Delayed Neurological Sequelae Successfully Treated with Adjuvant, Prolonged Hyperbaric Oxygen Therapy: Review and Case Report
Abstract
1. Introduction
2. Case Reports
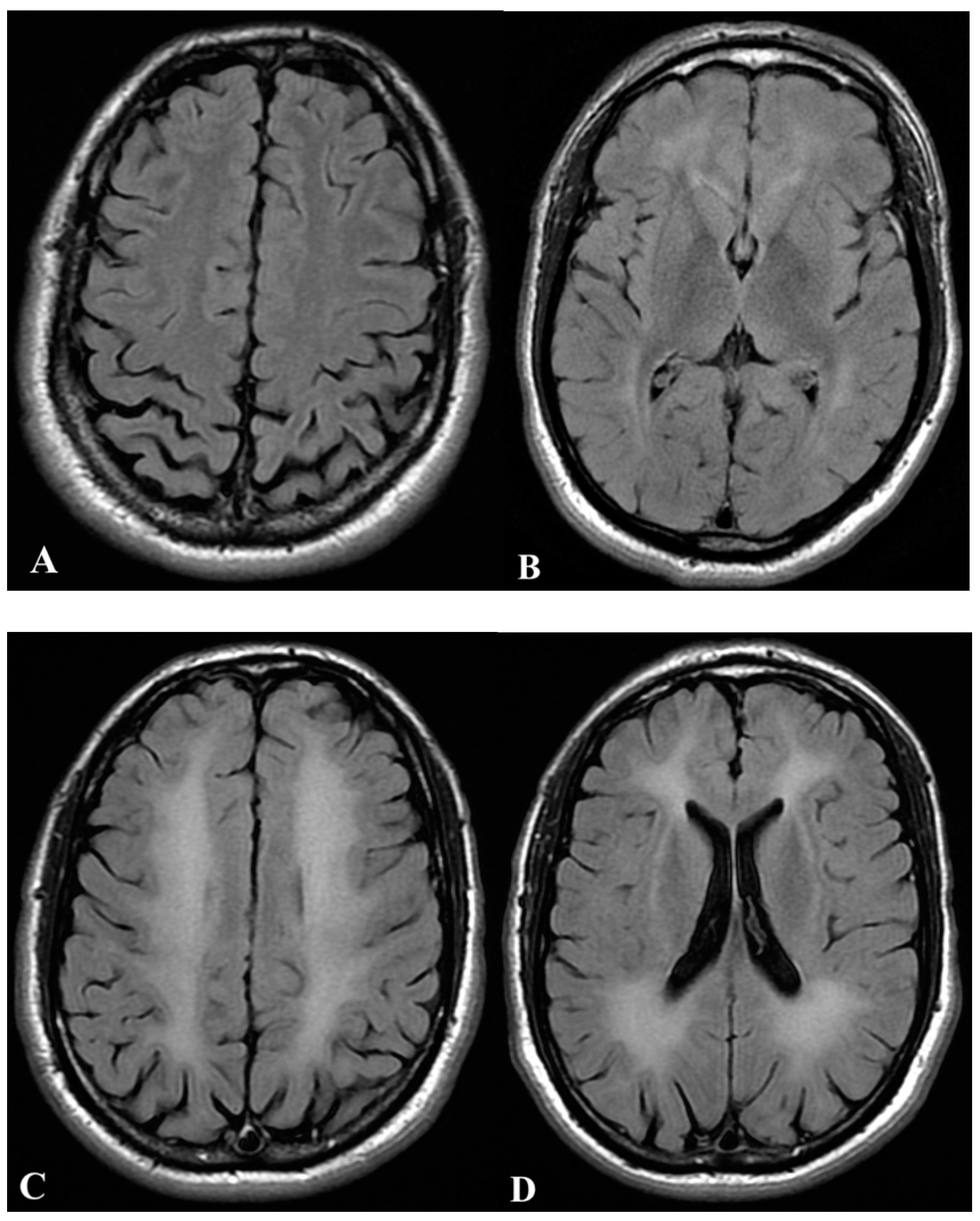
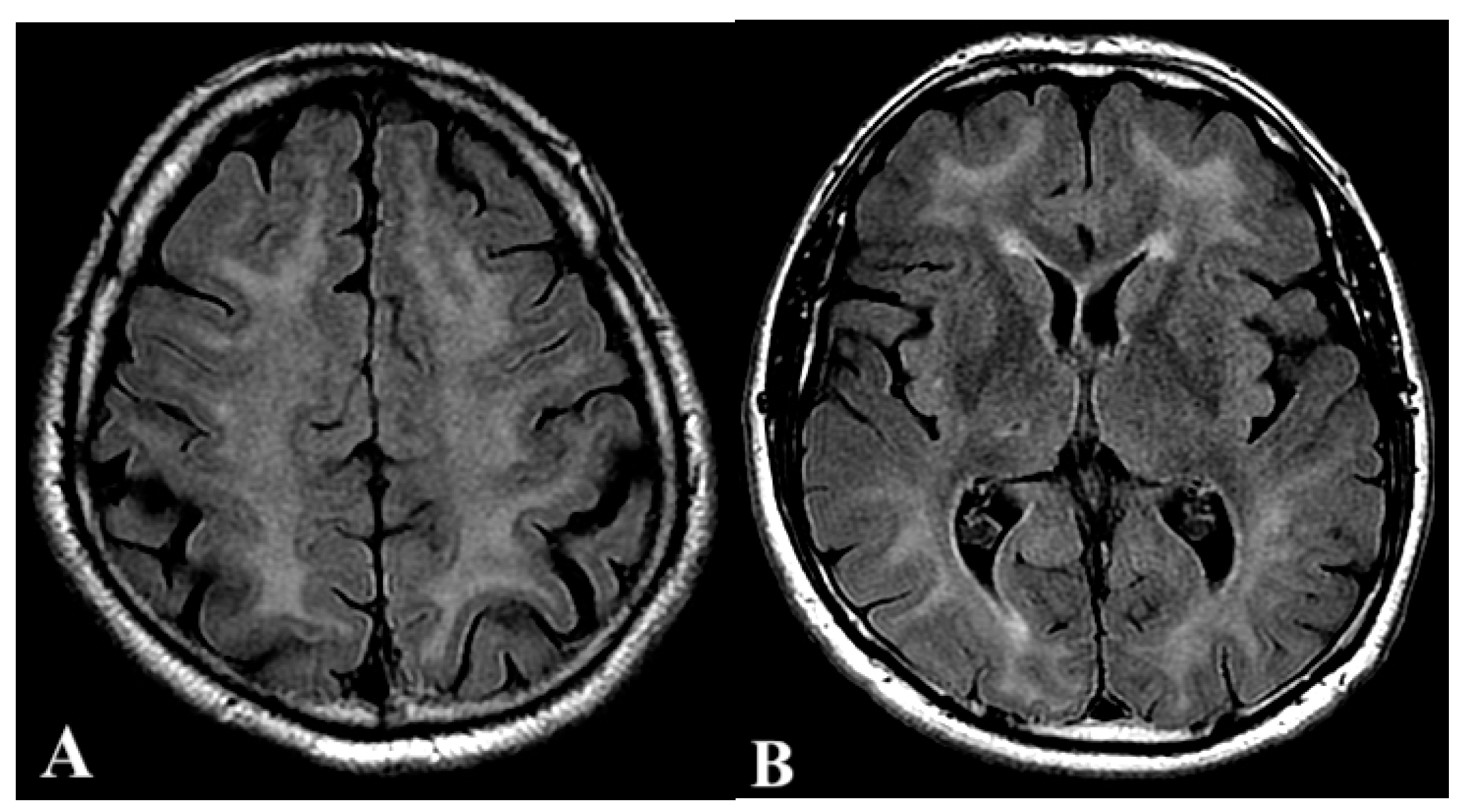
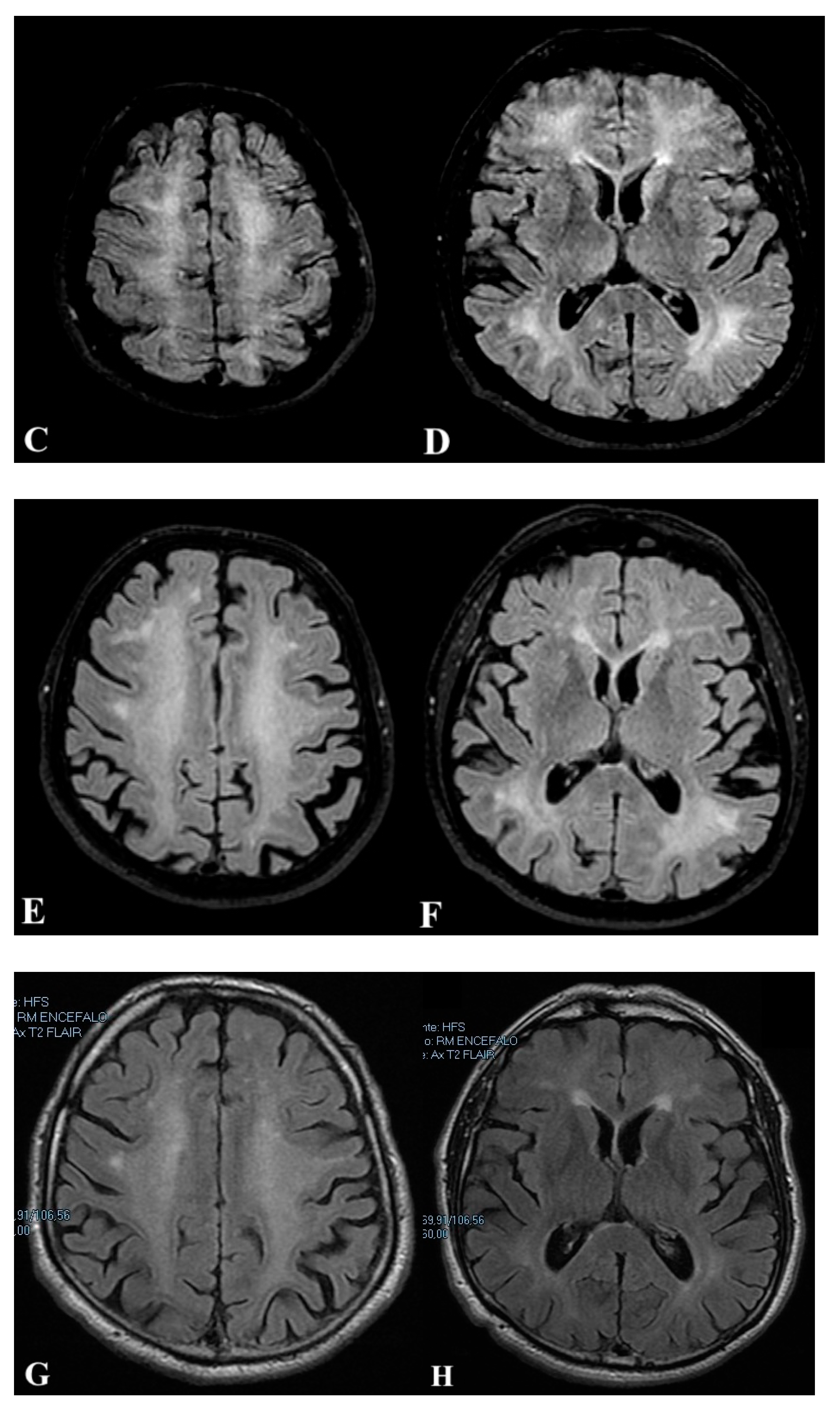
2.1. First Case
2.2. Second Case
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hampson, N.B.; Weaver, L.K. Carbon Monoxide Poisoning: A New Incidence for an Old Disease. Undersea Hyperb. Med. 2007, 34, 163–168. [Google Scholar]
- Hampson, N.B. U.S. Mortality Due to Carbon Monoxide Poisoning, 1999-2014. Accidental and Intentional Deaths. Ann. Am. Thorac. Soc. 2016, 13, 1768–1774. [Google Scholar] [CrossRef]
- Hampson, N.B.; Hauff, N.M. Carboxyhemoglobin Levels in Carbon Monoxide Poisoning: Do They Correlate with the Clinical Picture? Am. J. Emerg. Med. 2008, 26, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Hampson, N.B.; Piantadosi, C.A.; Thom, S.R.; Weaver, L.K. Practice Recommendations in the Diagnosis, Management, and Prevention of Carbon Monoxide Poisoning. Am. J. Respir. Crit. Care Med. 2012, 186, 1095–1101. [Google Scholar] [CrossRef]
