. Exercise training and physiological responses to acute stress: Study protocol and methodological considerations of a randomised controlled trial. BMJ Open Sport Exerc. Med. 2018, 4, e000393. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Koo, J.; Lim, S.K. Associations between Stress and Physical Activity in Korean Adolescents with Atopic Dermatitis Based on the 2018–2019 Korea Youth Risk Behavior Web-Based Survey. Int. J. Environ. Res. Public Health 2020, 17, 8175. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Kater, C.E. Hormonal aspects of overtraining syndrome: A systematic review. BMC Sports Sci. Med. Rehabil. 2017, 9, 14. [Google Scholar] [CrossRef]
- Lehrer, H.M.; Steinhardt, M.A.; Dubois, S.K.; Laudenslager, M.L. Perceived stress, psychological resilience, hair cortisol concentration, and metabolic syndrome severity: A moderated mediation model. Psychoneuroendocrinology 2020, 113, 104510. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar]
- Peters, A.; Conrad, M.; Hubold, C.; Schweiger, U.; Fischer, B.; Fehm, H.L. The principle of homeostasis in the hypothalamus-pituitary-adrenal system: New insight from positive feedback. Am. J. Physiol Regul. Integr. Comp. Physiol. 2007, 293, R83–R98. [Google Scholar] [CrossRef]
- Rao, R.; Androulakis, I.P. Allostatic adaptation and personalized physiological trade-offs in the circadian regulation of the HPA axis: A mathematical modeling approach. Sci. Rep. 2019, 9, 11212. [Google Scholar] [CrossRef]
- Rimmele, U.; Zellweger, B.C.; Marti, B.; Seiler, R.; Mohiyeddini, C.; Ehlert, U.; Heinrichs, M. Trained men show lower cortisol, heart rate and psychological responses to psychosocial stress compared with untrained men. Psychoneuroendocrinology 2007, 32, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Mangine, G.T.; Van Dusseldorp, T.A.; Feito, Y.; Holmes, A.J.; Serafini, P.R.; Box, A.G.; Gonzalez, A.M. Testosterone and Cortisol Responses to Five High-Intensity Functional Training Competition Workouts in Recreationally Active Adults. Sports 2018, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Smilios, I.; Pilianidis, T.; Karamouzis, M.; Tokmakidis, S.P. Hormonal responses after various resistance exercise protocols. Med. Sci. Sports Exerc. 2003, 35, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.; Fortes, M.; de Mello, D.B. Concurrent Training Decreases Cortisol but Not Zinc Concentrations: Effects of Distinct Exercise Protocols. Scientifica 2016, 2016, 7643016. [Google Scholar] [CrossRef] [PubMed]
- Corazza, D.I.; Sebastião, É.; Pedroso, R.V.; Andreatto, C.A.; de Melo Cohelo, F.G.; Gobbi, S.; Teodorov, E.; Santoz-Galduroz, R.F. Influence of chronic exercise on serum cortisol levels in older adults. Eur. Rev. Aging Phys. Act. 2014, 11, 25–34. [Google Scholar] [CrossRef]
- Taha, M.; Mounir, K. Acute response of serum cortisol to different intensities of resisted exercise in the elderly. Bull. Fac. Phys. Ther. 2019, 24, 20–25. [Google Scholar] [CrossRef]
- Bonato, M.; La Torre, A.; Marventano, I.; Saresella, M.; Merati, G.; Banfi, G.; Vitale, J.A. Effect of High-Intensity Interval Training versus Small-Sided Games Training on Sleep and Salivary Cortisol Level. Int. J. Sports Physiol. Perform. 2020, 15, 1237–1244. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org (accessed on 30 August 2020).
