Effects and Causes of Detraining in Athletes Due to COVID-19: A Review
Abstract
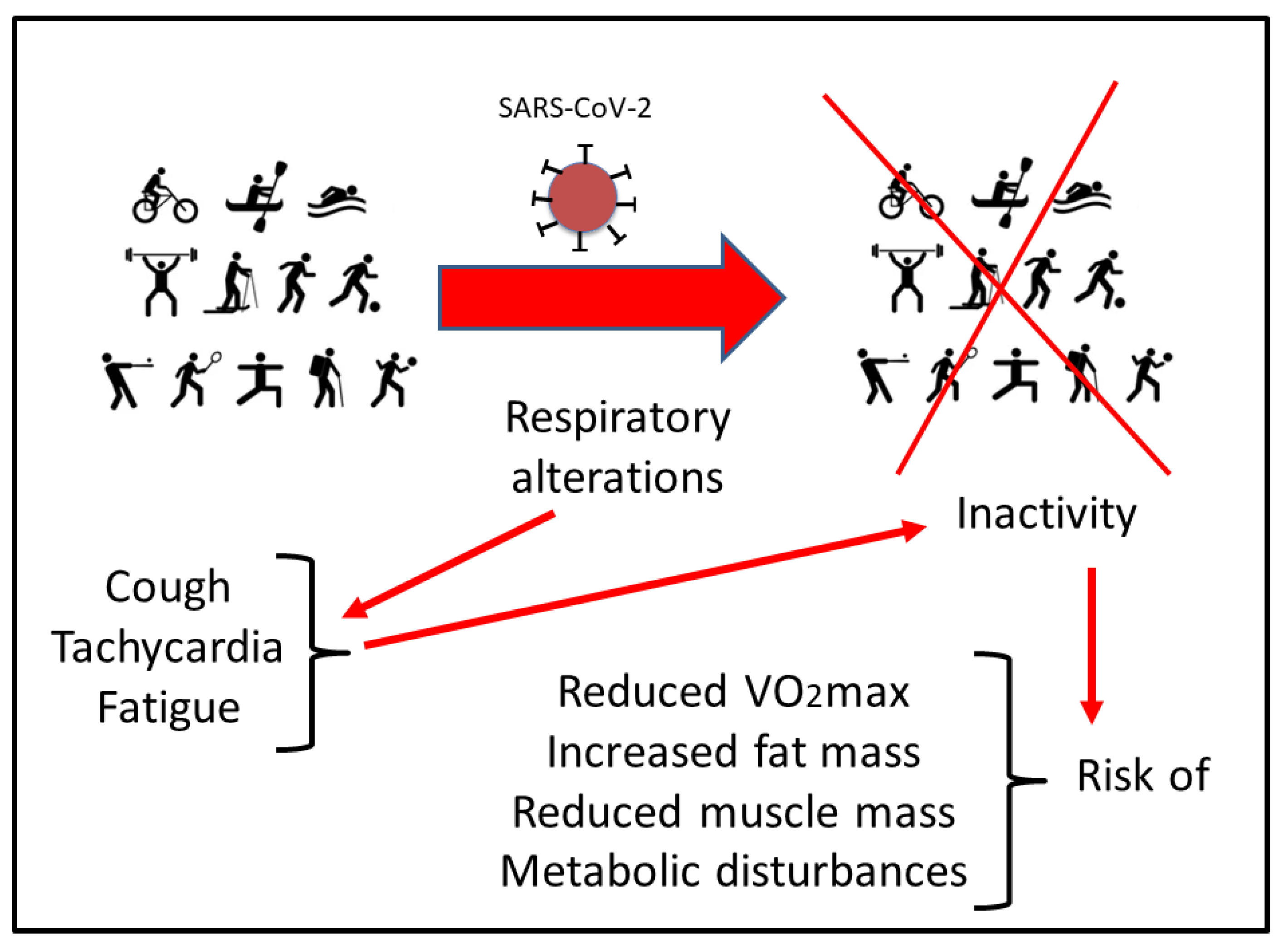
1. Introduction
2. Respiratory Disturbances
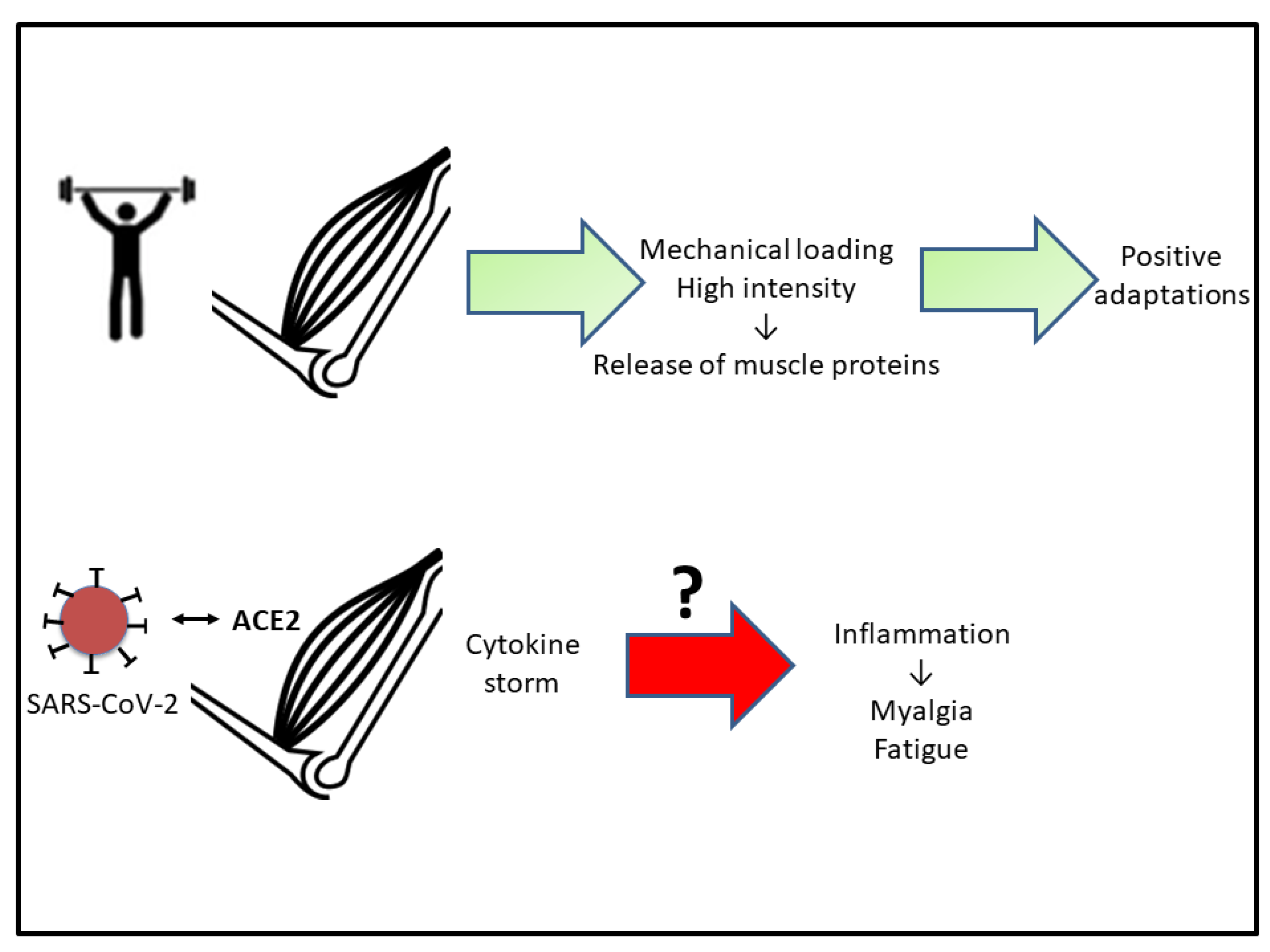
3. Muscular Repercussions
4. Cardiac Consequences
5. Neurological Involvement
6. State of Stress
7. Proposals for Reintegration into Sport Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demarie, S.; Galvani, C.; Billat, V.L. Horse-Riding Competitions Pre and Post COVID-19: Effect of Anxiety, sRPE and HR on Performance in Eventing. Int. J. Environ. Res. Public Health 2020, 17, 8648. [Google Scholar] [CrossRef]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz Martínez, B.J.; Bernal-Morel, E.; Courel-Ibáñez, J. Post-COVID-19 syndrome and the potential benefits of exercise. Int. J. Environ. Res. Public Health 2021, 18, 5329. [Google Scholar] [CrossRef]
- Dauty, M.; Menu, P.; Fouasson-Chailloux, A. Effects of the COVID-19 confinement period on physical conditions in young elite soccer players. J. Sports Med. Phys. Fit. 2021, 61, 1252–1257. [Google Scholar] [CrossRef]
- Spyrou, K.; Alcaraz, P.E.; Marín-Cascales, E.; Herrero-Carrasco, R.; Cohen, D.D.; Calleja-Gonzalez, J.; Pereira, L.A.; Loturco, I.; Freitas, T.T. Effects of the COVID-19 Lockdown on Neuromuscular Performance and Body Composition in Elite Futsal Players. J. Strength Cond. Res. 2021, 35, 2309–2315. [Google Scholar] [CrossRef]
- Font, R.; Irurtia, A.; Gutierrez, J.; Salas, S.; Vila, E.; Carmona, G. The effects of COVID-19 lockdown on jumping performance and aerobic capacity in elite handball players. Biol. Sport 2021, 38, 753–759. [Google Scholar] [CrossRef]
- Corsini, A.; Bisciotti, G.N.; Eirale, C.; Volpi, P. Football cannot restart soon during the COVID-19 emergency! A critical perspective from the Italian experience and a call for action. Br. J. Sports Med. 2020, 54, 1186–1187. [Google Scholar] [CrossRef]
- Brackbill, R.M.; Thorpe, L.E.; DiGrande, L.; Perrin, M.; Sapp, J.H.; Wu, D.; Campolucci, S.; Walker, D.J.; Cone, J.; Pulliam, P.; et al. Surveillance for World Trade Center Disaster Health Effects Among Survivors of Collapsed and Damaged Buildings. MMWR Surveill. Summ. 2006, 55, 1–18. [Google Scholar] [CrossRef]
- Mills, M.A.; Edmondson, D.; Park, C.L. Trauma and Stress Response Among Hurricane Katrina Evacuees. Am. J. Public Health 2007, 97, S116–S123. [Google Scholar] [CrossRef]
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.S.; Ho, R.C. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729. [Google Scholar] [CrossRef]
- Park, S.-C.; Park, Y.C. Mental Health Care Measures in Response to the 2019 Novel Coronavirus Outbreak in Korea. Psychiatry Investig. 2020, 17, 85–86. [Google Scholar] [CrossRef]
- Martínez-Patiño, M.J.; Blas Lopez, F.J.; Dubois, M.; Vilain, E.; Fuentes-García, J.P. Effects of COVID-19 Home Confinement on Behavior, Perception of Threat, Stress and Training Patterns of Olympic and Paralympic Athletes. Int. J. Environ. Res. Public Health 2021, 18, 12780. [Google Scholar] [CrossRef]
