Hydration Status in Men Working in Different Thermal Environments: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
- A pacemaker or other electronic devices implanted under the skin;
- Metal elements under the skin (e.g., plates, screws, etc.);
- Symptoms of an infection on the day of the examination;
- Chronic diseases, e.g., diseases of the urinary system, metabolic diseases, cardiovascular diseases;
- Use of medications, e.g., diuretics [32];
- Over 45 years of age.
2.2. Study Design
- Urine color (Ucol);
- Urine osmolality (Uosm);
- Urine-specific gravity (USG);
- Serum osmolality (Sosm);
- Body mass (BM);
- Total body water (TBW);
- Thirst (questionnaire).
2.3. Measured Parameters
2.3.1. Workplace Microclimate
2.3.2. Thirst
2.3.3. Anthropometric Measurements (Total Body Water and Body Mass)
2.3.4. Urine Indices of Hydration Status
2.3.5. Serum Hydration Indices
2.4. Measured Hydration Indices
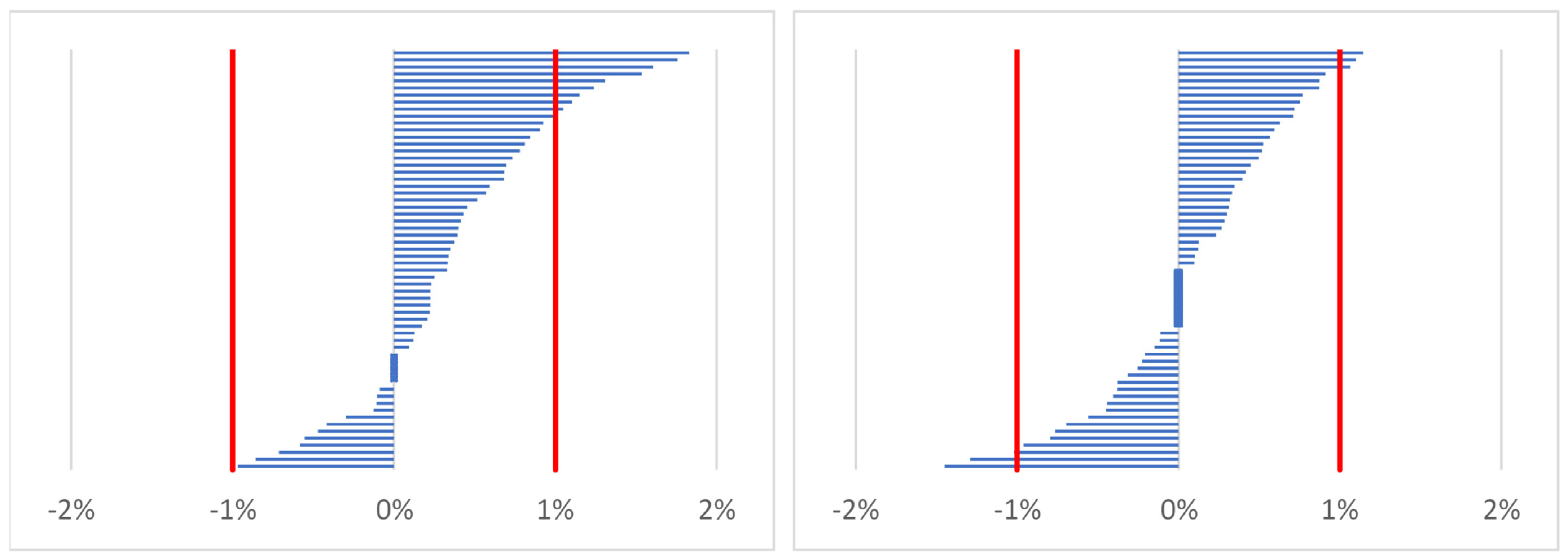
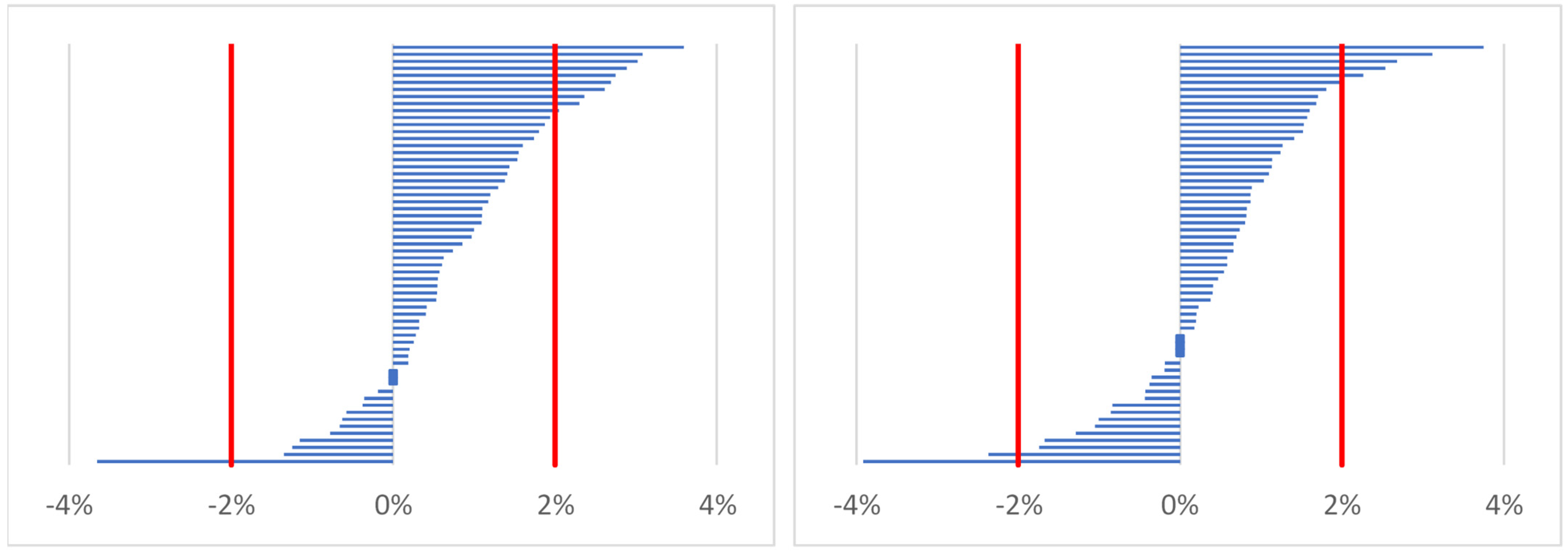
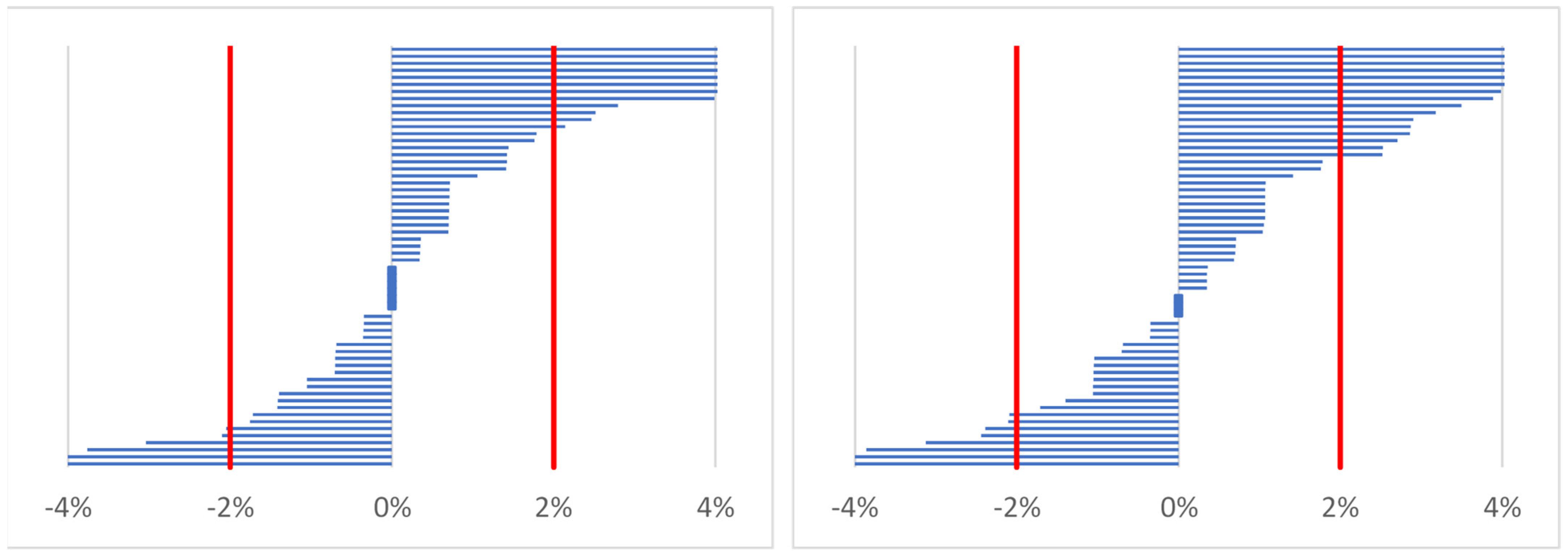
2.4.1. Estimating Percentage Changes of Indices (Body Mass, Total Body Water, Serum Osmolality)
