COVID-19 Lockdown in Israel: The Environmental Effect on Ultrafine Particle Content in the Airway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. EBC Sample Collection
2.3. Peripheral Blood Samples
2.4. UFP Measurement and Analysis in Biological Samples
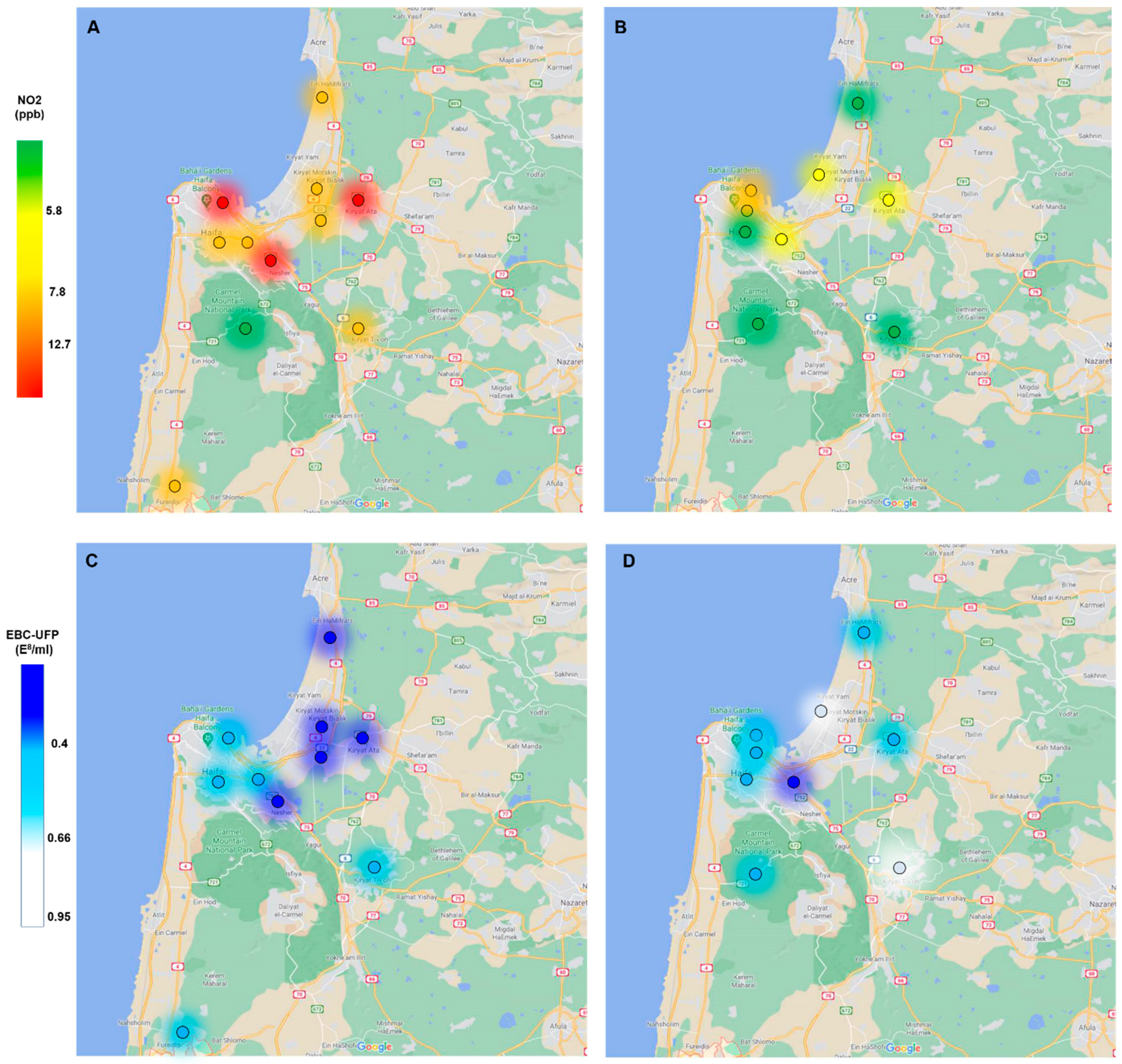
2.5. Environmental Ambient Air Pollution Data
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Air Pollutants
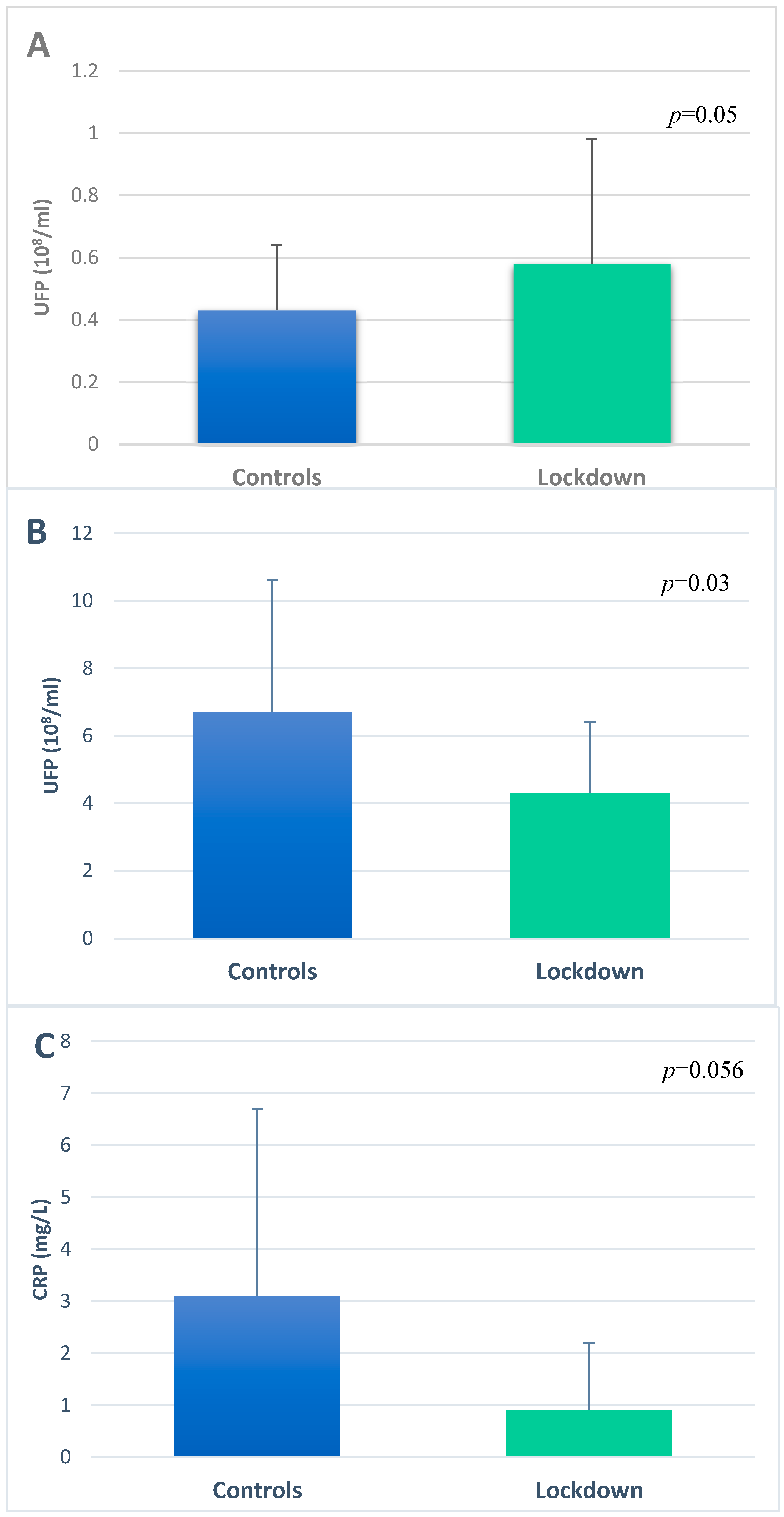