- Şenışık, S.; Denerel, N.; Köyağasıoğlu, O.; Tunç, S. The effect of isolation on athletes’ mental health during the COVID-19 pandemic. Phys. Sportsmed. 2021, 49, 187–193. [Google Scholar] [CrossRef]
- Uroh, C.C.; Adewunmi, C.M. Psychological Impact of the COVID-19 Pandemic on Athletes. Front. Sports Act. Living 2021, 3, 603415. [Google Scholar] [CrossRef]
- Melnyk, Y.B.; Stadnik, A.V.; Pypenko, I.S.; Kostina, V.V.; Yevtushenko, D.O. Impact of COVID-19 on the social and psychological state of athletes. J. Sports Med. Phys. Fit. 2022, 62, 297–299. [Google Scholar] [CrossRef]
- Lima, Y.; Denerel, N.; Öz, N.D.; Senisik, S. The psychological impact of COVID-19 infection on athletes: Example of professional male football players. Sci. Med. Footb. 2021, 5, 53–61. [Google Scholar] [CrossRef]
- Garfin, D.R.; Thompson, R.R.; Holman, E.A. Acute stress and subsequent health outcomes: A systematic review. J. Psychosom. Res. 2018, 112, 107–113. [Google Scholar] [CrossRef]
- Kirwan, R.; McCullough, D.; Butler, T.; de Heredia, F.P.; Davies, I.G.; Stewart, C. Sarcopenia during COVID-19 lockdown restrictions: Long-term health effects of short-term muscle loss. Geroscience 2020, 42, 1547–1578. [Google Scholar] [CrossRef]
- Palmer, K.; Monaco, A.; Kivipelto, M.; Onder, G.; Maggi, S.; Michel, J.-P.; Prieto, R.; Sykara, G.; Donde, S. The potential long-term impact of the COVID-19 outbreak on patients with non-communicable diseases in Europe: Consequences for healthy ageing. Aging Clin. Exp. Res. 2020, 32, 1189–1194. [Google Scholar] [CrossRef]
- Magdy, D.M.; Metwally, A.; Tawab, D.A.; Hassan, S.A.; Makboul, M.; Farghaly, S. Long-term COVID-19 effects on pulmonary function, exercise capacity, and health status. Ann. Thorac. Med. 2022, 17, 28–36. [Google Scholar] [CrossRef]
- Fabre, J.-B.; Grelot, L.; Vanbiervielt, W.; Mazerie, J.; Manca, R.; Martin, V. Managing the combined consequences of COVID-19 infection and lock-down policies on athletes: Narrative review and guidelines proposal for a safe return to sport. BMJ Open Sport Exerc. Med. 2020, 6, e000849. [Google Scholar] [CrossRef]
- Córdova, A. Fisiología Deportiva; Síntesis: Madrid, Spain, 2013. [Google Scholar]
- Mascia, G.; Pescetelli, F.; Baldari, A.; Gatto, P.; Seitun, S.; Sartori, P.; Pieroni, M.; Calò, L.; Della Bona, R.; Porto, I. Interpretation of elevated high-sensitivity cardiac troponin I in elite soccer players previously infected by severe acute respiratory syndrome coronavirus 2. Int. J. Cardiol. 2021, 326, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.G.; Hull, J.H.; Rogers, J.; Pollock, N.; Dodd, M.; Haines, J.; Harris, S.; Loosemore, M.; Malhotra, A.; Pieles, G.; et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: A practical guide for sport and exercise medicine physicians. Br. J. Sports Med. 2020, 54, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Toresdahl, B.G.; Asif, I.M. Coronavirus Disease 2019 (COVID-19): Considerations for the Competitive Athlete. Sports Health A Multidiscip. Approach 2020, 12, 221–224. [Google Scholar] [CrossRef]