- Hampson, N.B.; Hauff, N.M. Risk Factors for Short-Term Mortality from Carbon Monoxide Poisoning Treated with Hyperbaric Oxygen. Crit. Care Med. 2008, 36, 2523–2527. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Taber, R.L.; Mendiguren, I.I.; Clark, J.M.; Hardy, K.R.; Fisher, A.B. Delayed Neuropsychologic Sequelae After Carbon Monoxide Poisoning: Prevention by Treatment With Hyperbaric Oxygen. Ann. Emerg. Med. 1995, 25, 474–480. [Google Scholar] [CrossRef]
- Weaver, L.K.; Hopkins, R.O.; Chan, K.J.; Churchill, S.; Elliott, C.G.; Clemmer, T.P.; Orme, J.F.; Thomas, F.O.; Morris, A.H. Hyperbaric Oxygen for Acute Carbon Monoxide Poisoning. N. Engl. J. Med. 2002, 347, 1057–1067. [Google Scholar] [CrossRef]
- Rose, J.J.; Wang, L.; Xu, Q.; McTiernan, C.F.; Shiva, S.; Tejero, J.; Gladwin, M.T. Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy. Am. J. Respir. Crit. Care Med. 2017, 195, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Maffi, L.; Paganini, M.; Vezzani, G.; Soumelis, A.; Group, A.R.; Camporesi, E.M.; Bosco, G. Hyperbaric Oxygen Treatment for Carbon Monoxide Poisoning in Italy: Retrospective Validation of a Data Collection Tool for the Italian Registry of Carbon Monoxide Poisonings (IRCOP). Int. J. Environ. Res. Public Health 2020, 17, 574. [Google Scholar] [CrossRef] [PubMed]