3.3. Ultrafine Particles in EBC and Serum
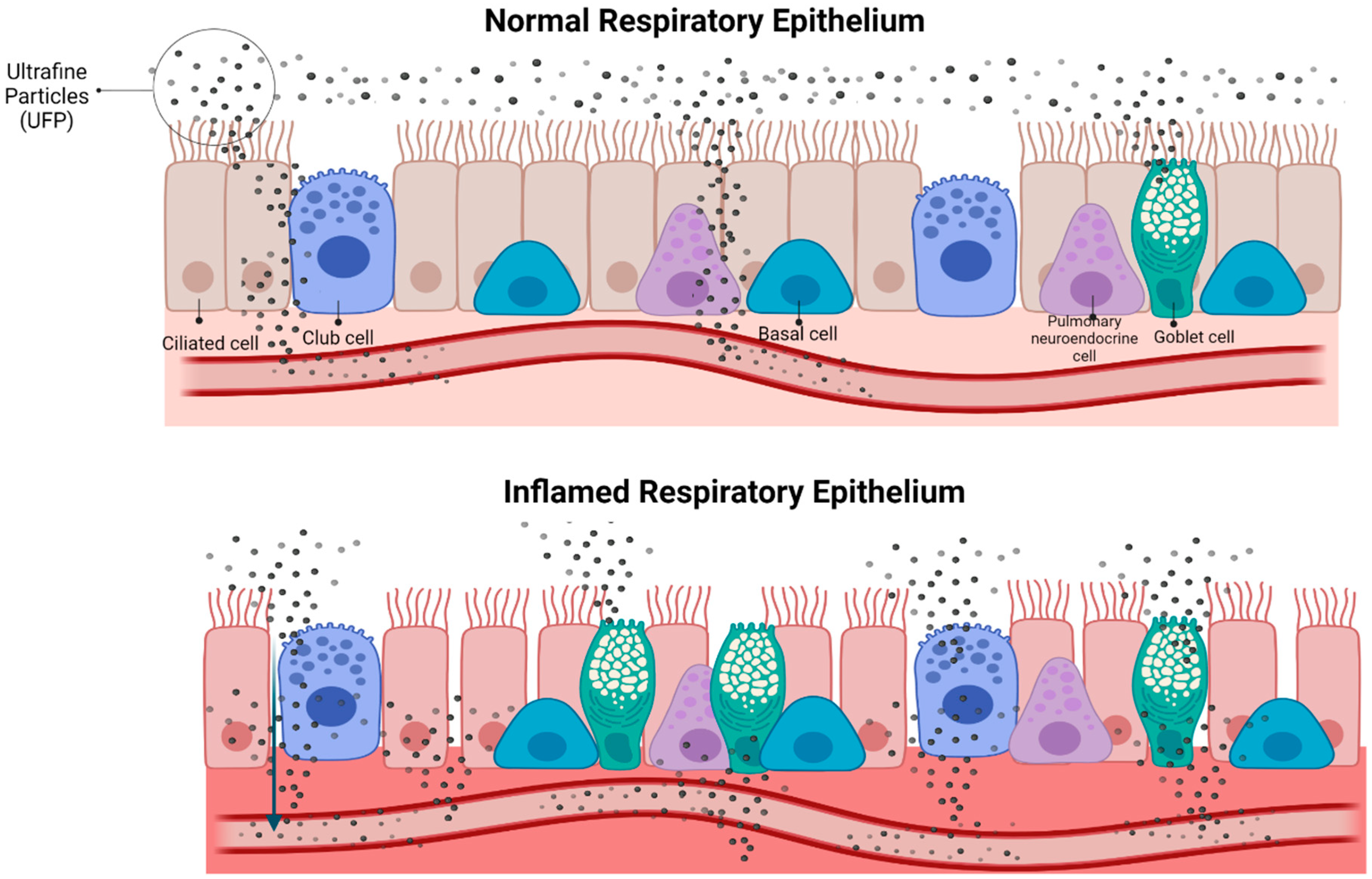
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Air Pollution. Available online: http://www.who.int/airpollution/en/ (accessed on 1 September 2020).
