Contamination Assessment of Mangrove Ecosystems in the Red Sea Coast by Polycyclic Aromatic Hydrocarbons
Abstract
:1. Introduction
2. Materials and Methods
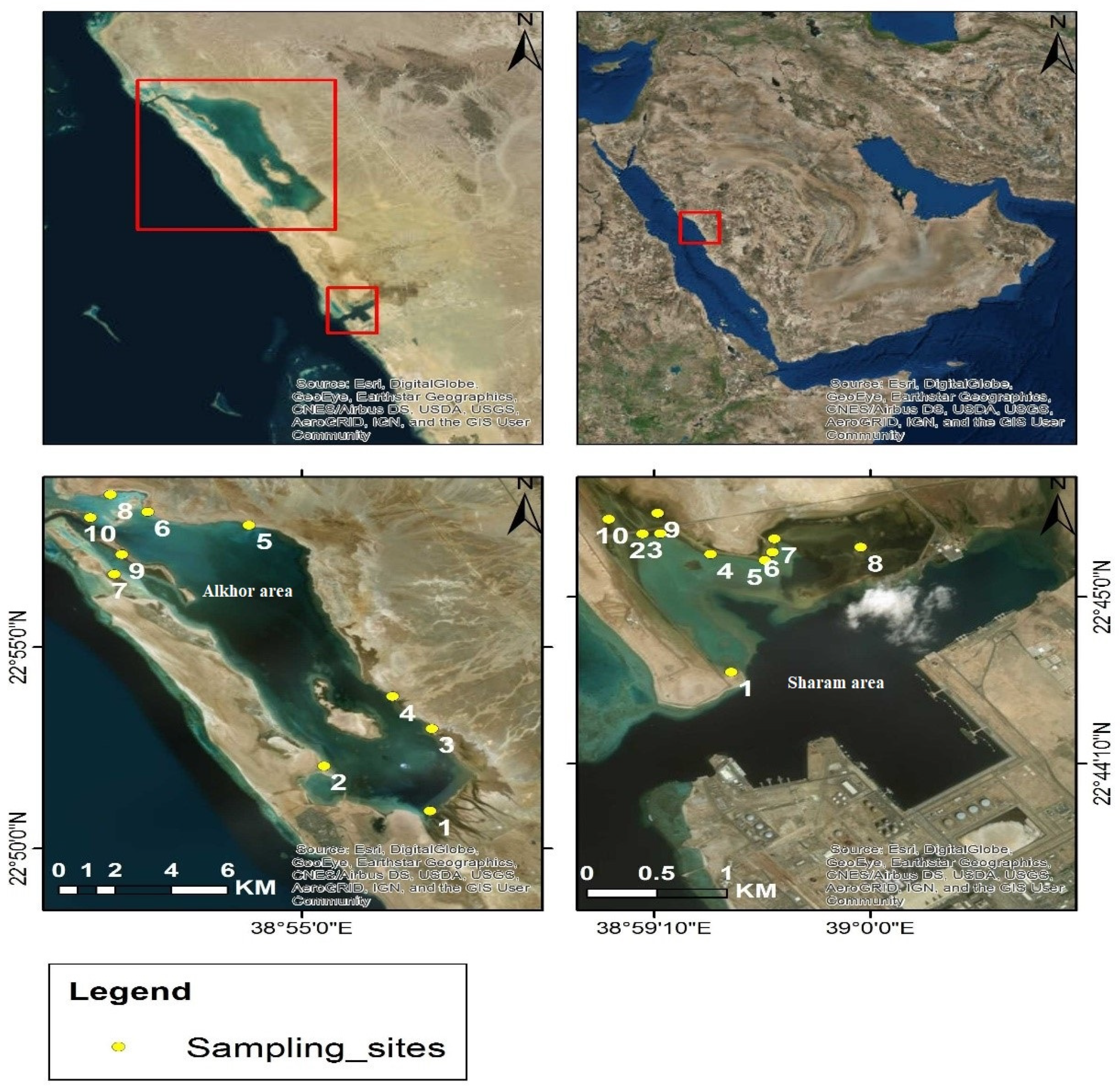
2.1. Study Area
2.2. Meteorology Measurements
2.3. Sampling
2.4. PAH Analysis
2.5. Degree of Similarity/Dissimilarity
2.6. Quality Control Procedures
2.7. PAH Diagnostic Ratios
3. Results and Discussion
3.1. Meteorology Measurements
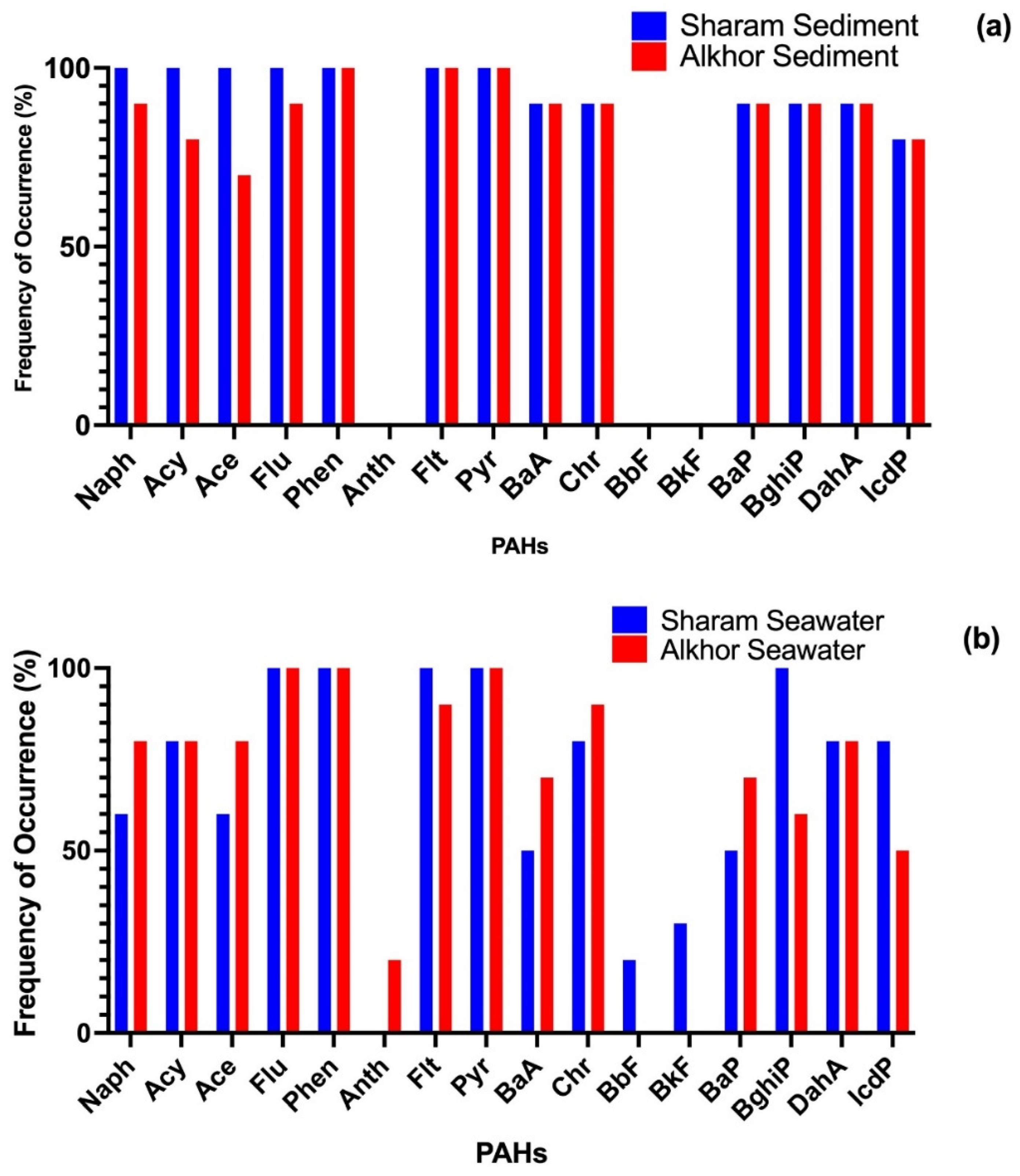
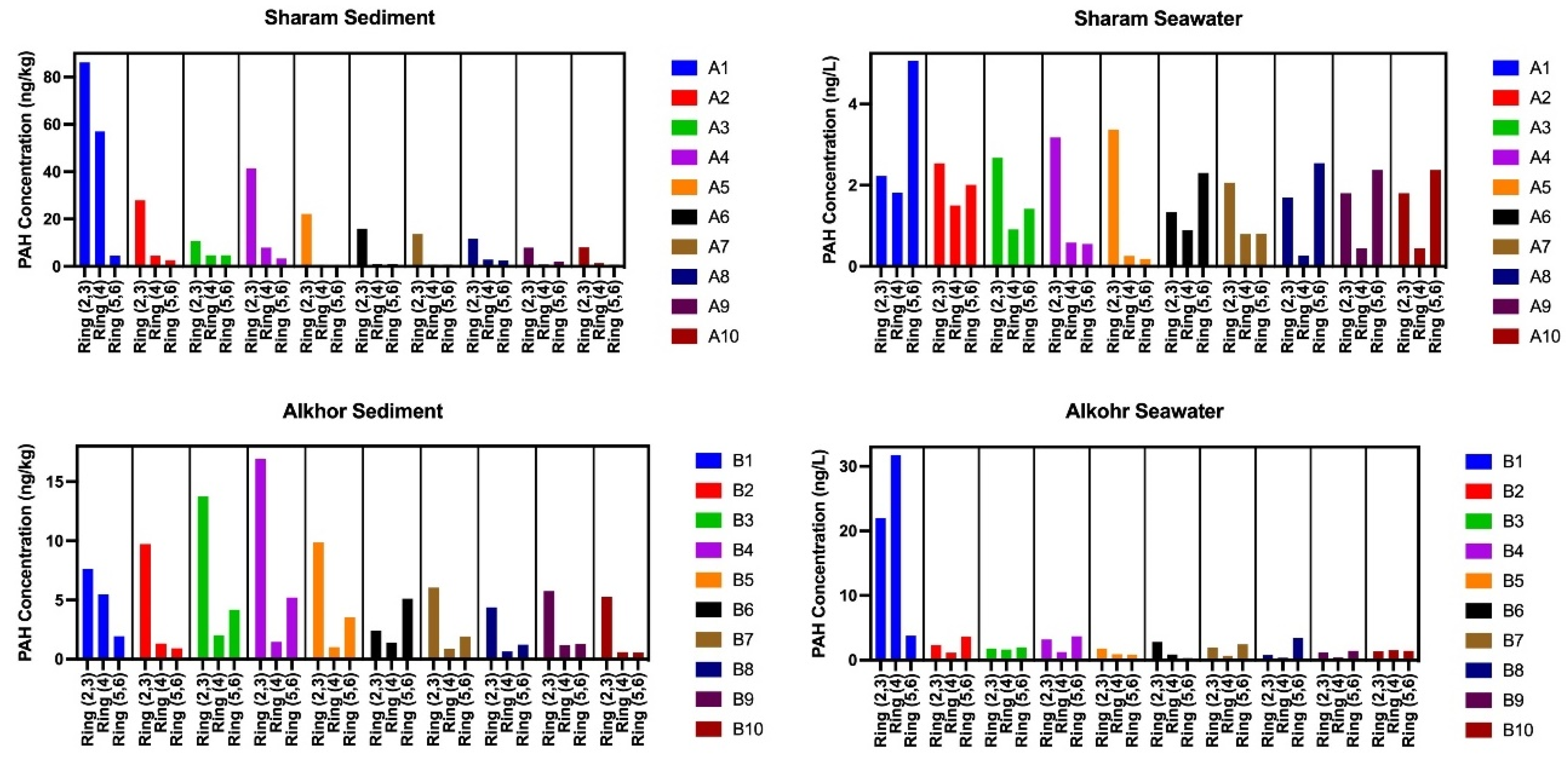
3.2. Concentrations and Composition of PAHs
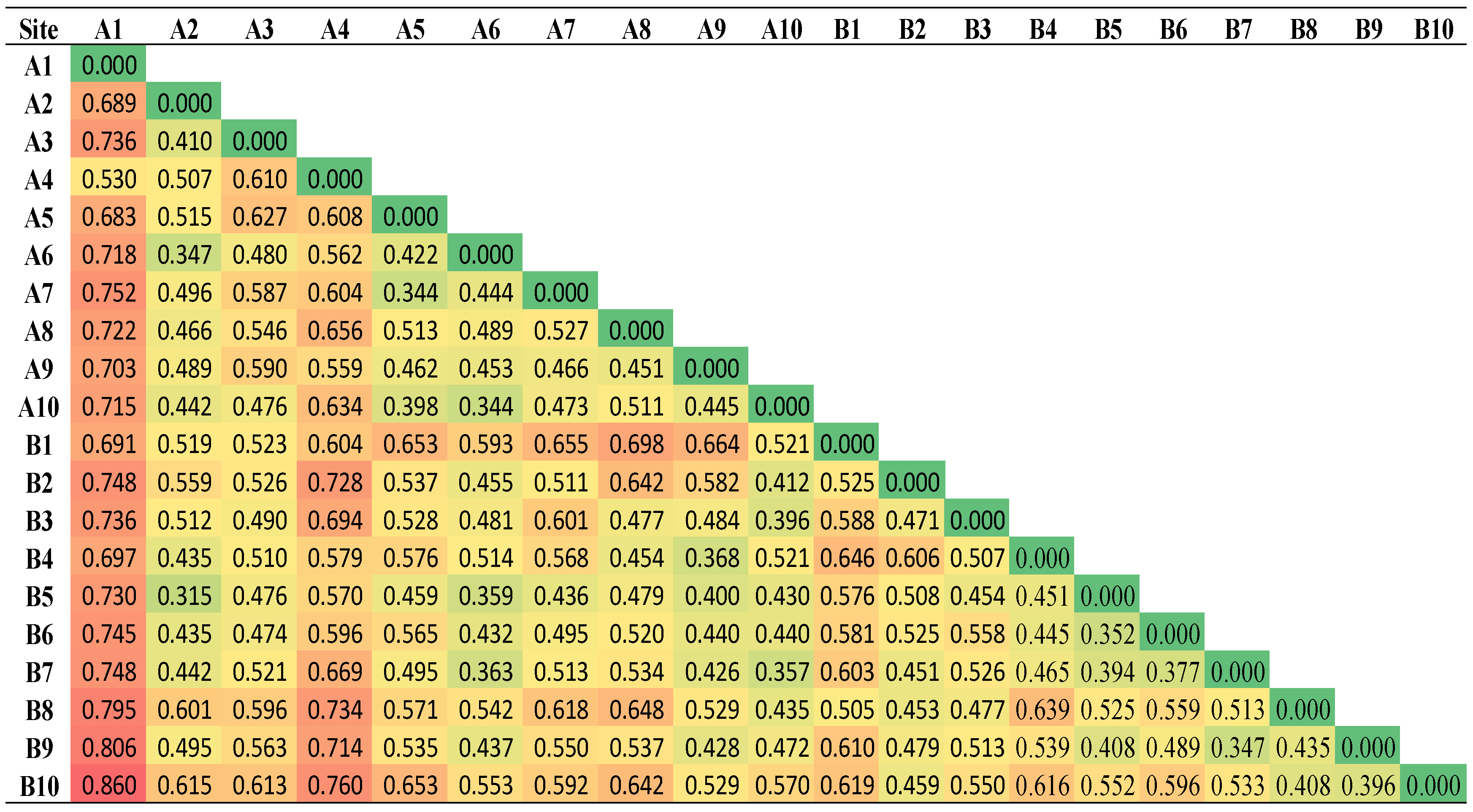
3.3. Dissimilarity between the PAH Contamination in the Two Areas
3.4. Potential Sources of PAHs Pollution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| Month | Temperature (°C) | Humidity (%) | Wind Speed (m/s) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. | Mean | |
| January | 24 | 34 | 16 | 51 | 91 | 10 | 0 | 7.20 | 1.90 |
| February | 25 | 37 | 17 | 46 | 88 | 8 | 0 | 5.80 | 2.10 |
| March | 26 | 36 | 16 | 53 | 89 | 4 | 0 | 6.20 | 2.03 |
| April | 30 | 39 | 19 | 50 | 91 | 13 | 0 | 6.70 | 2.10 |
| May | 30 | 44 | 23 | 53 | 93 | 5 | 0.04 | 6.80 | 2.10 |
| June | 32 | 46 | 25 | 47 | 89 | 2 | 0.10 | 5.3 | 2.10 |
| July | 32 | 46 | 27 | 57 | 88 | 14 | 0 | 5.6 | 2.20 |
| August | 33 | 44 | 27 | 57 | 87 | 18 | 0.20 | 5.9 | 2.30 |
| September | 32 | 44 | 24 | 59 | 94 | 9 | 0 | 5.50 | 2.10 |
| November | 30 | 42 | 22 | 61 | 91 | 6 | 0 | 4.90 | 1.80 |
| December | 28 | 38 | 21 | 45 | 81 | 3 | 0 | 5.80 | 1.80 |
| WIND SPEED (M/S) | |||||
|---|---|---|---|---|---|
| Wind Direction | 0–1.37 | 1.37–3.06 | 3.06–5.28 | >5.28 | Total |
| N | 82 | 49 | 26 | 0 | 157 (1.9%) |
| NNE | 621 | 321 | 17 | 0 | 959 (11.3%) |
| NE | 66 | 150 | 22 | 0 | 238 (2.8%) |
| ENE | 151 | 167 | 166 | 2 | 486 (5.7%) |
| E | 393 | 189 | 36 | 6 | 624 (7.4%) |
| ESE | 6 | 7 | 0 | 0 | 13 (0.2%) |
| SE | 8 | 11 | 1 | 0 | 20 (0.2%) |
