Evaluation of a Novel Tool for Apical Plug Formation during Apexification of Immature Teeth
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection, Preparation, and Distribution
2.2. Amalgam Carrier Method
2.3. MAP System Method
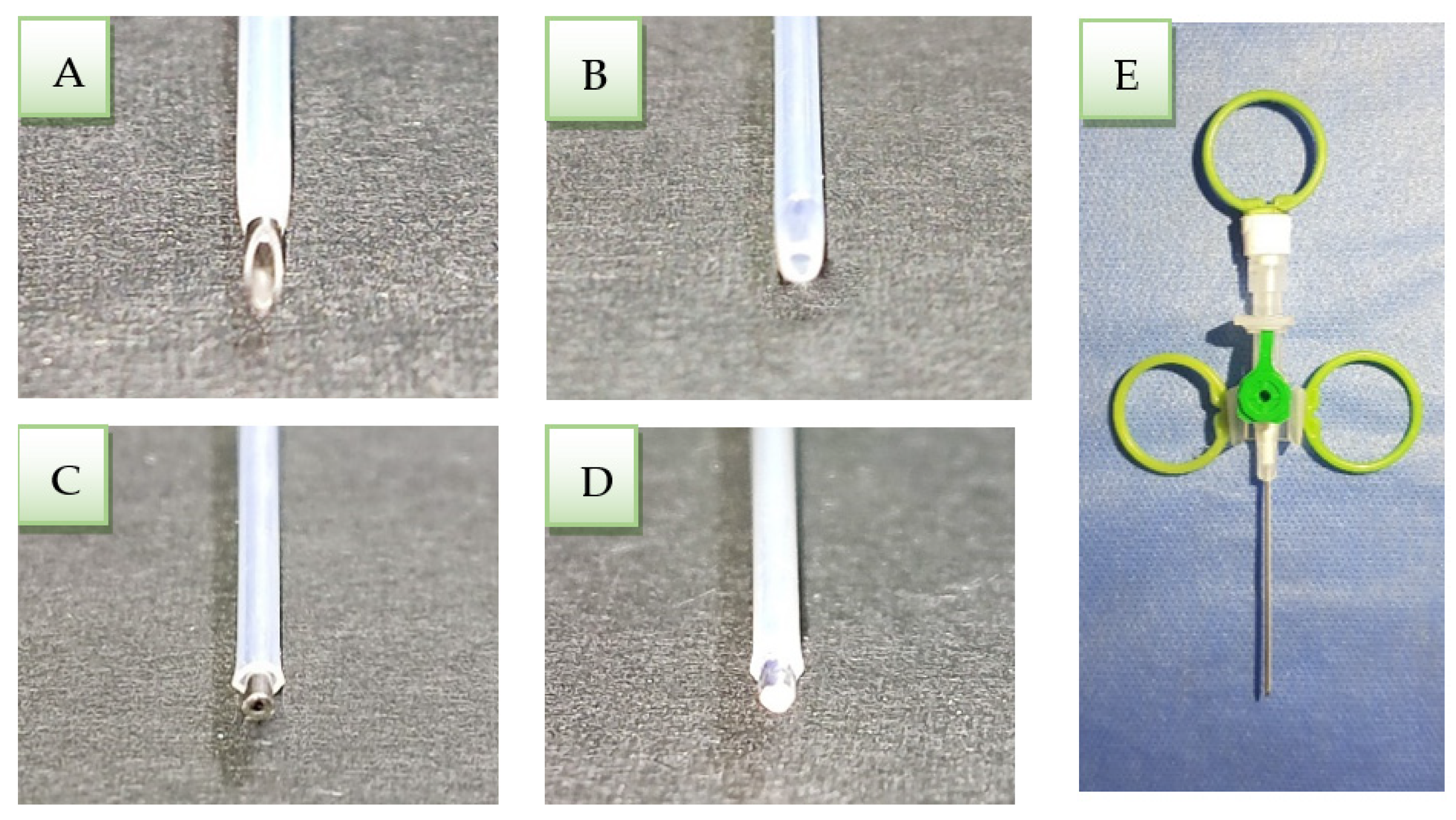
2.4. Modified Cannula Method
- A 2 mm cannula needle was cut with a diamond bur mounted on a high-speed handpiece perpendicularly to the vertical axis of the needle (Figure 1B).