- Dores, H.; Cardim, N. Return to play after COVID-19: A sport cardiologist’s view. Br. J. Sports Med. 2020, 54, 1132–1133. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.W.; Tucker, A.M.; Bloom, O.J.; Green, G.; DiFiori, J.P.; Solomon, G.; Phelan, D.; Kim, J.H.; Meeuwisse, W.; Sills, A.K.; et al. Prevalence of Inflammatory Heart Disease Among Professional Athletes with Prior COVID-19 Infection Who Received Systematic Return-to-Play Cardiac Screening. JAMA Cardiol. 2021, 6, 745–752. [Google Scholar] [CrossRef]
- Phelan, D.; Kim, J.H.; Chung, E.H. A Game Plan for the Resumption of Sport and Exercise After Coronavirus Disease 2019 (COVID-19) Infection. JAMA Cardiol. 2020, 5, 1085. [Google Scholar] [CrossRef] [PubMed]
- Salman, D.; Vishnubala, D.; Le Feuvre, P.; Beaney, T.; Korgaonkar, J.; Majeed, A.; McGregor, A.H. Returning to physical activity after covid-19. BMJ 2021, 372, m4721. [Google Scholar] [CrossRef]
- Schellhorn, P.; Klingel, K.; Burgstahler, C. Return to sports after COVID-19 infection. Eur. Heart J. 2020, 41, 4382–4384. [Google Scholar] [CrossRef]
- Santos-Ferreira, D.; Tomás, R.; Dores, H. Return-to-Play Guidelines for Athletes After COVID-19 Infection. JAMA Cardiol. 2021, 6, 478–479. [Google Scholar] [CrossRef]
- Olsen, R.H.; Krogh-Madsen, R.; Thomsen, C.; Booth, F.W.; Pedersen, B.K. Metabolic Responses to Reduced Daily Steps in Healthy Nonexercising Men. JAMA 2008, 299, 1261–1263. [Google Scholar] [CrossRef]
- Mikus, C.R.; Oberlin, D.J.; Libla, J.L.; Taylor, A.M.; Booth, F.W.; Thyfault, J.P. Lowering Physical Activity Impairs Glycemic Control in Healthy Volunteers. Med. Sci. Sports Exerc. 2012, 44, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ferran, M.; De La Guía-Galipienso, F.; Sanchis-Gomar, F.; Pareja-Galeano, H. Metabolic Impacts of Confinement during the COVID-19 Pandemic Due to Modified Diet and Physical Activity Habits. Nutrients 2020, 12, 1549. [Google Scholar] [CrossRef] [PubMed]
- Astrand, P.O. Quantification of exercise capability and evaluation of physical capacity in man. Prog. Cardiovasc. Dis. 1976, 19, 51–67. [Google Scholar] [CrossRef]
- Yu, C.; Li, A.M.; So, R.C.H.; McManus, A.; Ng, P.C.; Chu, W.; Chan, D.; Cheng, F.; Chiu, W.K.; Leung, C.W.; et al. Longer term follow up of aerobic capacity in children affected by severe acute respiratory syndrome (SARS). Thorax 2006, 61, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.; De Vito, G.; Franchi, M.; Paoli, A.; Moro, T.; Marcolin, G.; Grassi, B.; Baldassarre, G.; Zuccarelli, L.; Biolo, G.; et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2021, 21, 614–635. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z.; et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424. [Google Scholar] [CrossRef]
- Zhu, J.; Ji, P.; Pang, J.; Zhong, Z.; Li, H.; He, C.; Zhang, J.; Zhao, C. Clinical characteristics of 3062 COVID-19 patients: A meta analysis. J. Med. Virol. 2020, 92, 1902–1914. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, X.; Xiong, L.; Cai, K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J. Med. Virol. 2020, 92, 1449–1459. [Google Scholar] [CrossRef]