- Namgung, M.; Oh, J.; Ahn, C.; Kim, C.W.; Lee, H.; Kang, H. Association between Glasgow Coma Scale in Early Carbon Monoxide Poisoning and Development of Delayed Neurological Sequelae: A Meta-Analysis. JPM 2022, 12, 635. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Milovanova, T.M.; Hardy, K.R.; Logue, C.J.; Lambert, D.S.; Troxel, A.B.; Ballard, K.; Eisinger, D. Plasma Biomarkers in Carbon Monoxide Poisoning. Clin. Toxicol. 2010, 48, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Messier, L.D.; Myers, R.A.M. A Neuropsychological Screening Battery for Emergency Assessment of Carbon-Monoxide-Poisoned Patients. J. Clin. Psychol. 1991, 47, 675–684. [Google Scholar] [CrossRef]
- Yang, S.; Liu, H.; Peng, Q.; Li, J.; Liu, Q. Predicting Scale of Delayed Neuropsychiatric Sequelae in Patients with Acute Carbon Monoxide Poisoning: A Retrospective Study. Am. J. Emerg. Med. 2022, 52, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, T.; Tsuda, M.; Kato, M.; Saito, F.; Kamijo, Y.; Kinoshita, T. Risk Factors for the Delayed Onset of Neuropsychologic Sequelae Following Carbon Monoxide Poisoning. Acute Med. Surg. 2016, 3, 315–319. [Google Scholar] [CrossRef]