- The catheter was cut until 1 mm of the clipped needle appeared outside to push out the entire amount of cement inside the catheter when it reached the apical third of the canal (Figure 1C).
- The clipped needle tip was closed with a silver weld to push the cement inside the catheter and condense the apical plug (Figure 1D).
- Plastic rings were fixed to the cannula wings, and Luer lock plugs with silicon to make them easier to catch and use (Figure 1E).
- The modified cannula was immersed in glutaraldehyde solution 2% for 24 h, then dried by air and kept in plastic wraps until use.
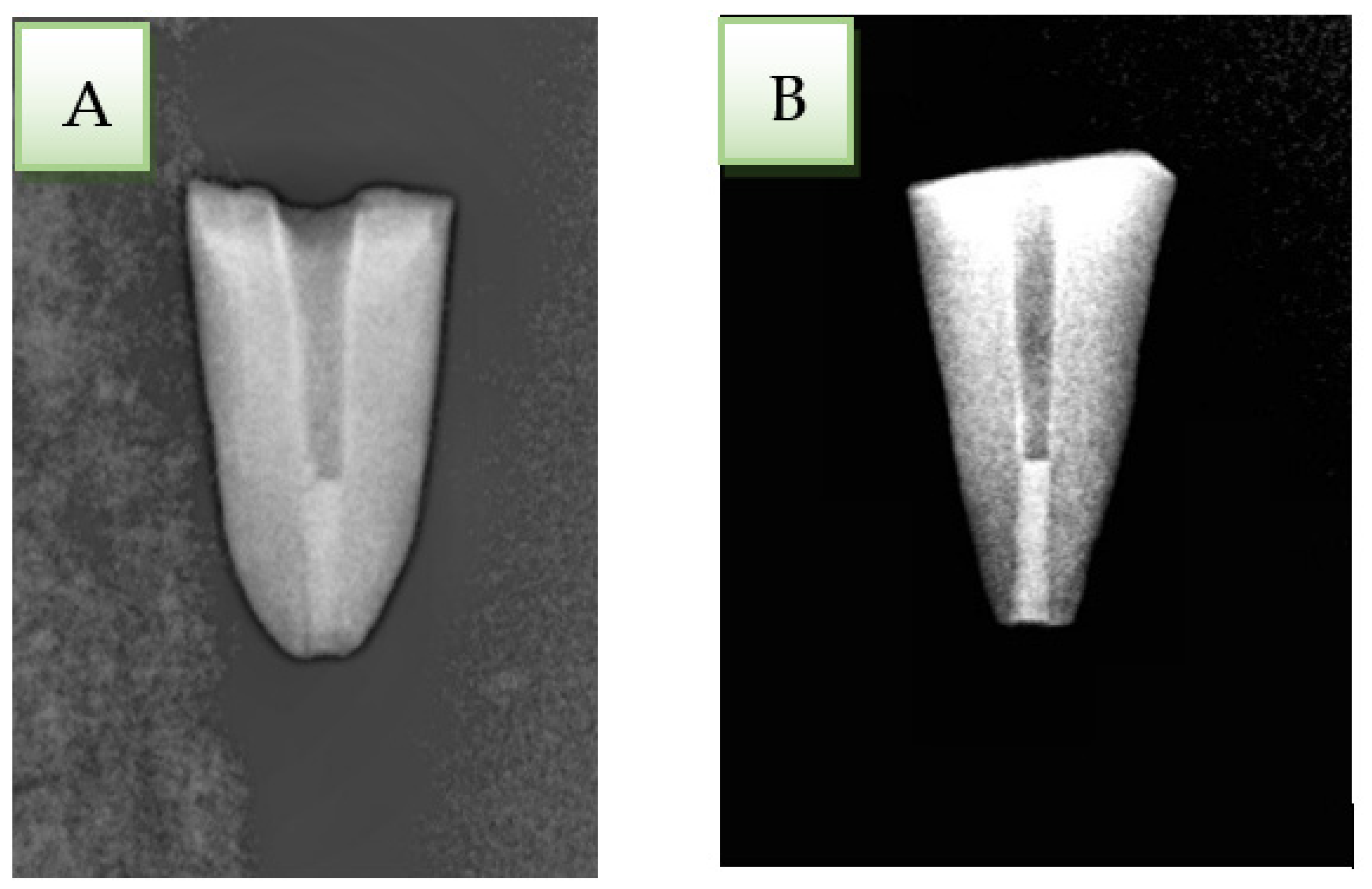
2.5. Marginal Leakage Testing
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trope, M. Treatment of the Immature Tooth with a Non–Vital Pulp and Apical Periodontitis. Dent. Clin. N. Am. 2010, 54, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Paulindraraj, S.; Venkatesan, R.; Suprakasam, S.; Christopher, A. Apexification-Then and Now: A Review. Int. J. Dent. Med. Res. 2015, 1, 193–196. [Google Scholar]
- Demirci, G.K.; Kaval, M.E.; Güneri, P.; Çalışkan, M.K. Treatment of immature teeth with nonvital pulps in adults: A prospective comparative clinical study comparing MTA with Ca(OH)2. Int. Endod. J. 2020, 53, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Rilliard, F.; Berdal, A.; Machtou, P. The use of mineral trioxide aggregate in one-visit apexification treatment: A prospective study. Int. Endod. J. 2007, 40, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Ghaziani, P.; Aghasizadeh, N.; Sheikh-Nezami, M. Endodontic treatment with MTA apical plugs: A case report. J. Oral Sci. 2007, 49, 325–329. [Google Scholar] [CrossRef][Green Version]
- Jitaru, S.; Hodisan, I.; Timis, L.; Lucian, A.; Bud, M. The Use of Bioceramics in Endodontics–Literature Review. Med. Pharm. Rep. 2016, 89, 470–473. [Google Scholar] [CrossRef]
- Nayak, G.; Hasan, M.F. Biodentine-a novel dentinal substitute for single visit apexification. Restor. Dent. Endod. 2014, 39, 120–125. [Google Scholar] [CrossRef]