| SSE | 33 | 29 | 1 | 0 | 63 (0.7%) |
| S | 24 | 32 | 4 | 0 | 60 (0.7%) |
| SSW | 35 | 22 | 5 | 0 | 62 (0.7%) |
| SW | 12 | 14 | 6 | 0 | 32 (0.40%) |
| WSW | 9 | 23 | 0 | 0 | 32 (0.40%) |
| W | 466 | 388 | 97 | 20 | 971 (11.5%) |
| WNW | 215 | 341 | 150 | 6 | 712 (8.4%) |
| NW | 166 | 852 | 889 | 11 | 1918 (22.6%) |
| NNW | 750 | 842 | 529 | 2 | 2123 (25.1%) |
| Total | 3037 (35.9%) | 3437 (40.6%) | 1949 (23%) | 47 (0.6%) | 8470 (100%) |
| Study | Location | Concentrations | Number of PAH |
|---|---|---|---|
| This study | Rabigh, Saudi Arabia | <0.001–0.073 ng/g | 16 PAH |
| Viguri, J. et al. (2002) [30] | Santander Bay, Spain | 0.02–344.6 μg/g | 16 PAH |
| McCready, S. et al. (2000) [31] | Sydney, Australia | <100–380,000 μg/kg | 16 PAH |
| Dong, Cheng-Di et al., (2012) [32] | Taiwan | 4425–51,261 ng/g | 16 PAH |
| Qiao, Min et al. (2006) [14] | Taihu Lake, China | 1207–4754 ng/g | 16 PAH |
| Adami, Gianpiero et al. (2000) [33] | Trieste, Adriatic Sea | 2.37–64.56 μg/g | 16 PAH |
| Oen, Amy MP et al., (2006) [34]: 370–380. | Norway | 2–113 μg/g | 16 PAH |
| De Luca, Giuseppe et al. (2004) [35] | Sardinia, Italy | 0.07–1.21 μg/g | 16 PAH |
| Simpson, Christopher D. et al. (1996) [36] | Kitimat, Canada | <1–10,000 μg/g | 16 PAH |
| Witt, G. (1995) [37] | Baltic Sea | 9–29 ng/g | 15 PAH (sandy areas) |
| Witt, G. (1995) [37] | Baltic Sea | 800–1900 ng/g | 15 PAH (sediment) |
References
- Burke, L.; Kura, Y.; Kassem, K.; Revenya, C.; Spalding, M.; McAllister, D. Pilot Analysis of Global Ecosystems: Coastal Ecosystems; World Resources Institute: Washington, DC, USA, 2001. [Google Scholar]
- Lewis, M.; Pryor, R.; Wilking, L. Fate and effects of anthropogenic chemicals in mangrove ecosystems: A review. Environ. Pollut. 2011, 159, 2328–2346. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.; Chaer, G.M.; Carmo, F.L.; Araújo, F.V.; Paes, J.E.; Volpon, A.; Santiago, G.A.; Rosado, A.S. Bacterial communities reflect the spatial variation in pollutant levels in Brazilian mangrove sediment. Antonie Van Leeuwenhoek 2011, 99, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Penha-Lopes, G.; Torres, P.; Cannicci, S.; Narciso, L.; Paula, J. Monitoring anthropogenic sewage pollution on mangrove creeks in southern Mozambique: A test of Palaemon concinnus Dana, 1852 (Palaemonidae) as a biological indicator. Environ. Pollut. 2011, 159, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.; Lagomasino, D.; Thomas, N.; Fatoyinbo, T. Global declines in human-driven mangrove loss. Glob. Change Biol. 2020, 26, 5844–5855. [Google Scholar] [CrossRef] [PubMed]
- Agrawala, S.; Moehner, A.; El Raey, M.; Conway, D.; Van Aalst, M.; Hagenstad, M.; Smith, J. Development and Climate Change in Egypt: Focus on Coastal Resources and The Nile. (Project Report); Organization for Economic Co-Operation and Development (OECD): Paris, France, 2004. [Google Scholar]
- Neff, J.M. Polycyclic Aromatic Hydrocarbons Sources Fates and Biological Effects; Applied Science Publishers: London, UK, 1980. [Google Scholar]
- Bernard, D.; Pascaline, H.; Jeremie, J.J. Distribution and origin of hydrocarbons in sediments from lagoons with fringing mangrove communities. Mar. Pollut. Bull. 1996, 32, 734–739. [Google Scholar] [CrossRef]
- Ke, L.; Yu KS, H.; Wong, Y.S.; Tam, N.F.Y. Spatial and vertical distribution of polycyclic aromatic hydrocarbons in mangrove sediments. Sci. Total Environ. 2005, 340, 177–187. [Google Scholar] [CrossRef]
- Cavalcante, R.M.; Sousa, F.W.; Nascimento, R.F.; Silveira, E.R.; Freire, G.S.S. The impact of urbanization on tropical mangroves (Fortaleza, Brazil): Evidence from PAH distribution in sediments. J. Environ. Manag. 2009, 9, 328–335. [Google Scholar] [CrossRef]
- Zakaria, M.P.; Takada, H.; Tsutsumi, S.; Ohno, K.; Yamada, J.; Kouno, E.; Kumata, H. Distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in Rivers and Estuaries in Malaysia: A Widespread Input of Petrogenic PAHs. Environ. Sci. Technol. 2002, 36, 1907–1918. [Google Scholar] [CrossRef]