- Ning, K.; Zhou, Y.-Y.; Zhang, N.; Sun, X.-J.; Liu, W.-W.; Han, C.-H. Neurocognitive Sequelae after Carbon Monoxide Poisoning and Hyperbaric Oxygen Therapy. Med. Gas Res. 2020, 10, 30. [Google Scholar] [CrossRef]
- Marcinkowska, A.B.; Mankowska, N.D.; Kot, J.; Winklewski, P.J. Impact of Hyperbaric Oxygen Therapy on Cognitive Functions: A Systematic Review. Neuropsychol. Rev. 2022, 32, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Nah, S.; Choi, S.W.; Kim, G.W.; Lee, Y.H.; Moon, J.E.; Han, S. Objective Predictors of Delayed Neurological Sequelae in Patients with Altered Mental Status after Carbon Monoxide Poisoning. Undersea Hyperb. Med. 2021, 49, 569–577. [Google Scholar]
- Thom, S.R.; Bhopale, V.M.; Fisher, D.; Zhang, J.; Gimotty, P. Delayed Neuropathology after Carbon Monoxide Poisoning Is Immune-Mediated. Proc. Natl. Acad. Sci. USA 2004, 101, 13660–13665. [Google Scholar] [CrossRef] [PubMed]
- Betterman, K.; Patel, S. Neurologic Complications of Carbon Monoxide Intoxication. Handb. Clin. Neurol. 2014, 120, 971–979. [Google Scholar] [CrossRef]