- Kadoguchi, T.; Kinugawa, S.; Takada, S.; Fukushima, A.; Furihata, T.; Homma, T.; Masaki, Y.; Mizushima, W.; Nishikawa, M.; Takahashi, M.; et al. Angiotensin II can directly induce mitochondrial dysfunction, decrease oxidative fibre number and induce atrophy in mouse hindlimb skeletal muscle. Exp. Physiol. 2015, 100, 312–322. [Google Scholar] [CrossRef]
- Kim, J.-H.; Thompson, L.V. Inactivity, age, and exercise: Single-muscle fiber power generation. J. Appl. Physiol. 2013, 114, 90–98. [Google Scholar] [CrossRef]
- Jee, H.; Kim, J.-H. A mini-overview of single muscle fibre mechanics: The effects of age, inactivity and exercise in animals and humans. Swiss. Med. Wkly. 2017, 147, w14488. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Josephson, G.; Sunbuli, M.; Chadaga, A.R. Spontaneous Pneumothorax in an Elderly Patient with Coronavirus Disease (COVID-19) Pneumonia. Ochsner J. 2020, 20, 343–345. [Google Scholar] [CrossRef]
- Wang, J.-T.; Sheng, W.-H.; Fang, C.-T.; Chen, Y.-C.; Wang, J.-L.; Yu, C.-J.; Chang, S.-C.; Yang, P.-C. Clinical Manifestations, Laboratory Findings, and Treatment Outcomes of SARS Patients. Emerg. Infect. Dis. 2004, 10, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Hsu, C.-W.; Tian, Y.-C.; Fang, J.-T. Rhabdomyolysis associated with acute renal failure in patients with severe acute respiratory syndrome. Int. J. Clin. Pract. 2005, 59, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Kley, R.A.; Schmidt-Wilcke, T.; Vorgerd, M. Differential Diagnosis of HyperCKemia. Neurol. Int. Open 2018, 2, E72–E83. [Google Scholar] [CrossRef][Green Version]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Córdova, A.; Martin, J.F.; Reyes, E.; Alvarez-Mon, M. Protection against muscle damage in competitive sports players: The effect of the immunomodulator AM3. J. Sports Sci. 2004, 22, 827–833. [Google Scholar] [CrossRef]
- Cordova, A.; Monserrat, J.; Villa, G.; Reyes, E.; Soto, M.A.-M. Effects of AM3 (Inmunoferon) on increased serum concentrations of interleukin-6 and tumour necrosis factor receptors I and II in cyclists. J. Sports Sci. 2006, 24, 565–573. [Google Scholar] [CrossRef]
- Córdova-Martínez, A.; Martorell-Pons, M.; Sureda-Gomila, A.; Tur-Marí, J.A.; Pons-Biescas, A. Changes in circulating cytokines and markers of muscle damage in elite cyclists during a multi-stage competition. Clin. Physiol. Funct. Imaging 2015, 35, 351–358. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.C.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A. Fisiología Dinámica; Ed Elsevier-Masson: Barcelona, Spain, 2003. [Google Scholar]
- Majano, P.; Roda-Navarro, P.; Alonso-Lebrero, J.L.; Brieva, A.; Casal, C.; Pivel, J.P.; López-Cabrera, M.; Moreno-Otero, R. AM3 inhibits HBV replication through activation of peripheral blood mononuclear cells. Int. Immunopharmacol. 2004, 4, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Draganidis, D.; Karagounis, L.G.; Athanailiidis, I.; Chatzinikolaou, A.; Jamurtas, A.Z.; Fatouros, I.G. Inflammaging and Skeletal Muscle: Can Protein Intake Make a Difference? J. Nutr. 2016, 146, 1940–1952. [Google Scholar] [CrossRef]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Linossier, M.-T.; Dormois, D.; Perier, C.; Frey, J.; Geyssant, A.; Denis, C. Enzyme adaptations of human skeletal muscle during bicycle short-sprint training and detraining. Acta Physiol. Scand. 1997, 161, 439–445. [Google Scholar] [CrossRef]