- Boonyatumanond, R.; Wattayakorn, G.; Togo, A.; Takada, H. Distribution and origins of polycyclic aromatic hydrocarbons (PAHs) in riverine, estuarine, and marine sediments in Thailand. Mar. Pollut. Bull. 2006, 52, 942–956. [Google Scholar] [CrossRef]
- Chen, C.W.; Chen, C.F. Distribution, origin, and potential toxicological significance of polycyclic aromatic hydrocarbons (PAHs) in sediments of Kaohsiung Harbor, Taiwan. Mar. Pollut. Bull. 2011, 63, 417–423. [Google Scholar] [CrossRef]
- Qiao, M.; Wang, C.; Huang, S.; Wang, D.; Wang, Z. Composition, sources, and potential toxicological significance of PAHs in the surface sediments of the Meiliang Bay, Taihu Lake, China. Environ. Int. 2006, 32, 28–33. [Google Scholar] [CrossRef]
- Sany, B.T.; Salleh, A.; Sulaiman, A.H.; Tehrani, G.M. Ecological risk assessment of poly aromatic hydrocarbons in the North Port, Malaysia. World Acad. Sci. Eng. Technol. 2012, 69, 43–46. [Google Scholar]
- Montero, N.; Belzunce-Segarra, M.J.; Gonzalez, J.-L.; Menchaca, I.; Garmendia, J.M.; Etxebarria, N.; Nieto, O.; Franco, J. Application of Toxicity Identification Evaluation (TIE) procedures for the characterization and management of dredged harbor sediments. Mar. Pollut. Bull. 2013, 71, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.O.; Mostafa, A.; Wade, T.L.; Sweet, S.T.; El Sayed, N.B. Distribution and characteristics of PAHs in sediments from the Mediterranean coastal environment of Egypt. Mar. Pollut. Bull. 2011, 62, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Zeng, E.Y.; Vista, C.L. Organic pollutants in the coastal environment off San Diego, California. 1. Source identification and assessment by compositional indices of polycyclic aromatic hydrocarbons. Environ. Toxicol. Chem. 1997, 16, 179–188. [Google Scholar] [CrossRef]