- Geraldo, A.F.; Silva, C.; Neutel, D.; Neto, L.; Albuquerque, L. Delayed Leukoencephalopathy after Acute Carbon Monoxide Intoxication. Radiol. Case 2014, 8, 1–8. [Google Scholar] [CrossRef]
- Mizuno, Y.; Sakurai, Y.; Sugimoto, I.; Ichinose, K.; Ishihara, S.; Sanjo, N.; Mizusawa, H.; Mannen, T. Delayed Leukoencephalopathy after Carbon Monoxide Poisoning Presenting as Subacute Dementia. Intern. Med. 2014, 53, 1441–1445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Guo, Y.; Li, W.; Li, G.; Chen, Y. The Efficacy of N-Butylphthalide and Dexamethasone Combined with Hyperbaric Oxygen on Delayed Encephalopathy After Acute Carbon Monoxide Poisoning. DDDT 2020, 14, 1333–1339. [Google Scholar] [CrossRef]
- Wolf, S.J.; Lavonas, E.J.; Sloan, E.P.; Jagoda, A.S. Clinical Policy: Critical Issues in the Management of Adult Patients Presenting to the Emergency Department with Acute Carbon Monoxide Poisoning. Ann. Emerg. Med. 2008, 51, 138–152. [Google Scholar] [CrossRef] [PubMed]
- De Iaco, G.; Infascelli, R.M.; Nasole, E.; Zanon, V. Linee Guida 2015. SIMSI. Available online: https://simsi.it/linee-guida/ (accessed on 26 April 2022).