- %X—percentage change of body mass or percentage change of total body water or percentage change of serum osmolality;
- Xpost—marker of hydration status after work (time point 2) or before work on the following day (time point 3);
- Xpre—marker of hydration status before work on the first day (time point 1).
2.4.2. Criteria for Hydration Status Indices
2.5. Statistical Analysis
3. Results
3.1. General Information
3.1.1. Participants
3.1.2. Environmental Conditions
3.2. Hydration Markers in All Participants at Time Points
3.2.1. Thirst
3.2.2. Body Mass (BM)
3.2.3. Total Body Water (TBW)
3.2.4. Urine Color (Ucol)
3.2.5. Urine-Specific Gravity (USG)
3.2.6. Urine Osmolality (Uosm)
3.2.7. Serum Osmolality (Sosm)
3.2.8. Correlations between Urine and Serum Markers
3.3. The Impact of the Season of the Year on Hydration Markers
3.4. Comparison of the Percentage of Workers Who Qualified as Dehydrated Depending on the Hydration Status Indices
4. Discussion
4.1. The Impact of the Time of Day: Time Points (before or after Work)
4.2. The Impact of Season of the Year on Hydration Status
4.3. The Effect of Selected Hydration Status Markers on the Prevalence of Dehydration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheung, S.S.; Lee, J.K.; Oksa, J. Thermal stress, human performance, and physical employment standards. Appl. Physiol. Nutr. Metab. 2016, 41, S148–S164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute for Occupational Safety and Health. NIOSH. HEAT STRESS. Available online: https://www.cdc.gov/niosh/topics/heatstress/default.html (accessed on 27 March 2022).
- Ioannou, L.G.; Mantzios, K.; Tsoutsoubi, L.; Nintou, E.; Vliora, M.; Gkiata, P.; Dallas, C.N.; Gkikas, G.; Agaliotis, G.; Sfakianakis, K.; et al. Occupational Heat Stress: Multi-Country Observations and Interventions. Int. J. Environ. Res. Public Health 2021, 18, 6303. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.D.; Dumitrascu, A.E.; Ciobanu, V.D. Chainsaw operators’ exposure to occupational risk factors and incidence of professional diseases specific to the forestry field. Int. J. Occup. Saf. Ergon. 2022, 28, 8–19. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Occupational Safety and Health. NIOSH. The Physiological Response of Working in Cold Environments and How Your PPE Can Help. Available online: https://blogs.cdc.gov/niosh-science-blog/2021/02/24/cold_ppe/ (accessed on 27 March 2022).