- Boonyatumanond, R.; Murakami, M.; Wattayakorn, G.; Togo, A.; Takada, H. Sources of polycyclic aromatic hydrocarbons (PAHs) in street dust in a tropical Asian mega-city, Bangkok, Thailand. Sci. Total Environ. 2007, 384, 420–432. [Google Scholar] [CrossRef]
- Farrington John, W.; Westall, J. Organic Chemical Pollutants in the Oceans and Groundwater: A Review of Fundamental Chemical Properties and Biogeochemistry. In The Role of the Oceans as a Waste Disposal Option; Kullenberg, G., Ed.; Springer: Dordrecht, The Netherlands, 1986; pp. 361–425. [Google Scholar] [CrossRef]
- Pastorok, R.A.; Sampson, J.R.; Jacobson, M.A.; Peek, D.C. Ecological risk assessment for river sediments contaminated by creosote. Environ. Toxicol. Chem. 1994, 13, 1929–1941. [Google Scholar] [CrossRef]
- Gustafsson, Ö.; Gschwend, P.M.; Buesseler, K.O. Using 234Th disequilibria to estimate the vertical removal rates of polycyclic aromatic hydrocarbons from the surface ocean. Mar. Chem. 1997, 57, 11–23. [Google Scholar] [CrossRef]
- Bashe, W.J.; Baker, T.V. Determination of Polycyclic Aromatic Hydrocarbons in Drinking Water by Liquid-Solid Extraction and HPLC with Coupled Ultraviolet and Fluorescence Detection; Environmental Monitoring Systems Laboratory: Cincinnati, OH, USA, 1990. [Google Scholar]
- Agudelo-Castañeda, D.M.; Teixeira, E.C. Seasonal changes, identification and source apportionment of PAH in PM1.0. Atmos. Environ. 2014, 96, 186–200. [Google Scholar] [CrossRef]
- Matos, J.; Silveira, C.; Cerqueira, M. Particle-bound polycyclic aromatic hydrocarbons in a rural background atmosphere of southwestern Europe. Sci. Total Environ. 2021, 787, 147666. [Google Scholar] [CrossRef]