- Lo Pardo, D.; Amedola, D.; Senatore, G.; Damiano, A.; Pezzuti, G.; Pugliese, N.; Locatelli, G.; Siani, A.; Vitola, N.M. Delayed Neuropsychiatric Syndrome after Carbon Monoxide Poisoning: Inclusion of Hyperbaric Oxygen Therapy in the Recovery Protocol. Emerg. Care J. 2016, 1. [Google Scholar] [CrossRef][Green Version]
- Wang, W.; Cheng, J.; Zhang, J.; Wang, K. Effect of Hyperbaric Oxygen on Neurologic Sequelae and All-Cause Mortality in Patients with Carbon Monoxide Poisoning: A Meta-Analysis of Randomized Controlled Trials. Med. Sci. Monit. 2019, 25, 7684–7693. [Google Scholar] [CrossRef] [PubMed]
- Akyol, S.; Erdogan, S.; Idiz, N.; Celik, S.; Kaya, M.; Ucar, F.; Dane, S.; Akyol, O. The Role of Reactive Oxygen Species and Oxidative Stress in Carbon Monoxide Toxicity: An in-Depth Analysis. Redox Rep. 2014, 19, 180–189. [Google Scholar] [CrossRef]
- Spina, V.; Tomaiuolo, F.; Celli, L.; Bonfiglio, L.; Cecchetti, L.; Carboncini, M.C. A Case of Carbon Monoxide-Induced Delayed Neurological Sequelae Successfully Treated with Hyperbaric Oxygen Therapy, N-Acetylcysteine, and Glucocorticoids: Clinical and Neuroimaging Follow-Up. Case Rep. Neurol. Med. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Xue, H.; Wang, B.; Li, Y.; Zhang, J.; Jiang, C.; Liang, F.; Pang, J.; Yu, L. Combined Application of Dexamethasone and Hyperbaric Oxygen Therapy Yields Better Efficacy for Patients with Delayed Encephalopathy after Acute Carbon Monoxide Poisoning. DDDT 2017, 11, 513–519. [Google Scholar] [CrossRef]
- Keim, L.; Koneru, S.; Ramos, V.; Murr, N.; Hoffnung, D.S.; Murman, D.L.; Cooper, J.S.; Torres-Russotto, D. Hyperbaric oxygen for late sequelae of carbon monoxide poisoning enhances neurological recovery: Case report. Undersea Hyperb. Med. 2018, 45, 83–87. [Google Scholar] [CrossRef]
- Fineschi, V.; Agricola, E.; Baroldi, G.; Bruni, G.; Cerretani, D.; Mondillo, S.; Parolini, M.; Turillazzi, E. Myocardial Findings in Fatal Carbon Monoxide Poisoning: A Human and Experimental Morphometric Study. Int. J. Leg. Med. 2000, 113, 276–282. [Google Scholar] [CrossRef]
- Mizrak, B.; Celbiş, O.; Parlakpinar, H.; Olmez, E. Effect of melatonin and atenolol on carbon monoxide cardiotoxicity: An experimental study in rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 565–568. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martani, L.; Giovanniello, A.; Bosco, G.; Cantadori, L.; Calissi, F.; Furfaro, D.; Pedrazzini, M.; Vaschetto, R.; Camporesi, E.M.; Paganini, M. Delayed Neurological Sequelae Successfully Treated with Adjuvant, Prolonged Hyperbaric Oxygen Therapy: Review and Case Report. Int. J. Environ. Res. Public Health 2022, 19, 5300. https://doi.org/10.3390/ijerph19095300
Martani L, Giovanniello A, Bosco G, Cantadori L, Calissi F, Furfaro D, Pedrazzini M, Vaschetto R, Camporesi EM, Paganini M. Delayed Neurological Sequelae Successfully Treated with Adjuvant, Prolonged Hyperbaric Oxygen Therapy: Review and Case Report. International Journal of Environmental Research and Public Health. 2022; 19(9):5300. https://doi.org/10.3390/ijerph19095300
Chicago/Turabian StyleMartani, Luca, Andrea Giovanniello, Gerardo Bosco, Luca Cantadori, Francesca Calissi, Dany Furfaro, Massimo Pedrazzini, Rosanna Vaschetto, Enrico Mario Camporesi, and Matteo Paganini. 2022. "Delayed Neurological Sequelae Successfully Treated with Adjuvant, Prolonged Hyperbaric Oxygen Therapy: Review and Case Report" International Journal of Environmental Research and Public Health 19, no. 9: 5300. https://doi.org/10.3390/ijerph19095300
APA StyleMartani, L., Giovanniello, A., Bosco, G., Cantadori, L., Calissi, F., Furfaro, D., Pedrazzini, M., Vaschetto, R., Camporesi, E. M., & Paganini, M. (2022). Delayed Neurological Sequelae Successfully Treated with Adjuvant, Prolonged Hyperbaric Oxygen Therapy: Review and Case Report. International Journal of Environmental Research and Public Health, 19(9), 5300. https://doi.org/10.3390/ijerph19095300








