Abstract
Over 300 million patients with coronavirus disease 2019 (COVID-19) have been reported worldwide since the outbreak of the pandemic in Wuhan, Hubei Province, China. COVID-19 is induced by the acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The effect of SARS-CoV-2 infection on the male reproductive system is unclear. The aim of this review is to assess the effect of SARS-CoV-2 infection on male fertility and the impact of possible mediators, such as metabolic, oxidative and psychological stress. SARS-CoV-2 infection aggravates metabolic stress and directly or indirectly affects male fertility by reducing seminal health. In addition, SARS-CoV-2 infection leads to excessive production of reactive oxygen species (ROS) and increased psychological distress. These data suggest that SARS-CoV-2 infection reduces male fertility, possibly by means of metabolic, oxidative and psychological stress. Therefore, among other consequences, the possibility of COVID-19-induced male infertility should not be neglected.
1. Introduction
Since the outbreak of the coronavirus disease 2019 (COVID-19) pandemic in Wuhan, Hubei Province, China, over 300 million cases have been reported worldwide [1,2]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, is an RNA virus and a member of the beta-coronavirus family consisting of 29,891 nucleotides and 9860 amino acids [3]. The virus, which has a diameter of 60–140 nm, acquired this name from its crown-like shape [4]. Virus transmission among humans occurs either through direct contact or with airborne transmission through aerosols. The most common ways of spread are coughing, sneezing, or oral, nasal or eye membrane contact [5]. The most common clinical presentation of COVID-19 includes fatigue, cough and fever. Less often, patients complain of headache, dizziness or gastrointestinal symptoms such as nausea, vomiting and abdominal pain. Other symptoms are anosmia and ageusia [6]. Age, obesity, comorbidities and indicators of organ dysfunction are risk factors for increased COVID-19 severity [7]. Furthermore, severe disease and fatality are more prevalent in males compared with females [8,9,10]. Consequently, the reproductive hormones have been implicated in SARS-CoV-2 infection pathophysiology.
Semen quality deteriorates immediately after the SARS-CoV-2 infection. These effects on semen quality may be the direct effect of the virus on semen or an indirect effect of the cytokine storm, which is induced by the infection and the increased oxidative stress [11].
All types of stress (metabolic, oxidative, psychological) affect male fertility adversely [12,13]. Infertility is defined as failure to achieve a clinical pregnancy after 12 months of unprotected sexual intercourse and affects 8–12% of couples worldwide. It is estimated that males are solely responsible for 20–30% of infertility cases, and they contribute to 50% of all cases. There are several causes of infertility that affect both sexes, such as hypogonadotrophic hypogonadism, hyperprolactinemia, infections, systemic diseases, cystic fibrosis and, in some cases, lifestyle factors. However, some causes of infertility affect males only, such as testicular deficiencies and semen deterioration due to aging and endocrine disruptors [14]. To understand the pathophysiology of male infertility, knowledge of the testicular structure is necessary. The testes are oval-shaped structures, covered by the tunica vaginalis, the outer layer, and the tunica albuginea, the inner layer. The testes consist of lobules that contain seminiferous tubules. The tubular epithelium consists of spermatogenic and Sertoli cells; the Leydig cells are located in the interstitial space among the seminiferous tubules [15]. Vascular endothelial and perivascular cells, as well as immune cells (mostly macrophages), are also present in the interstitial space [16]. Sperm is released by the Sertoli cells into the lumen of the seminiferous tubules for transport towards the epididymides. The latter, located on the posterior testicular margin, are connected to the vas deferens [17]. The epididymides are responsible for sperm maturation. Following this process, sperm is forwarded towards the seminal vesicles, a pair of glands behind the urinary bladder which secrete alkaline fluid that partly composes the semen. The prostate gland also produces fluid that, combined with the output of the seminal vesicles and the sperm, forms the semen [18]. Male infertility and male functional (non-classical) hypogonadism are often diagnosed during the investigation of couple infertility; they have been associated with oxidative and psychological stress as well as metabolic disorders (metabolic stress) [19].
Based on the hypothesis that SARS-CoV-2 infection impairs male fertility by increasing oxidative, metabolic and psychological stress, this manuscript aims to assess the impact of COVID-19 on male infertility (via the effects on testosterone secretion or semen parameters) and study the contribution of various types of stress as possible mediators (Figure 1).
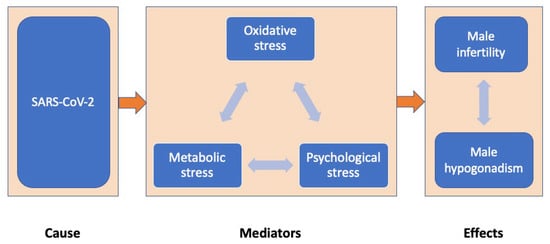
Figure 1.