- Adel, M.; Nima, M.M.; Kojoori, S.S.; Oliaie, H.N.; Naghavi, N.; Asgary, S. Comparison of Endodontic Biomaterials as Apical Barriers in Simulated Open Apices. ISRN Dent. 2012, 2012, 359873. [Google Scholar] [CrossRef]
- Chang, S.-W.; Oh, T.-S.; Lee, W.; Cheung, G.S.-P.; Kim, H.-C. Long-term observation of the mineral trioxide aggregate extrusion into the periapical lesion: A case series. Int. J. Oral Sci. 2013, 5, 54–57. [Google Scholar] [CrossRef]
- Günes, B.; Aydinbelge, H.A. Mineral trioxide aggregate apical plug method for the treatment of nonvital immature permanent maxillary incisors: Three case reports. J. Conserv. Dent. 2012, 15, 73–76. [Google Scholar] [CrossRef]
- Raldi, D.P.; Mello, I.; Habitante, S.M.; Lage-Marques, J.L.; Coil, J. Treatment options for teeth with open apices and apical peri-odontitis. J. Can. Dent. Assoc. 2009, 75, 591–596. [Google Scholar]
- Berástegui, E.; Juez, M.; Ballester, M. In vitro comparison of apical microleakage by spectrophotometry in simulated apexification using White Mineral Trioxide Aggregate, TotalFill Bioceramic Root Repair material, and BioDentine. J. Conserv. Dent. 2019, 22, 237–240. [Google Scholar] [CrossRef]
- Pereira, A.C.; Pallone, M.V.; Marciano, M.A.; Cortellazzi, K.L.; Frozoni, M.; Gomes, B.P.F.A.; De Almeida, J.F.A.; Soares, A.D.J. Effect of intracanal medications on the interfacial properties of reparative cements. Restor. Dent. Endod. 2019, 44, e21. [Google Scholar] [CrossRef] [PubMed]
- Tolibah, Y.A.; Kouchaji, C.; Lazkani, T.; Abbara, M.; Jbara, S.; Baghdadi, Z. Dental Care for a Child with Congenital Hydrocephalus: A Case Report with 12-Month Follow-Up. Int. J. Environ. Res. Public Health 2021, 18, 1209. [Google Scholar] [CrossRef] [PubMed]
- Giovarruscio, M.; Uccioli, U.; Malentacca, A.; Koller, G.; Foschi, F.; Mannocci, F. A technique for placement of apical MTA plugs using modified Thermafil carriers for the filling of canals with wide apices. Int. Endod. J. 2012, 46, 88–97. [Google Scholar] [CrossRef]
- Rosaline, H.; Rajan, M.; Deivanayagam, K.; Reddy, S.Y. BioRoot inlay: An innovative technique in teeth with wide open apex. Indian J. Dent. Res. 2018, 29, 521–524. [Google Scholar] [CrossRef]