- Freund, B.J.; Sawka, M.N. Influence of Cold Stress on Human Fluid Balance. In Nutritional Needs in Cold and in High-Altitude Environments: Applications for Military Personnel in Field Operations, Institute of Medicine (US) Committee on Military Nutrition Research; Marriott, B.M., Carlson, S.J., Eds.; National Academies Press: Washington, DC, USA, 1996; Chapter 9; pp. 161–179. [Google Scholar]
- Castellani, J.W.; O’Brien, C.; Baker-Fulco, C.; Sawka, M.N.; Young, A.J. Sustaining Health & Performance in Cold Weather Operations; Technical Note; US Army Research Institute of Environmental Medicine Natick: Natick, MA, USA, 2001; TN/02-2 AD; Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/a395745.pdf (accessed on 27 March 2022).
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A multidisciplinary consensus on dehydration: Definitions, diagnostic methods and clinical implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, S.A. Hydration, dehydration, underhydration, optimal hydration: Are we barking up the wrong tree? Eur. J. Nutr. 2019, 58, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Parsons, K. Dehydration and water requirements. In Human Thermal Environments, 2nd ed.; Parsons, K., Ed.; Taylor and Francis: London, UK; New York, NY, USA, 2003; pp. 71–92. [Google Scholar]
- Kenefick, R.W.; Sawka, M.N. Hydration at the work site. J. Am. Coll. Nutr. 2007, 26, 597S–603S. [Google Scholar] [CrossRef]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar]
- Liska, D.; Mah, E.; Brisbois, T.; Barrios, P.L.; Baker, L.B.; Spriet, L.L. Narrative Review of Hydration and Selected Health Outcomes in the General Population. Nutrients 2019, 11, 70. [Google Scholar] [CrossRef] [Green Version]
- Parker, R.; Ashby, L.; Bates, G. Dehydration in loggers—Effects of season and time of day. COHFE Rep. 2002, 3, 1174–1234. [Google Scholar]
- Armstrong, L.E. Assessing hydration status: The elusive gold standard. J. Am. Coll. Nutr. 2007, 26, 575S–584S. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Pumerantz, A.C.; Fiala, K.A.; Roti, M.W.; Kavouras, S.A.; Casa, D.J.; Maresh, C.M. Human hydration indices: Acute and longitudinal reference values. Int. J. Sport. Nutr. Exerc. Metab. 2010, 20, 145–153. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, A.J.; Allanson, B.A.; Caldwell Odgers, J.N.; Cort, M.M.; Costa, R.; Cox, G.R.; Crawshay, S.T.; Desbrow, B.; Freney, E.G.; Gaskell, S.K.; et al. Sports Dietitians Australia Position Statement: Nutrition for Exercise in Hot Environments. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 83–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheuvront, S.N.; Sawka, M.N. Hydration Assessment of Athletes. Sports Sci. Exch. 2006, 25, 238. [Google Scholar]