- Dawson, B.; Fitzsimons, M.; Green, S.; Goodman, C.; Carey, M.; Cole, K. Changes in performance, muscle metabolites, enzymes and fibre types after short sprint training. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 78, 163–169. [Google Scholar] [CrossRef]
- Izquierdo, M.; Ibañez, J.; Calbet, J.A.L.; González-Izal, M.; Navarro-Amézqueta, I.; Granados, C.; Malanda, A.; Idoate, F.; González-Badillo, J.J.; Häkkinen, K.; et al. Neuromuscular Fatigue after Resistance Training. Int. J. Sports Med. 2009, 30, 614–623. [Google Scholar] [CrossRef]
- Bogdanis, G.C.P. Effects of Physical Activity and Inactivity on Muscle Fatigue. Front. Physiol. 2012, 3, 142. [Google Scholar] [CrossRef]
- Demarie, S.; Chirico, E.; Galvani, C. Prediction and Analysis of Tokyo Olympic Games Swimming Results: Impact of the COVID-19 Pandemic on Swimmers’ Performance. Int. J. Environ. Res. Public Health 2022, 19, 2110. [Google Scholar] [CrossRef] [PubMed]
- Rampinini, E.; Donghi, F.; Martin, M.; Bosio, A.; Riggio, M.; Maffiuletti, N.A. Impact of COVID-19 Lockdown on Serie a Soccer Players’ Physical Qualities. Int. J. Sports Med. 2021, 42, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.R.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.-F.; Charles, P.; Richaud, C.; Caussin, C.; Diakov, C. Myocarditis revealing COVID-19 infection in a young patient. Eur. Heart. J. Cardiovasc. Imaging 2020, 21, 776. [Google Scholar] [CrossRef]
- Nieman, D.C. Is infection risk linked to exercise workload? Med. Sci. Sports Exerc. 2000, 32, S406–S411. [Google Scholar] [CrossRef]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar]
- Doyen, D.; Moceri, P.; Ducreux, D.; Dellamonica, J. Myocarditis in a patient with COVID-19: A cause of raised troponin and ECG changes. Lancet 2020, 395, 1516. [Google Scholar] [CrossRef]
- Boraita-Pérez, A.; Serratosa-Fernández, L. Sudden death (IV). Sudden death in the athlete. The minimal requirements before performing a competitive sport. Rev. Esp. Cardiol. 1999, 52, 1139–1145. [Google Scholar]
- Emery, M.S.; Kovacs, R.J. Sudden Cardiac Death in Athletes. JACC Heart Fail. 2018, 6, 30–40. [Google Scholar] [CrossRef]
- Corrado, D.; Zorzi, A. Sudden death in athletes. Int. J. Cardiol. 2017, 237, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Martínez, F.A.; Rubio-Arias, J.Á.; Ramos-Campo, D.J.; Alcaraz, P.E. Effectiveness of Resistance Circuit-Based Training for Maximum Oxygen Uptake and Upper-Body One-Repetition Maximum Improvements: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 2553–2568. [Google Scholar] [CrossRef] [PubMed]