- Křůmal, K.; Mikuška, P.; Horák, J.; Hopan, F.; Kuboňová, L. Influence of boiler output and type on gaseous and particulate emissions from the combustion of coal for residential heating. Chemosphere 2021, 278, 130402. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, E.C.; Agudelo-Castañeda, D.M.; Mattiuzi, C.D.P. Contribution of polycyclic aromatic hydrocarbon (PAH) sources to the urban environment: A comparison of receptor models. Sci. Total Environ. 2015, 538, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Berto, D.; Cacciatore, F.; Ausili, A.; Sunseri, G.; Bellucci, L.; Frignani, M.; Albertazzi, S.; Giani, M. Polycyclic Aromatic Hydrocarbons (PAHs) from Diffuse Sources in Coastal Sediments of a Not Industrialised Mediterranean Island. Water Air Soil Pollut. 2008, 200, 199–209. [Google Scholar] [CrossRef]
- Guo, W.; He, M.; Yang, Z.; Lin, C.; Quan, X.; Wang, H. Distribution of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River watershed, China. Chemosphere 2007, 68, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Viguri, J.; Verde, J.; Irabien, A. Environmental assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Santander Bay, Northern Spain. Chemosphere 2002, 48, 157–165. [Google Scholar] [CrossRef]
- McCready, S.; Slee, D.J.; Birch, G.F.; Taylor, S.E. The distribution of polycyclic aromatic hydrocarbons in surficial sediments of Sydney Harbour, Australia. Mar. Pollut. Bull. 2000, 40, 999–1006. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.F.; Chen, C.W. Determination of polycyclic aromatic hydrocarbons in industrial harbor sediments by GC-MS. Int. J. Environ. Res. Public Health 2012, 9, 2175. [Google Scholar] [CrossRef] [Green Version]
- Adami, G.; Barbieri, P.; Piselli, S.; Predonzani, S.; Reisenhofer, E. Detecting and characterising sources of persistent organic pollutants (PAHs and PCBs) in surface sediments of an industrialized area (harbour of Trieste, northern Adriatic Sea). J. Environ. Monit. 2000, 2, 261–265. [Google Scholar] [CrossRef]
- Oen, A.M.; Cornelissen, G.; Breedveld, G.D. Relation between PAH and black carbon contents in size fractions of Norwegian harbor sediments. Environ. Pollut. 2006, 141, 370–380. [Google Scholar] [CrossRef]