- Mujica-Parodi, L.R.; Renelique, R.; Taylor, M.K. Higher body fat percentage is associated with increased cortisol reactivity and impaired cognitive resilience in response to acute emotional stress. Int. J. Obes 2009, 33, 157–165. [Google Scholar] [CrossRef]
- Woods, A.L.; Rice, A.J.; Garvican-Lewis, L.A.; Wallett, A.M.; Lundy, B.; Rogers, M.A.; Welvaert, M.; Halson, S.; McKune, A.; Thompson, K.G. The effects of intensified training on resting metabolic rate (RMR), body composition and performance in trained cyclists. PLoS ONE 2018, 13, e0191644. [Google Scholar] [CrossRef]
- Cselkó, A.; Szabó, E.I.; Váczi, M.; Kőszegi, T.; Tékus, E.; Wilhelm, M. Relationship between Anthropometric, Physical and Hormonal Parameters among Pre-Pubertal Handball Players. Int. J. Environ. Res. Public Health 2021, 18, 9977. [Google Scholar] [CrossRef]
- Caplin, A.; Chen, F.S.; Beauchamp, M.R.; Puterman, E. The effects of exercise intensity on the cortisol response to a subsequent acute psychosocial stressor. Psychoneuroendocrinology 2021, 131, 105336. [Google Scholar] [CrossRef]
- Gottschall, J.S.; Davis, J.J.; Hastings, B.; Porter, H.J. Exercise Time and Intensity: How Much Is Too Much? Int. J. Sports Physiol. Perform. 2020, 15, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Minetto, M.A.; Lanfranco, F.; Baldi, M.; Termine, A.; Kuipers, H.; Ghigo, E.; Rainoldi, A. Corticotroph axis sensitivity after exercise: Comparison between elite athletes and sedentary subjects. J. Endocrinol. Investig. 2007, 30, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Gatti, R.; De Palo, E.F. An update: Salivary hormones and physical exercise. Scand. J. Med. Sci. Sports 2011, 21, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Basset, F.A.; Kelly, L.P.; Hohl, R.; Kaushal, N. Type of self-talk matters: Its effects on perceived exertion, cardiorespiratory, and cortisol responses during an iso-metabolic endurance exercise. Psychophysiology 2022, 59, e13980. [Google Scholar] [CrossRef]
- Zapater-Fajarí, M.; Crespo-Sanmiguel, I.; Pulopulos, M.M.; Hidalgo, V.; Salvador, A. Resilience and Psychobiological Response to Stress in Older People: The Mediating Role of Coping Strategies. Front. Aging Neurosci. 2021, 13, 632141. [Google Scholar] [CrossRef] [PubMed]

| 1st Week | Last Week | |||||
|---|---|---|---|---|---|---|
| Training times [min/Day] | Days per Week | Training times [min/Day] | Days per Week | |||
| Type of Exertion | Mean | SD | Mean | SD | ||
| Training for force development | 80.00 | 14.14 | 2 | 75.00 | 7.07 | 2 |
| Extensive endurance rowing | 77.00 | 17.31 | 7 | 78.83 | 17.09 | 6 |
| High-intensity endurance rowing | 25.25 | 10.50 | 4 | 87.86 | 16.66 | 7 |
| Very high-intensity endurance rowing | 0 | 0 | 0 | 31.67 | 11.59 | 3 |
| Unspecific training (running, among others) | 26.43 | 24.10 | 7 | 22.86 | 25.63 | 7 |
| LA concentration [mmol/L] | LA concentration [mmol/L] | |||||
| Lactate acid measurement | Mean | SD | Mean | SD | ||