- Costill, D.L.; Fink, W.J.; Hargreaves, M.; King, D.S.; Thomas, R.; Fielding, R. Metabolic characteristics of skeletal muscle during detraining from competitive swimming. Med. Sci. Sports Exerc. 1985, 17, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Neufer, P.D.; Costill, D.L.; Fielding, R.A.; Flynn, M.G.; Kirwan, J.P. Effect of reduced training on muscular strength and endurance in competitive swimmers. Med. Sci. Sports Exerc. 1987, 19, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Daou, B.J.; Koduri, S.; Palmateer, G.; Thompson, B.G.; Chaudhary, N.; Gemmete, J.J.; Pandey, A.S. Letter: Neurological Implications of COVID-19 and Lessons Learned From Prior Epidemics and Pandemics. Neurosurgery 2020, 87, E234–E238. [Google Scholar] [CrossRef]
- Montalvan, V.; Lee, J.; Bueso, T.; De Toledo, J.; Rivas, K. Neurological manifestations of COVID-19 and other coronavirus infections: A systematic review. Clin. Neurol. Neurosurg. 2020, 194, 105921. [Google Scholar] [CrossRef]
- Rahimi, K. Guillain-Barre syndrome during COVID-19 pandemic: An overview of the reports. Neurol. Sci. 2020, 41, 3149–3156. [Google Scholar] [CrossRef]
- Toscano, G.; Palmerini, F.; Ravaglia, S.; Ruiz, L.; Invernizzi, P.; Cuzzoni, M.G.; Franciotta, D.; Baldanti, F.; Daturi, R.; Postorino, P.; et al. Guillain–Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2574–2576. [Google Scholar] [CrossRef]
- Gutiérrez-Ortiz, C.; Méndez-Guerrero, A.; Rodrigo-Rey, S.; San Pedro-Murillo, E.; Bermejo-Guerrero, L.; Gordo-Mañas, R.; de Aragón-Gómez, F.; Benito-León, J. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 2020, 95, e601–e605. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, D.; Zhou, H.; Liu, J.; Chen, S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol. 2020, 19, 383–384. [Google Scholar] [CrossRef]
- Harapan, B.N.; Yoo, H.J. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19). J. Neurol. 2021, 268, 3059–3071. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Valnet, R.; Pizzarotti, B.; Anichini, A.; Demars, Y.; Russo, E.; Schmidhauser, M.; Cerutti-Sola, J.; Rossetti, A.O.; Du Pasquier, R. Two patients with acute meningo-encephalitis concomitant to SARS-CoV-2infection. Eur. J. Neurol. 2020, 27, e43–e44. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Sharifian-Dorche, M.; Huot, P.; Osherov, M.; Wen, D.; Saveriano, A.; Giacomini, P.S.; Antel, J.P.; Mowla, A. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J. Neurol. Sci. 2020, 417, 117085. [Google Scholar] [CrossRef] [PubMed]
- Doobay, M.F.; Talman, L.S.; Obr, T.D.; Tian, X.; Davisson, R.L.; Lazartigues, E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R373–R381. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, Y.; Zhang, W.; Chen, A.F.; Lin, S.; Morris, M. RNA interference shows interactions between mouse brainstem angiotensin AT1 receptors and angiotensin-converting enzyme 2. Exp. Physiol. 2008, 93, 676–684. [Google Scholar] [CrossRef]