- Cheuvront, S.N.; Ely, B.R.; Kenefick, R.W.; Sawka, M.N. Biological variation and diagnostic accuracy of dehydration assessment markers. Am. J. Clin. Nutr. 2010, 92, 565–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, L.E.; Maughan, R.J.; Senay, L.C.; Shirreffs, S.M. Limitations to the use of plasma osmolality as a hydration biomarker. Am. J. Clin. Nutr. 2013, 98, 503–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrier, E.; Vergne, S.; Klein, A.; Poupin, M.; Rondeau, P.; Le Bellego, L.; Armstrong, L.E.; Lang, F.; Stookey, J.; Tack, I. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br. J. Nutr. 2013, 109, 1678–1687. [Google Scholar] [CrossRef] [Green Version]
- Bates, G.; Parker, R.; Ashby, L.; Bentley, T. Fluid intake and hydration status of forest workers—A preliminary investigation. J. For. Eng. 2001, 12, 27–32. [Google Scholar] [CrossRef]
- Bates, G.P.; Schneider, J. Hydration status and physiological workload of UAE construction workers: A prospective longitudinal observational study. J. Occup. Med. Toxicol. 2008, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Bates, G.P.; Miller, V.S.; Joubert, D.M. Hydration status of expatriate manual workers during summer in the middle East. Ann. Occup. Hyg. 2010, 54, 137–143. [Google Scholar]
- Biggs, C.; Paterson, M.; Maunder, E. Hydration status of South African forestry workers harvesting trees in autumn and winter. Ann. Occup. Hyg. 2011, 55, 6–15. [Google Scholar]
- Raines, J.; Snow, R.; Petersen, A.; Harvey, J.; Nichols, D.; Aisbett, B. Pre-shift fluid intake: Effect on physiology, work and drinking during emergency wildfire fighting. Appl. Ergon. 2012, 43, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Montazer, S.; Farshad, A.A.; Monazzam, M.R.; Eyvazlou, M.; Yaraghi, A.A.; Mirkazemi, R. Assessment of construction workers’ hydration status using urine specific gravity. Int. J. Occup. Med. Environ. Health 2013, 26, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Sakakibara, Y.; Hisanaga, N.; Oka, T.; Yamaguchi-Sekino, S. Heat Strain and Hydration of Japanese Construction Workers during Work in Summer. Ann. Work. Expo. Health 2018, 62, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Edwin, M.; Moore, D.; Guard, D. Dehydration in New Zealand fishing vessel crews. Saf. Sci. 2019, 117, 314–319. [Google Scholar] [CrossRef]
- Wagoner, R.S.; López-Gálvez, N.I.; de Zapien, J.G.; Griffin, S.C.; Canales, R.A.; Beamer, P.I. An Occupational Heat Stress and Hydration Assessment of Agricultural Workers in North Mexico. Int. J. Environ. Res. Public Health 2020, 17, 2102. [Google Scholar] [CrossRef] [Green Version]
- Mansor, Z.; Rosnah, I.; Ismail, N.H.; Hashim, J.H. Effects of hydration practices on the severity of heat-related illness among municipal workers during a heat wave phenomenon. Med. J. Malays. 2019, 74, 275–280. [Google Scholar]
- Ellis, L.A.; Yates, B.A.; McKenzie, A.L.; Muñoz, C.X.; Casa, D.J.; Armstrong, L.E. Effects of Three Oral Nutritional Supplements on Human Hydration Indices. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 356–362. [Google Scholar] [CrossRef]
- Institute of Meteorology and Water Management—National Research Institute. Instytut Meteorologii i Gospodarki Wodnej—Państwowy Instytut Badawczy. IMGW-PIB. Available online: https://danepubliczne.imgw.pl/datastore (accessed on 27 March 2022).
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 27 March 2022).