The impact of COVID-19 on male reproduction (via effects on testosterone secretion or semen parameters) through oxidative, metabolic and psychological stress mediators.
2. SARS-CoV-2 and Male Infertility
2.1. SARS-CoV-2 and Testosterone
Although androgens, such as testosterone (T) and dihydrotestosterone (DHT), are produced in both sexes, their concentrations in males are approximately ten times those of females. The testes and the adrenal cortex secrete T, an anabolic hormone. After the age of 40 years, T serum concentrations decrease approximately 2% per year, leading to a 9.5% increase in hypogonadism in men over 40 years of age [20]. Νormal T production is inextricably linked to normal hypothalamic–pituitary–gonadal axis function. SARS-CoV-2 crosses the blood–brain barrier and results in neuroinflammation through the infected angiotensin-converting enzyme 2 (ACE2)–expressing cells. Hormonal imbalance could manifest due to this inflammation [21]. Male hypogonadism has been correlated with poor prognosis and mortality in patients with COVID-19 admitted to intensive care units [22]. Low T concentrations in men represent a risk factor for high morbidity and mortality [23]. More specifically, among 89 patients with COVID-19, 30 patients with non-COVID-19 respiratory tract infections and 143 age-matched controls, serum T concentrations were 186, 289 and 332 ng/dL, respectively (median values, p < 0.0001) [21]. Recently, a comparison between 286 patients with COVID-19 and 281 healthy controls showed lower T concentrations in the former (p < 0.0001) [24]. Another study reported that T concentrations fluctuated between patients with COVID-19 of varying severity: patients with more severe disease showed lower T concentrations compared with patients with disease of mild-to-moderate severity [median (range) 85.1 (0.21–532) vs. 315 (0.88–486) ng/dL, p < 0.001, respectively). When the T concentrations of patients with COVID-19 who were admitted to the intensive care unit were compared with those who did not need admission, the results were similar [median (range) 64.0 (0.21–337) vs. 286 (0.88–532) ng/dL, p < 0.001, respectively). Differences in T concentrations were also demonstrated between patients with COVID-19 who died and survivors [median (range) 82.9 (2.63–165) vs. 166 (0.21–532) ng/dL, p < 0.001, respectively) [25]. Another study confirmed the above results, where patients with severe COVID-19 had lower T concentrations (1.4 ng/mL) compared with patients with mild COVID-19 (3.5 ng/mL) (p = 0.005) [26]. While T concentrations are lower in males with more severe COVID-19 disease than those with milder disease, there are no differences in women with severe and mild COVID-19 disease [27]. Among hospitalized men with COVID-19, those with T concentrations <100 ng/dL had higher mortality risk [odds ratio (OR) 18.2, 95% confidence interval (CI) 2.3–144.6, p = 0.006] than those with T concentrations >230 ng/dL [28]. It is of note that T exerts biological actions other than those of reproduction, playing, inter alia, an anti-inflammatory role. Obesity and low T concentrations could trigger the cytokine storm that leads to the deterioration of COVID-19 patients’ clinical conditions [29]. Moreover, it is speculated that SARS-CoV-2 infection per se could rapidly cause depletion of androgenic action, resulting in severe or even fatal disease [30]. Further studies are needed to clarify whether T replacement therapy (TRT) could be beneficial in severely hypogonadal men with COVID-19 [31]. Furthermore, the SARS-CoV-2 spike protein exacerbates endothelial injury when the virus interacts with DHT [32,33].