- Aminoshariae, A.; Hartwell, G.R.; Moon, P.C. Placement of Mineral Trioxide Aggregate Using Two Different Techniques. J. Endod. 2003, 29, 679–682. [Google Scholar] [CrossRef]
- Adel, M.; Salmani, Z.; Youssefi, N.; Heidari, B. Comparison of Microleakage of Mineral Trioxide Aggregate Apical Plug Applied by the Manual Technique and Indirect Use of Ultrasonic with Different Powers. J. Dent. 2021, 22, 290–295. [Google Scholar] [CrossRef]
- Kahler, B. Chapter 21: ‘The role of endodontics after dental traumatic injuries’. In Cohen’s Pathways of the Pulp, 12th ed.; Hagreaves, K.M., Berman, L.H., Eds.; Elsvier: Amsterdam, The Netherlands, 2021; pp. 2555–2594. ISBN 9780323673044. [Google Scholar]
- Bachoo, I.K.; Seymour, D.; Brunton, P. A biocompatible and bioactive replacement for dentine: Is this a reality? The properties and uses of a novel calcium-based cement. Br. Dent. J. 2013, 214, E5. [Google Scholar] [CrossRef]
- Chia, M.S.Y.; Parolia, A.; Lim, B.S.H.; Jayaraman, J.; Porto, I.C.C.D.M. Effect of QMix irrigant in removal of smear layer in root canal system: A systematic review of in vitro studies. Restor. Dent. Endod. 2020, 45, e28. [Google Scholar] [CrossRef]
- Kottoor, J.; Mathew, J.; Kumar, S.; George, S.; Das, A. Dentine microhardness changes following conventional and alternate irrigation regimens: An in vitro study. J. Conserv. Dent. 2014, 17, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Elnaghy, A.M. Influence of QMix Irrigant on the Micropush-out Bond Strength of Biodentine and White Mineral Trioxide Aggregate. J. Adhes. Dent. 2014, 16, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.M. The Effect of Root Canal Irrigants on the Push–out Bond Strength of Biodentine. Tikrit J. Dent. Sci. 2015, 3, 70–75. [Google Scholar]
- Torabinejad, M.; Parirokh, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part II: Leakage and Biocompatibility Investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef]
- Veríssimo, D.M.; Vale, M.S.D. Methodologies for assessment of apical and coronal leakage of endodontic filling materials: A critical review. J. Oral Sci. 2006, 48, 93–98. [Google Scholar] [CrossRef]