- Matz, C.J.; Egyed, M.; Hocking, R.; Seenundun, S.; Charman, N.; Edmonds, N. Human health effects of traffic-related air pollution (TRAP): A scoping review protocol. Syst. Rev. 2019, 8, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Georas, S.; Alexis, N.; Fritz, P.; Xia, T.; Williams, M.A.; Horner, E.; Nel, A. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J. Allergy Clin. Immunol. 2016, 138, 386–396. [Google Scholar] [PubMed] [Green Version]
- Guan, W.-J.; Zheng, X.-Y.; Chung, K.F.; Zhong, N.S. Impact of air pollution on the burden of chronic respiratory diseases in China: Time for urgent action. Lancet 2016, 388, 1939–1951. [Google Scholar] [CrossRef]
- Parker, J.D.; Kravets, N.; Vaidyanathan, A. Particulate matter air pollution exposure and heart disease mortality risks by race and ethnicity in the United States. Circulation 2018, 137, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Kown, H.S.; Ryu, M.H.; Carlsten, C. Ultrafine particles: Unique physicochemical properties relevant to health and disease. Exp. Mol. Med. 2020, 52, 318–328. [Google Scholar] [CrossRef]
- Konstantinidi, E.M.; Lappas, A.S.; Tzortzi, A.S.; Behrakis, P.K. Exhaled Breath Condensate: Technical and Diagnostic Aspects. Sci. World J. 2015, 2015, 435160. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Fujimoto, K.; Kitaguchi, Y.; Horiuchi, T.; Kubo, K.; Honda, T. Hydrogen peroxide content and pH of expired breath condensate from patients with asthma and COPD. COPD J. Chronic. Obstr. Pulm. Dis. 2014, 11, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Warwick, G.; Thomas, P.S.; Yates, D.H. Non-invasive biomarkers in exacerbations of obstructive lung disease. Respirology 2013, 18, 874–884. [Google Scholar] [CrossRef]
- Antus, B.; Barta, I.; Kullmann, T.; Lazar, Z.; Valyon, M.; Horvath, I.; Csiszer, E. Assessment of exhaled breath condensate pH in exacerbations of asthma and chronic obstructive pulmonary disease: A longitudinal study. Am. J. Respir. Crit. Care Med. 2010, 182, 1492–1497. [Google Scholar] [CrossRef]
- Klein, E.F.; Adir, Y.; Krencel, A.; Peri, R.; Vasserman, B.; Fireman, E.; Kessel, A. Ultrafine particles in airways: A novel marker of COPD exacerbation risk and inflammatory status. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 557–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fireman, E.K.; Adir, Y.; Fireman, E.; Kessel, A. Cigarette-related cadmium and environmental pollution exposure are reflected in airway ultrafine particle content. ERJ Open Res. 2020, 6, 00361–02019. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [PubMed]
- Venter, Z.S.; Aunan, K.; Chowdhury, S.; Lelieveld, J. COVID-19 lockdowns cause global air pollution declines. Proc. Natl. Acad. Sci. USA 2020, 117, 18984–18990. [Google Scholar] [CrossRef] [PubMed]
- Tobías, A.; Carnerero, C.; Reche, C.; Massagué, J.; Via, M.; Minguillón, M.C.; Alastuey, A.; Querol, X. Changes in air quality during the lockdown in Barcelona (Spain) one month into the SARS-CoV-2 epidemic. Sci. Total Environ. 2020, 726, 138540. [Google Scholar] [CrossRef]
- Mahato, S.; Pal, S.; Ghosh, K.G. Effect of lockdown amid COVID-19 pandemic on air quality of the megacity Delhi, India. Sci. Total Environ. 2020, 730, 139086. [Google Scholar] [CrossRef]
- Horvath, I.; Hunt, J.; Barnes, P.J.; Alving, K.; Antczak, A.; Baraldi, E.; Becher, G.; van Beurden, W.J.; Corradi, M.; Dekhuijzen, R.; et al. 2005 ATS/ERS Task Force on Exhaled Breath Condensate. Exhaled breath condensate: Methodological recommendations and unresolved questions. Eur. Respir. J. 2005, 26, 523–548. [Google Scholar]
- Schwarz, K.; Biller, H.; Windt, H.; Koch, W.; Hohlfeld, J.M. Characterization of exhaled particles from the healthy human lung-a systematic analysis in relation to pulmonary function variables. J. Aerosol. Med. Pulm. Drug Deliv. 2010, 23, 371–379. [Google Scholar] [CrossRef]
- Steinvil, A.; Cohen, M.; Shapira, I.; Berliner, S.; Rogowski, O.; Fireman, E.; Kordova-Biezuner, L. Environmental air pollution has decremental effects on pulmonary function test parameters up to one week after exposure. Am. J. Med. Sci. 2009, 338, 273–279. [Google Scholar] [CrossRef]