- Armstrong, L.E.; Maresh, C.M.; Castellani, J.W.; Bergeron, M.F.; Kenefick, R.W.; LaGasse, K.E.; Riebe, D. Urinary indices of hydration status. Int. J. Sport Nutr. 1994, 4, 265–279. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W.; Charkoudian, N.; Sawka, M.N. Physiologic basis for understanding quantitative dehydration assessment. Am. J. Clin. Nutr. 2013, 97, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Gunawan, A.A.S.; Brandon, D.; Puspa, V.D.; Wiweko, B. Development of Urine Hydration System Based on Urine Color and Support Vector Machine. Procedia Comput. Sci. 2018, 135, 481–489. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Kavouras, S.A.; Walsh, N.P.; Roberts, W.O. Diagnosing dehydration? Blend evidence with clinical observations. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 434–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrier, E.; Demazières, A.; Girard, N.; Pross, N.; Osbild, D.; Metzger, D.; Guelinckx, I.; Klein, A. Circadian variation and responsiveness of hydration biomarkers to changes in daily water intake. Eur. J. Appl. Physiol. 2013, 113, 2143–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheuvront, S.N.; Kenefick, R.W. Am I Drinking Enough? Yes, No, and Maybe. J. Am. Coll. Nutr. 2016, 35, 185–192. [Google Scholar] [CrossRef]
- Barley, O.R.; Chapman, D.W.; Abbiss, C.R. Reviewing the current methods of assessing hydration in athletes. J. Int. Soc. Sports Nutr. 2020, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, C.; Aragón, A.; González, M.; Weiss, I.; Glaser, J.; Rivard, C.J.; Roncal-Jiménez, C.; Correa-Rotter, R.; Johnson, R.J. Heat stress, hydration and uric acid: A cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open 2016, 6, e011034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hew-Butler, T.; Smith-Hale, V.; Pollard-McGrandy, A.; VanSumeren, M. Of Mice and Men-The Physiology, Psychology, and Pathology of Overhydration. Nutrients 2019, 11, 1539. [Google Scholar] [CrossRef] [Green Version]
- Kenefick, R.W. Drinking Strategies: Planned Drinking Versus Drinking to Thirst. Sports Med. 2018, 48, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Cheuvront, S.N.; Kenefick, R.W.; Zambraski, E.J. Spot Urine Concentrations Should Not be Used for Hydration Assessment: A Methodology Review. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 293–297. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Weisz, K. The hydration sweet spot: Importance of Aquaporins. Clin. Lab. Sci. 2016, 29, 186–193. [Google Scholar] [CrossRef]
- Popowski, L.A.; Oppliger, R.A.; Patrick Lambert, G.; Johnson, R.F.; Kim Johnson, A.; Gisolf, C.V. Blood and urinary measures of hydration status during progressive acute dehydration. Med. Sci. Sports Exerc. 2001, 33, 747–753. [Google Scholar] [CrossRef]