- Nepal, M.; Shubham, S.; Tripathi, R.; Khadka, J.; Kunwar, D.; Gautam, V.; Gautam, N. Spectrophotometric analysis evaluating apical microleakage in retrograde filling using GIC, MTA and biodentine: An in-vitro study. BMC Oral Health 2020, 20, 37. [Google Scholar] [CrossRef]
- Park, S.-M.; Yoo, Y.-J.; Lee, I.-B.; Lee, W. Real-time nanoleakage and the flow characteristics of calcium silicate root canal filling materials. J. Mech. Behav. Biomed. Mater. 2020, 112, 104111. [Google Scholar] [CrossRef]
- De-Deus, G.; Murad, C.; Paciornik, S.; Reis, C.M.; Coutinho-Filho, T. The effect of the canal-filled area on the bacterial leakage of oval-shaped canals. Int. Endod. J. 2008, 41, 183–190. [Google Scholar] [CrossRef]
- Jovanović, L.Z.; Bajkin, B.V. Scanning electron microscopy analysis of marginal adaptation of mineral trioxide aggregate, tricalcium silicate cement, and dental amalgam as a root end filling materials. Microsc. Res. Tech. 2021, 84, 2068–2074. [Google Scholar] [CrossRef]
- Tolibah, Y.A.; Kouchaji, C.; Lazkani, T.; Ahmad, I.A.; Baghdadi, Z.D. Comparison of MTA versus Biodentine in Apexification Procedure for Nonvital Immature First Permanent Molars: A Randomized Clinical Trial. Children 2022, 9, 410. [Google Scholar] [CrossRef]
- Baghdadi, Z.D. Microleakage of a single-bottle adhesive system with 3 restorative mate-rials: In vitro study and clinical considerations. Compend. Contin. Educ. Dent. 2003, 24, 755–758, 760, 763 passim, quiz 772. [Google Scholar] [PubMed]
- Baghdadi, Z.D. The clinical evaluation of a single-bottle adhesive system with three restorative materials in children: Six-month results. Gen. Dent. 2005, 53, 357–365, quiz 366–368. [Google Scholar] [PubMed]
| Group (n = 10) | Plug Material | Delivery Method | |
|---|---|---|---|
| 1 | (MTA-AC) | MTA | Amalgam carrier |
| 2 | (MTA-MAP) | MTA | MAP System |
| 3 | (MTA-MC) | MTA | Modified cannula |
| 4 | (Biodentine-AC) | Biodentine | Amalgam carrier |
| 5 | (Biodentine-MAP) | Biodentine | MAP System |
| 6 | (Biodentine-MC) | Biodentine | Modified cannula |
| Group (n = 10) | Mean ± SD | Range | |
|---|---|---|---|
| 1 | (MTA-AC) | 444.7 ± 155.2 (7.4 ± 2.5 min) | 260–731 |