- Hernán, M.A.; Robins, J.M. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am. J. Epidemiol. 2016, 183, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Gianicolo, E.A.L.; Eichler, M.; Muensterer, O.; Strauch, K.; Blettner, M. Methods for Evaluating Causality in Observational Studies. Dtsch. Arztebl. Int. 2020, 116, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hufstedler, H.; Rahman, S.; Danzer, A.M.; Goymann, H.; de Jong, V.M.; Campbell, H.; Gustafson, P.; Debray, T.P.; Jaenisch, T.; Maxwell, L.; et al. Systematic Review Reveals Lack of Causal Methodology Applied to Pooled Longitudinal Observational Infectious Disease Studies. J. Clin. Epidemiol. 2022, 145, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sokhi, R.S.; Singh, V.; Querol, X.; Finardi, S.; Targino, A.C.; Andrade, M.D.F.; Pavlovic, R.; Garland, R.M.; Massagué, J.; Kong, S.; et al. A global observational analysis to understand changes in air quality during exceptionally low anthropogenic emission conditions. Environ. Int. 2021, 157, 106818. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.; Jorba, O.; Soret, A.; Petetin, H.; Bowdalo, D.; Serradell, K.; Tena, C.; van der Gon, H.D.; Kuenen, J.; Peuch, V.-H.; et al. Time-resolved emission reductions for atmospheric chemistry modelling in Europe during the COVID-19 lockdowns. Atmos. Chem. Phys. 2021, 21, 773–797. [Google Scholar] [CrossRef]
- Biswal, A.; Singh, V.; Singh, S.; Kesarkar, A.P.; Ravindra, K.; Sokhi, R.S.; Chipperfield, M.P.; Dhomse, S.S.; Pope, R.J.; Singh, T.; et al. COVID-19 lockdown-induced changes in NO 2 levels across India observed by multi-satellite and surface observations. Atmos. Chem. Phys. 2021, 21, 5235–5251. [Google Scholar] [CrossRef]
- Usmani, O.S.; Han, M.K.; Kaminsky, D.A.; Hogg, J.; Hjoberg, J.; Patel, N.; Hardin, M.; Keen, C.; Rennard, S.; Blé, F.X.; et al. Seven Pillars of Small Airways Disease in Asthma and COPD: Supporting Opportunities for Novel Therapies. Chest 2021, 160, 114–134. [Google Scholar] [CrossRef]
- Benor, S.; Alcalay, Y.; Domany, K.A.; Gut, G.; Soferman, R.; Kivity, S.; Fireman, E. Ultrafine particle content in exhaled breath condensate in airways of asthmatic children. J. Breath Res. 2015, 9, 026001. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Seaton, A.; MacNee, W. Ambient particle inhalation and the cardiovascular system: Potential mechanisms. Environ. Health Perspect. 2001, 109, 523–527. [Google Scholar]
- Fazlollahi, F.; Kim, Y.H.; Sipos, A.; Hamm-Alvarez, S.F.; Borok, Z.; Kim, K.-J.; Crandall, E.D. Nanoparticle translocation across mouse alveolar epithelial cell monolayers: Species-specific mechanisms. Nanomedicine 2013, 9, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Nemmar, A.; Hoet, P.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Lunts, A.; Kreyling, W.; Cox, C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J. Toxicol. Environ. Health A 2002, 65, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Raftis, J.B.; Langrish, J.P.; McLean, S.G.; Samutrtai, P.; Connell, S.; Wilson, S.; Vesey, A.T.; Fokkens, P.H.B.; Boere, A.J.F.; et al. Inhaled nanoparticles accumulate at sites of vascular disease. ACS Nano 2017, 11, 4542–4552. [Google Scholar] [CrossRef] [Green Version]
- Bar-Shai, A.; Alcalay, Y.; Sagiv, A.; Rotem, M.; Feigelson, S.W.; Alon, R.; Fireman, E. Fingerprint of lung fluid ultrafine particles, a novel marker of acute lung inflammation. Respiration 2015, 90, 74–84. [Google Scholar] [CrossRef]
- Ophir, N.; Shai, A.B.; Korenstein, R.; Kramer, M.R.; Fireman, E. Functional, inflammatory and interstitial impairment due to artificial stone dust ultrafine particles exposure. Occup. Environ. Med. 2019, 76, 875–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, B.; Roebbel, N.; Gumy, S.; Forastiere, F.; Brunekreef, B.; Jarosinska, D.; Walker, K.D.; van Erp, A.M.; O’Keefe, R.; Greenbaum, D.; et al. Air pollution and health: Recent advances in air pollution epidemiology to inform the European Green Deal: A joint workshop report of ERS, WHO, ISEE and HEI. Eur. Respir. J. 2020, 56, 2002575. [Google Scholar] [CrossRef]
- COVID-19 Overview and Infection Prevention and Control Priorities in Non-U.S. Healthcare Settings. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/overview/index.html#standard-based-precautions (accessed on 1 September 2020).