- Sepehrinezhad, A.; Shahbazi, A.; Negah, S.S. COVID-19 virus may have neuroinvasive potential and cause neurological complications: A perspective review. J. Neurovirol. 2020, 26, 324–329. [Google Scholar] [CrossRef]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe Acute Respiratory Syndrome Coronavirus Infection Causes Neuronal Death in the Absence of Encephalitis in Mice Transgenic for Human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef]
- Dubé, M.; Le Coupanec, A.; Wong, A.H.M.; Rini, J.M.; Desforges, M.; Talbot, P.J. Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43. J. Virol. 2018, 92, e00404–e00418. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses 2019, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Swanson, P.A.; McGavern, D.B. Viral diseases of the central nervous system. Curr. Opin. Virol. 2015, 11, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; on behalf of the HLH Across Speciality Collaboration UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Sankowski, R.; Mader, S.; Valdés-Ferrer, S.I. Systemic Inflammation and the Brain: Novel Roles of Genetic, Molecular, and Environmental Cues as Drivers of Neurodegeneration. Front. Cell. Neurosci. 2015, 9, 28. [Google Scholar] [CrossRef]
- McNeil, J.B.; Hughes, C.G.; Girard, T.; Ware, L.B.; Ely, E.W.; Chandrasekhar, R.; Han, J.H. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS ONE 2019, 14, e0226412. [Google Scholar] [CrossRef]
- Li, Y.; Fu, L.; Gonzales, D.M.; Lavi, E. Coronavirus Neurovirulence Correlates with the Ability of the Virus to Induce Proinflammatory Cytokine Signals from Astrocytes and Microglia. J. Virol. 2004, 78, 3398–3406. [Google Scholar] [CrossRef]
- Bohmwald, K.; Gálvez, N.M.S.; Ríos, M.; Kalergis, A.M. Neurologic Alterations Due to Respiratory Virus Infections. Front. Cell. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Kuo, W.-C.; Bratzke, L.C.; Oakley, L.D.; Kuo, F.; Wang, H.; Brown, R.L. The association between psychological stress and metabolic syndrome: A systematic review and meta-analysis. Obes. Rev. 2019, 20, 1651–1664. [Google Scholar] [CrossRef]
- Pillay, L.; van Rensburg, D.C.C.J.; van Rensburg, A.J.; Ramagole, D.A.; Holtzhausen, L.; Dijkstra, H.P.; Cronje, T. Nowhere to hide: The significant impact of coronavirus disease 2019 (COVID-19) measures on elite and semi-elite South African athletes. J. Sci. Med. Sport 2020, 23, 670–679. [Google Scholar] [CrossRef]
- Mukhtar, S. Psychological health during the coronavirus disease 2019 pandemic outbreak. Int. J. Soc. Psychiatry 2020, 66, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Cordova, A.; Alvarez-Mon, M. Inmunidad y Deporte; Ed Gymnos: Madrid, Spain, 2001. [Google Scholar]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, A.; Wu, Y.; Han, N.; Huang, H. Impact of the COVID-19 Pandemic on the Mental Health of College Students: A Systematic Review and Meta-Analysis. Front. Psychol. 2021, 12, 669119. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Allostasis and Allostatic Load Implications for Neuropsychopharmacology. Neuropsychopharmacology 2000, 22, 108–124. [Google Scholar] [CrossRef]
- Maugeri, G.; Castrogiovanni, P.; Battaglia, G.; Pippi, R.; D’Agata, V.; Palma, A.; Di Rosa, M.; Musumeci, G. The impact of physical activity on psychological health during Covid-19 pandemic in Italy. Heliyon 2020, 6, e04315. [Google Scholar] [CrossRef]