- Sommerfield, L.M.; McAnulty, S.R.; McBride, J.M.; Zwetsloot, J.J.; Austin, M.D.; Mehlhorn, J.D.; Calhoun, M.C.; Young, J.O.; Haines, T.L.; Utter, A.C. ValidIty of urine specific gravity when compared with plasma osmolality as a measure of hydration status in male and female NCAA Collegiate athletes. J. Strength Cond. Res. 2016, 30, 2219–2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hew-Butler, T.D.; Eskin, C.; Bickham, J.; Rusnak, M.; VanderMeulen, M. Dehydration is how you define it: Comparison of 318 blood and urine athlete spot checks. BMJ. Open. Sport Exerc. Med. 2018, 4, e000297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brake, D.J.; Bates, G.P. Fluid losses and hydration status of industrial workers under thermal stress working extended shifts. Occup. Environ. Med. 2003, 60, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, C.E.; Mitchell, D.C.; Armitage, T.L.; Moyce, S.C.; Tancredi, D.J.; Castro, J.; Vega-Arroyo, A.J.; Bennett, D.H.; Schenker, M.B. Are Cal/OSHA Regulations Protecting Farmworkers in California from Heat-Related Illness? J. Occup. Environ. Med. 2021, 63, 532–539. [Google Scholar] [CrossRef]
- Ayotte, D., Jr.; Corcoran, M.P. Individualized hydration plans improve performance outcomes for collegiate athletes engaging in in-season training. J. Int. Soc. Sports Nutr. 2018, 15, 27. [Google Scholar] [CrossRef] [Green Version]
- Cheuvront, S.N.; Kenefick, R.W. Personalized fluid and fuel intake for performance optimization in the heat. J. Sci. Med. Sport 2021, 24, 735–738. [Google Scholar] [CrossRef]
- Perrier, E.T.; Armstrong, L.E.; Bottin, J.H.; Clark, W.F.; Dolci, A.; Guelinckx, I.; Iroz, A.; Kavouras, S.A.; Lang, F.; Lieberman, H.R.; et al. Hydration for health hypothesis: A narrative review of supporting evidence. Eur. J. Nutr. 2021, 60, 1167–1180. [Google Scholar] [CrossRef]
| Hydration Indices | Cut-off Points for Dehydration |
|---|---|
| Thirst [18] | Answer “yes” |
| Urine color [19,37] | 5–8 |
| Urine osmolality [12,19] | ≥700 mOsmol/kg H2O |
| Urine-specific gravity [12,19] | ≥1.020 g/ml |
| Serum osmolality [12,19] | ≥290 mOsmol/kg H2O |
| Body mass (percentage change) [12,19] | ≥1% |
| Total body water (percentage change) [12] | ≥2% |
| Serum osmolality (percentage change) [36] | ≥2% |
| Summer (n = 20) | Autumn (n = 21) | Winter (n = 19) | All (n = 60) | |
|---|---|---|---|---|
| Age (years) | 30.2 ± 7.9 | 33.0 ± 6.2 | 33.9 ± 5.9 | 32.3 ± 6.8 |
| Body mass (kg) | 84.9 ± 14.0 | 93.3 ± 19.2 | 91.5 ± 10.6 | 89.9 ± 15.4 |
| Body height (cm) | 177.0 ± 7.3 | 179.8 ± 4.9 | 181.7 ± 6.3 | 179.5 ± 6.4 |
| BMI (kg/m2) | 27.1 ± 4.5 | 28.7 ± 5.1 | 27.7 ± 3.1 | 27.9 ± 4.3 |
| Body fat percentage | 23.9 ± 6.6 | 25.1 ± 6.7 | 22.0 ± 5.4 | 23.7 ± 6.3 |
| Hydration Status Indices | Time Point | ||
|---|---|---|---|
| 1—Before Work (Baseline) | 2—After Work | 3—Before Work on the Following Day | |
| Thirst | - 28 (47) | - 21 (35) | - 29 (48) |
| Body mass (kg) | 89.9 ± 15.4 - | 90.2 ± 15.4 * - | 90.0 ± 15.3 # - |
| Total body water (L) | 49.8 ± 6.2 - | 50.2 ± 6.2 * - | 50.0 ± 6.1 * - |
| Urine color | 4 ± 2 25 (42) | 4 ± 1 18 (30) | 4 ± 1 26 (43) |
| Urine-specific gravity (g/mL) | 1.022 ± 0.006 39 (65) | 1.019 ± 0.006 * 31 (52) | 1.020 ± 0.006 # 30 (50) |
| Urine osmolality (mOsmol/kg H2O) | 760 ± 217 39 (65) | 736 ± 213 37 (62) | 724 ± 219 32 (53) |
| Serum osmolality (mOsmol/kg H2O) | 283 ± 6 7 (12) | 285 ± 5 7 (12) | 286 ± 6 10 (7) |