| 2 | (MTA-MAP) | 484.0 ± 176.5 (8.0 ± 2.9 min) | 143–689 |
| 3 | (MTA-MC) | 516.3 ± 138.8 (8.6 ± 2.3 min) | 272–644 |
| 4 | (Biodentine-AC) | 364.9 ± 119.6 (6.0 ± 1.9 min) | 182–570 |
| 5 | (Biodentine-MAP) | 438.1 ± 145.1 (7.3 ± 2.4 min) | 271–697 |
| 6 | (Biodentine-MC) | 552.5 ± 178.9 (9.2 ± 2.9 min) | 261–829 |
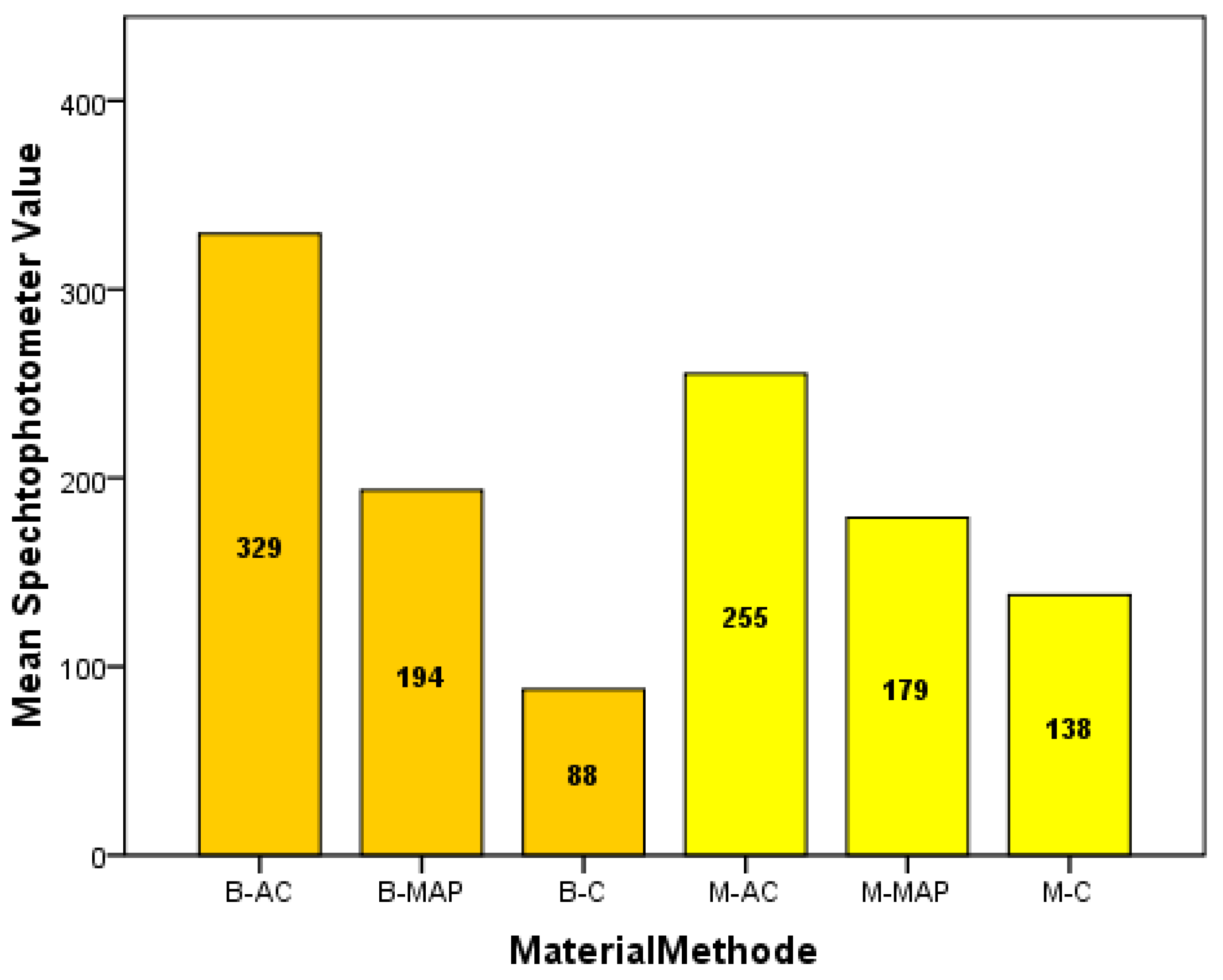
| Group (n = 10) | Mean ± SD | Range | |
|---|---|---|---|
| 1 | (MTA-AC) | 255.2 ± 174.0 | 45–482 |
| 2 | (MTA-MAP) | 179.0 ± 146.1 | 43–470 |
| 3 | (MTA-MC) | 138.0 ± 130.9 | 0–381 |
| 4 | (Biodentine-AC) | 329.4 ± 145.9 | 83–284 |
| 5 | (Biodentine-MAP) | 193.5 ± 128.9 | 42–426 |
| 6 | (Biodentine-MC) | 87.90 ± 73.3 | 22–237 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolibah, Y.A.; Droubi, L.; Alkurdi, S.; Abbara, M.T.; Bshara, N.; Lazkani, T.; Kouchaji, C.; Ahmad, I.A.; Baghdadi, Z.D. Evaluation of a Novel Tool for Apical Plug Formation during Apexification of Immature Teeth. Int. J. Environ. Res. Public Health 2022, 19, 5304. https://doi.org/10.3390/ijerph19095304
Tolibah YA, Droubi L, Alkurdi S, Abbara MT, Bshara N, Lazkani T, Kouchaji C, Ahmad IA, Baghdadi ZD. Evaluation of a Novel Tool for Apical Plug Formation during Apexification of Immature Teeth. International Journal of Environmental Research and Public Health. 2022; 19(9):5304. https://doi.org/10.3390/ijerph19095304
Chicago/Turabian StyleTolibah, Yasser Alsayed, Line Droubi, Saleh Alkurdi, Mohammad Tamer Abbara, Nada Bshara, Thuraya Lazkani, Chaza Kouchaji, Ibrahim Ali Ahmad, and Ziad D. Baghdadi. 2022. "Evaluation of a Novel Tool for Apical Plug Formation during Apexification of Immature Teeth" International Journal of Environmental Research and Public Health 19, no. 9: 5304. https://doi.org/10.3390/ijerph19095304
APA StyleTolibah, Y. A., Droubi, L., Alkurdi, S., Abbara, M. T., Bshara, N., Lazkani, T., Kouchaji, C., Ahmad, I. A., & Baghdadi, Z. D. (2022). Evaluation of a Novel Tool for Apical Plug Formation during Apexification of Immature Teeth. International Journal of Environmental Research and Public Health, 19(9), 5304. https://doi.org/10.3390/ijerph19095304