- Violant-Holz, V.; Gallego-Jiménez, M.G.; González-González, C.S.; Muñoz-Violant, S.; Rodríguez, M.J.; Sansano-Nadal, O.; Guerra-Balic, M. Psychological Health and Physical Activity Levels during the COVID-19 Pandemic: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 9419. [Google Scholar] [CrossRef]
- Mon-López, D.; de la Rubia Riaza, A.; Hontoria Galán, M.; Refoyo Roman, I. The Impact of Covid-19 and the Effect of Psychological Factors on Training Conditions of Handball Players. Int. J. Environ. Res. Public Health 2020, 17, 6471. [Google Scholar] [CrossRef]
- Cerón-Enríquez, N.; García-Saldivia, M.A.; Lara-Vargas, J.A.; Núñez-Urquiza, J.P.; Alonso-Sánchez, J.J.; Silva-Torres, J.J.; Pérez-Gámez, J.C.; Pacheco-Beltrán, N.; Alcocer-Gamba, M.A. Return to exercise after COVID-19. Statement of the Mexican Society of Cardiology. Arch. Cardiol. Mex. 2021, 91, 102–109. (In Spanish) [Google Scholar] [CrossRef]
- Rey, E.; Padrón-Cabo, A.; Penedo-Jamardo, E.; González-Víllora, S. Effect of the 11+ injury prevention programme on fundamental movement patterns in soccer players. Biol. Sport 2018, 35, 229–236. [Google Scholar] [CrossRef]
- Caldwell, B.P.; Peters, D.M. Seasonal Variation in Physiological Fitness of a Semiprofessional Soccer Team. J. Strength Cond. Res. 2009, 23, 1370–1377. [Google Scholar] [CrossRef]
- Greene, D.N.; Wu, A.H.B.; Jaffe, A.S. Return-to-Play Guidelines for Athletes After COVID-19 Infection. JAMA Cardiol. 2021, 6, 479. [Google Scholar] [CrossRef] [PubMed]
- Lodi, E.; Scavone, A.; Carollo, A.; Guicciardi, C.; Reggianini, L.; Savino, G.; Modena, M.G. Return to sport after the COVID-19 pandemic. How to behave? G. Ital. Cardiol. 2020, 21, 514–522. (In Italian) [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdova-Martínez, A.; Caballero-García, A.; Roche, E.; Pérez-Valdecantos, D.; Noriega, D.C. Effects and Causes of Detraining in Athletes Due to COVID-19: A Review. Int. J. Environ. Res. Public Health 2022, 19, 5400. https://doi.org/10.3390/ijerph19095400
Córdova-Martínez A, Caballero-García A, Roche E, Pérez-Valdecantos D, Noriega DC. Effects and Causes of Detraining in Athletes Due to COVID-19: A Review. International Journal of Environmental Research and Public Health. 2022; 19(9):5400. https://doi.org/10.3390/ijerph19095400
Chicago/Turabian StyleCórdova-Martínez, Alfredo, Alberto Caballero-García, Enrique Roche, Daniel Pérez-Valdecantos, and David C. Noriega. 2022. "Effects and Causes of Detraining in Athletes Due to COVID-19: A Review" International Journal of Environmental Research and Public Health 19, no. 9: 5400. https://doi.org/10.3390/ijerph19095400
APA StyleCórdova-Martínez, A., Caballero-García, A., Roche, E., Pérez-Valdecantos, D., & Noriega, D. C. (2022). Effects and Causes of Detraining in Athletes Due to COVID-19: A Review. International Journal of Environmental Research and Public Health, 19(9), 5400. https://doi.org/10.3390/ijerph19095400