| Hydration Status Indices | Summer | Autumn | Winter | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1—Before Work (Baseline) | 2—After Work | 3—Before Work on the Following Day | 1—Before Work (Baseline) | 2—After Work | 3—Before Work on the Following Day | 1—Before Work (Baseline) | 2—After Work | 3—Before Work on the Following Day | |
| Thirst | - 6 (30) | - 6 (30) | - 7 (35) | - 12 (57) | - 8 (38) | - 9 (43) | - 10 (53) | - 7 (37) | - 13 (68) |
| Body mass (kg) | 84.9 ± 14.0 - | 85.0 ± 13.8 - | 84.9 ± 13.8 - | 93.3 ± 19.2 - | 93.7 ± 19.4 - | 93.4 ± 19.3 - | 91.5 ± 10.6 - | 92.0 ± 10.5 - | 91.6 ± 10.4 - |
| Total body water (L) | 46.8 ± 5.5 - | 47.3 ± 5.4 - | 47.1 ± 5.1 - | 50.5 ± 7.3 - | 50.9 ± 7.5 - | 50.8 ± 7.5 - | 52.0 ± 4.2 * - | 52.4 ± 4.2 - | 52.1 ± 4.1 - |
| Urine color | 5 ± 1 8 (40) | 4 ± 1 8 (40) | 5 ± 1 11 (55) | 4 ± 1 9 (43) | 4 ± 1 5 (24) | 4 ± 1 6 (29) | 4 ± 2 8 (42) | 3 ± 1 5 (26) | 4 ± 1 9 (47) |
| Urine specific gravity (g/mL) | 1.022 ± 0.006 14 (70) | 1.019 ± 0.006 9 (45) | 1.021 ± 0.004 10 (50) | 1.021 ± 0.007 12 (57) | 1.018 ± 0.005 10 (48) | 1.019 ± 0.007 9 (49) | 1.022 ± 0.006 13 (68) | 1.020 ± 0.006 12 (63) | 1.021 ± 0.007 11 (42) |
| Urine osmolality (mOsmol/kg H2O) | 831 ± 184 15 (75) | 732 ± 233 11 (55) | 734 ± 174 10 (50) | 696 ± 244 11 (52) | 731 ± 210 13 (62) | 711 ± 264 11 (52) | 756 ± 206 13 (68) | 756 ± 206 13 (68) | 756 ± 206 11 (58) |
| Serum osmolality (mOsmol/kg H2O) | 282 ± 2 0 (0) | 282 ± 3 0 (0) | 283 ± 3 0 (0) | 284 ± 4 1 (5) | 286 ± 5 1 (5) | 287 ± 6 * 5 (24) * | 284 ± 10 6 (32) *# | 288 ± 6 * 6 (32) *# | 287 ± 9 5 (26) * |
| Hydration Status Indices | Index No. | Percentage of Dehydrated Participants (Time Point 2) | Percentage of Dehydrated Participants (Time Point 3) |
|---|---|---|---|
| Percentage change of body mass a | 1 | 0.0% | 5.0% |
| Urine color | 2 | 30.0% 1,4,7 | 43.3% 1,4,7 |
| Thirst | 3 | 35.0% 1,4,7 | 48.3% 1,4,7,8 |
| Percentage change of total body water a | 4 | 1.7% | 3.3% |
| Urine-specific gravity | 5 | 51.7% 1,2,4,7,8 | 50.0% 1,4,7,8 |
| Urine osmolality | 6 | 61.7% 1,2,3,4,7,8 | 53.3% 1,4,7,8 |
| Serum osmolality | 7 | 11.7% 1,4 | 16.7% 1,4 |
| Percentage change of serum osmolality a | 8 | 20.0% 1,4 | 27.0% 1,4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orysiak, J.; Młynarczyk, M.; Tomaszewski, P. Hydration Status in Men Working in Different Thermal Environments: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 5627. https://doi.org/10.3390/ijerph19095627
Orysiak J, Młynarczyk M, Tomaszewski P. Hydration Status in Men Working in Different Thermal Environments: A Pilot Study. International Journal of Environmental Research and Public Health. 2022; 19(9):5627. https://doi.org/10.3390/ijerph19095627
Chicago/Turabian StyleOrysiak, Joanna, Magdalena Młynarczyk, and Paweł Tomaszewski. 2022. "Hydration Status in Men Working in Different Thermal Environments: A Pilot Study" International Journal of Environmental Research and Public Health 19, no. 9: 5627. https://doi.org/10.3390/ijerph19095627
APA StyleOrysiak, J., Młynarczyk, M., & Tomaszewski, P. (2022). Hydration Status in Men Working in Different Thermal Environments: A Pilot Study. International Journal of Environmental Research and Public Health, 19(9), 5627. https://doi.org/10.3390/ijerph19095627