- De Luca, G.; Furesi, A.; Leardi, R.; Micera, G.; Panzanelli, A.; Piu, P.C.; Sanna, G. Polycyclic aromatic hydrocarbons assessment in the sediments of the Porto Torres Harbor (Northern Sardinia, Italy). Mar. Chem. 2004, 86, 15–32. [Google Scholar] [CrossRef]
- Simpson, C.D.; Mosi, A.A.; Cullen, W.R.; Reimer, K.J. Composition and distribution of polycyclic aromatic hydrocarbon contamination in surficial marine sediments from Kitimat Harbor, Canada. Sci. Total Environ. 1996, 181, 265–278. [Google Scholar] [CrossRef]
- Witt, G. Polycyclic aromatic hydrocarbons in water and sediment of the Baltic Sea. Mar. Pollut. Bull. 1995, 31, 237–248. [Google Scholar] [CrossRef]


| PAH Ratio | Value Ranges | Source |
|---|---|---|
| PAHs (L/H MW) | >1 <1 | Petrogenic Pyrogenic |
| Flt/(Flt + Pyr) | <0.4 0.4–0.5 >0.5 | Petrogenic Fossil fuel combustion grass/wood/coal combustion |
| BaA/(BaA + Chr) | <0.2 0.2–0.35 >0.35 | Petrogenic Mixed sources Combustion |
| IcdP/(IcdP+BghiP) | <0.2 0.2–0.35 >0.35 | Petrogenic Mixed sources Combustion |
| PAH | # Rings | Sharam Area | Alkhor Area | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sediment | Seawater | Sediment | Seawater | ||||||
| Mean (ng/kg) | Std. dev. | Mean (ng/L) | Std. dev. | Mean (ng/kg) | Std. dev. | Mean (ng/L) | Std. dev. | ||
| Naph | 2 | 7.49 | 7.53 | 0.14 | 0.16 | 2.02 | 1.32 | 0.23 | 0.33 |
| Acy | 3 | 0.23 | 0.21 | 0.09 | 0.08 | 0.10 | 0.10 | 0.10 | 0.08 |
| Ace | 3 | 0.65 | 0.87 | 0.08 | 0.08 | 0.12 | 0.16 | 0.19 | 0.16 |
| Flu | 3 | 0.44 | 0.25 | 0.22 | 0.10 | 0.20 | 0.11 | 0.30 | 0.19 |
| Phen | 3 | 2.18 | 2.09 | 1.06 | 0.32 | 0.82 | 0.30 | 0.36 | 0.18 |
| Anth | 3 | N/A | N/A | N/A | N/A | N/A | N/A | 0.03 | 0.08 |
| Flt | 4 | 3.17 | 7.75 | 0.40 | 0.29 | 0.36 | 0.50 | 2.29 | 5.88 |
| Pyr | 4 | 5.07 | 12.46 | 0.20 | 0.12 | 0.53 | 1.00 | 3.25 | 9.26 |
| BaA | 4 | 0.18 | 0.18 | 0.10 | 0.18 | 0.10 | 0.11 | 0.22 | 0.22 |