| Before exertion test at the end of a week | 1.43 | 0.20 | 1.64 | 0.57 | ||
| After exertion test at the end of a week | 16.42 | 3.43 | 15.05 | 3.02 | ||
| Before Training | After Training | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Cortisol concentration [nmol/L] | I | 146.0 [133.9, 158.1] | 22.78 | 160.1 [138.4, 181.8] | 40.69 |
| E | 121.2 [112.0, 130.4] | 17.31 | 151.9 [128.2, 175.6] | 44.51 | |
| R | 176.9 [163.6, 190.2] | 24.97 | 156.7 [140.2, 173.3] | 31.14 | |
| Reaction to exertion (%) | IE | −16.98 [−22.16, −11.79] | 9.73 | −8.59 [−14.92, −2.26] | 11.87 |
| IR | 19.87 [13.95, 25.80] | 11.12 | 1.42 [−10.36, 13.20] | 22.11 | |
| Training effects | Mean (%) | Cohen’s d | Cliff’s δ | p | |
| ΔI | 8.95 [−0.18, 18.09] | 0.52 [−0.01, 1.05] | 0.37 [−0.17, 0.74] | 0.054 | |
| ΔIE | 7.14 [−1.27, 15.56] | 0.45 [−0.08, 0.98] | 0.25 [−0.28, 0.66] | 0.090 | |
| ΔIR | −20.84 [−35.83, −5.85] | −0.74 [−1.27, −0.21] | −0.5 [−0.82, 0.05] | 0.010 | |
| ΔE | 19.07 [8.33, 29.82] | 0.95 [0.41, 1.48] | 0.75 [0.21, 0.94] | 0.002 | |
| ΔR | −13.79 [−23.02, −4.46] | −0.80 [−1.33, −0.26] | −0.5 [−0.82, 0.05] | 0.006 | |
| Before Training | After Training | Training Effect (Δ) | ||||
|---|---|---|---|---|---|---|
| τ | p | τ | p | τ | p | |
| I | 0.15 [−0.25, 0.51] | 0.417 | 0.10 [−0.30, 0.47] | 0.588 | −0.16 [−0.51, 0.24] | 0.392 |
| IE | −0.23 [−0.56, 0.17] | 0.224 | 0.41 [0.03, 0.69] | 0.027 | 0.56 [0.22, 0.78] | 0.003 |
| IR | 0.06 [−0.33, 0.43] | 0.752 | 0.02 [−0.36, 0.41] | 0.892 | 0.07 [−0.32, 0.45] | 0.685 |
| E | 0.14 [−0.26, 0.50] | 0.470 | 0.42 [0.04, 0.70] | 0.024 | 0.53 [0.18, 0.76] | 0.005 |
| R | 0.15 [−0.25, 0.51] | 0.417 | 0.13 [−0.27, 0.49] | 0.498 | 0.09 [−0.3, 0.46] | 0.620 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latour, E.; Arlet, J.; Latour, E.; Latour, M.; Basta, P.; Skarpańska-Stejnborn, A. Stressor-Induced Temporal Cortisol Deficiency as a Primary Trigger for Adaptation to Stress. Int. J. Environ. Res. Public Health 2022, 19, 5633. https://doi.org/10.3390/ijerph19095633
Latour E, Arlet J, Latour E, Latour M, Basta P, Skarpańska-Stejnborn A. Stressor-Induced Temporal Cortisol Deficiency as a Primary Trigger for Adaptation to Stress. International Journal of Environmental Research and Public Health. 2022; 19(9):5633. https://doi.org/10.3390/ijerph19095633
Chicago/Turabian StyleLatour, Ewa, Jarosław Arlet, Emilia Latour, Marianna Latour, Piotr Basta, and Anna Skarpańska-Stejnborn. 2022. "Stressor-Induced Temporal Cortisol Deficiency as a Primary Trigger for Adaptation to Stress" International Journal of Environmental Research and Public Health 19, no. 9: 5633. https://doi.org/10.3390/ijerph19095633
APA StyleLatour, E., Arlet, J., Latour, E., Latour, M., Basta, P., & Skarpańska-Stejnborn, A. (2022). Stressor-Induced Temporal Cortisol Deficiency as a Primary Trigger for Adaptation to Stress. International Journal of Environmental Research and Public Health, 19(9), 5633. https://doi.org/10.3390/ijerph19095633