| Control Group (n = 25) | Lockdown Group (n = 30) | |
|---|---|---|
| * Age, years (mean, range) | 41.9, 35–49 | 34, 22–48 |
| Male, n (%) | 14 (56) | 10 (33) |
| Smokers a, n (%) | 12 (48) | 12 (40) |
| Pack years, (mean ± SD) | 3.8 ± 5.5 | 1.7 ± 3.5 |
| Residence facing road, n (%) | 11 (44) | 19 (63) |
| Distance from monitoring station, km (mean ± SD) | 5 ± 3.3 | 6.2 ± 7.1 |
| TRAP, ppb (Mean ± SD) | Control Group (n = 25) | Lockdown Group (n = 30) | p-Value + |
|---|---|---|---|
| SO2 | 0.7± 0.64 | 0.36 ± 0.14 | 0.037 |
| NOX | 13.65 ± 8.7 | 6.7± 2.9 | 0.01 |
| NO2 | 11.24± 5 | 8.3± 3.2 | 0.01 |
| NO | 3.25 ± 3.6 | 1.27 ± 1.1 | 0.014 |
| O3 | 62.4 ± 17.4 | 34.1 ± 2.2 | <0.001 |
| Serum UFP Concentration (108/mL) | Serum CRP Level (mg/L) | |
|---|---|---|
| NO2—1 month of exposure (ppb) | r = 0.3, p = 0.04 | r = 0.23, p = 0.09 |
| SO2—1 month of exposure (ppb) | r = 0.2, p = 0.27 | r = 0.26, p = 0.14 |
| O3—1 month of exposure (ppb) | r = 0.34, p = 0.06 | r = 0.64, p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fireman Klein, E.; Elimeleh, Y.; Adir, Y.; Majdoub, L.; Shteinberg, M.; Kessel, A. COVID-19 Lockdown in Israel: The Environmental Effect on Ultrafine Particle Content in the Airway. Int. J. Environ. Res. Public Health 2022, 19, 5507. https://doi.org/10.3390/ijerph19095507
Fireman Klein E, Elimeleh Y, Adir Y, Majdoub L, Shteinberg M, Kessel A. COVID-19 Lockdown in Israel: The Environmental Effect on Ultrafine Particle Content in the Airway. International Journal of Environmental Research and Public Health. 2022; 19(9):5507. https://doi.org/10.3390/ijerph19095507
Chicago/Turabian StyleFireman Klein, Einat, Yotam Elimeleh, Yochai Adir, Lana Majdoub, Michal Shteinberg, and Aharon Kessel. 2022. "COVID-19 Lockdown in Israel: The Environmental Effect on Ultrafine Particle Content in the Airway" International Journal of Environmental Research and Public Health 19, no. 9: 5507. https://doi.org/10.3390/ijerph19095507
APA StyleFireman Klein, E., Elimeleh, Y., Adir, Y., Majdoub, L., Shteinberg, M., & Kessel, A. (2022). COVID-19 Lockdown in Israel: The Environmental Effect on Ultrafine Particle Content in the Airway. International Journal of Environmental Research and Public Health, 19(9), 5507. https://doi.org/10.3390/ijerph19095507