| Chr | 4 | 0.36 | 0.51 | 0.23 | 0.24 | 0.30 | 0.18 | 0.25 | 0.24 |
| BbF | 5 | N/A | N/A | 0.07 | 0.16 | N/A | N/A | N/A | N/A |
| BKF | 5 | N/A | N/A | 0.09 | 0.19 | N/A | N/A | N/A | N/A |
| BaP | 5 | 0.75 | 0.82 | 0.20 | 0.25 | 0.38 | 0.41 | 0.33 | 0.36 |
| DahA | 5 | 0.62 | 0.43 | 0.55 | 0.42 | 1.01 | 0.85 | 0.75 | 0.59 |
| IcdP | 6 | 0.41 | 0.68 | 0.18 | 0.15 | 0.26 | 0.18 | 0.23 | 0.29 |
| BghiP | 6 | 0.51 | 0.49 | 0.71 | 0.49 | 0.31 | 0.48 | 0.66 | 1.02 |
| ΣPAHs | 22.09 | 4.33 | 6.51 | 9.19 | |||||
| ΣLMW | 11.00 | 1.59 | 3.26 | 1.20 | |||||
| ΣHMW | 11.09 | 2.73 | 3.25 | 7.98 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhudhodi, A.H.; Alduwais, A.K.; Aldhafeeri, Z.M.; Al-Shamsi, M.A.S.; Alharbi, B.H. Contamination Assessment of Mangrove Ecosystems in the Red Sea Coast by Polycyclic Aromatic Hydrocarbons. Int. J. Environ. Res. Public Health 2022, 19, 5474. https://doi.org/10.3390/ijerph19095474
Alhudhodi AH, Alduwais AK, Aldhafeeri ZM, Al-Shamsi MAS, Alharbi BH. Contamination Assessment of Mangrove Ecosystems in the Red Sea Coast by Polycyclic Aromatic Hydrocarbons. International Journal of Environmental Research and Public Health. 2022; 19(9):5474. https://doi.org/10.3390/ijerph19095474
Chicago/Turabian StyleAlhudhodi, Abdulrahman H., Abdulilah K. Alduwais, Zaid M. Aldhafeeri, Mohammed Ahmad S. Al-Shamsi, and Badr H. Alharbi. 2022. "Contamination Assessment of Mangrove Ecosystems in the Red Sea Coast by Polycyclic Aromatic Hydrocarbons" International Journal of Environmental Research and Public Health 19, no. 9: 5474. https://doi.org/10.3390/ijerph19095474
APA StyleAlhudhodi, A. H., Alduwais, A. K., Aldhafeeri, Z. M., Al-Shamsi, M. A. S., & Alharbi, B. H. (2022). Contamination Assessment of Mangrove Ecosystems in the Red Sea Coast by Polycyclic Aromatic Hydrocarbons. International Journal of Environmental Research and Public Health, 19(9), 5474. https://doi.org/10.3390/ijerph19095474