2.2. SARS-CoV-2 and Semen
More than 20 viruses affecting fertility have been found in human semen [34]. However, as SARS-CoV-2 was not detected in prostatic secretions, semen or testes, virus transmission via the genital tract appears unlikely [35,36]. Prospective observational studies that assessed the semen 6–75 days after COVID-19 diagnosis failed to detect SARS-CoV-2 [37]. A study of 18 patients with COVID-19 demonstrated no presence of SARS-CoV-2 in semen during the acute or convalescent phase of COVID-19 [38]. Another study of 11 consecutive men with mean age 29.7 ± 4.5 recovering from COVID-19 proved that there was no presence of SARS-CoV-2 in semen by using reverse transcription polymerase chain reaction (RT-PCR). The samples were collected 19–59 days (median 44 days) after the positive test for SARS-CoV-2. Patients declared that there was no history of epididymo-orchitis or sexual dysfunction at the time of inclusion [39]. In 70 semen samples of patients with COVID-19, there was no detection of the viral nucleic acid; however, there was a deterioration in total sperm count and motility compared with healthy controls. Moreover, there was an association between recovery duration and semen parameters: the longer the recovery period, the worse the sperm quality [40]. Contradicting these results, one study reported several cases in which SARS-CoV-2 was detected in semen during the acute and the recovery phase of COVID-19 [41], implying potential sexual transmission. Another study of 32 men with COVID-19 revealed only one patient with SARS-CoV-2 in a semen sample [39]. The possibility that this rare finding was caused by contamination cannot be ruled out [42]. Saylem et al. evaluated the semen of 30 patients one day following their diagnosis with COVID-19 and were able to detect SARS-CoV-2 in four patients. In this study, all patients were advised to wash their hands and use masks prior to and during masturbation to avoid contamination [43]. To conclude, the evidence for the presence of SARS-CoV-2 in semen has been provided by case-control studies mainly, while no research has been conducted to assess the semen before and after SARS-CoV-2 infection.
The testes of patients with COVID-19 display seminiferous tubular injury, Sertoli cell swelling, reduced Leydig cell number and mild lymphocytic inflammation [44,45]. More specifically, in microscopic examination of the testes from 12 deceased COVID-19 patients, Sertoli cell swelling, vacuolation and cytoplasmic rarefaction and detachment from tubular basement membranes were observed. Moreover, in COVID-19 patients’ testes, there were lower Leydig cell numbers compared with deceased individuals without COVID-19 (2.2 vs. 7.8, p < 0.001). There was also edema and mild inflammation with T-lymphocyte and histiocyte infiltration in the interstitium. The presence of the virus was confirmed by RT-PCR in only 1 of 12 cases [46]. A small study showed several spermatogenesis abnormalities in 50% of autopsy specimens from men who died of COVID-19 [47]. Studies in humans have reported that infection with SARS-CoV-2 results in a deterioration of seminal parameters such as total sperm number (millions/ejaculation) and sperm concentration (millions/mL) [37]. Summarizing the evidence, it is more likely for SARS-CoV-2 exerts a direct effect on spermatogenesis rather than an interaction with sperm in semen.
The wide distribution of ACE2 receptors in the testes enable virus entry into the cells and its replication, together with ACE2 mRNA in spermatogonia, seminiferous duct cells, Leydig cells and Sertoli cells [48]. ACE2 receptors are differently distributed and regulated in men and women, with their expression being age-dependent, reaching the highest levels at around 30 years of age and the lowest at 60 years, in both sexes [49]. A new method was developed using a short synthetic peptide which targets ACE2 in human serum. This peptide connects to ACE2 from the serum and forms a protein complex that can be detected by electrochemical assays. In this way, the viral load of SARS-CoV-2 could be quantified, as the human body increases ACE2 serum concentrations to minimize the entry of the virus into the cells [50]. Therefore, as indicated by some recent clinical reports, serum ACE2 may serve as an index of viral load and its associated complications. Presence of ACE2 in the testes is more frequent in infertility, and infertile men are more susceptible to viral infection compared to fertile men [48,51].
A trial including 120 patients with a history of SARS-CoV-2 infection reported decreased semen parameters post-SARS-CoV-2 infection [52], while semen quality was restored 2 months after the infection. The above process is thought to be immunologically mediated, as increased anti-sperm IgA and IgG concentrations have been detected in the semen [52]. Another trial of 43 male patients with proven recovery from COVID-19 (age range 30–64 years) showed an increased risk of developing oligo- or cryptozoospermia [53]. Apart from COVID-19 per se, fever is a well-established risk factor that can impair sperm parameters. Fever leads to increased temperature in the testes that can deteriorate sperm quality. The detrimental effects of fever in the semen appear 74 days after the onset of fever (one spermatogenesis cycle) [54]. Commonly used medications (e.g., antibiotics, steroids) [55] in cases of SARS-CoV-2 infection may have a negative impact on male hormonal profiles and reproductive potential [56]. For example, steroids interfere with the hypothalamic–pituitary–gonadal axis [57].
More studies evaluating the semen of patients before and after the infection are needed to clarify the impact of SARS-CoV-2 on semen, with special focus on potential long-term effects and the reversibility of semen parameters [58]. Despite yet inconclusive data, it is being debated whether semen cryopreservation should be considered during the pandemic, given that SARS-CoV-2 infection is highly likely to lead to long-term detrimental effects on semen [59]. The Italian Society of Andrology and Sexual Medicine (SIAMS) suggests that patients recovered from COVID-19, especially those of reproductive age, should undergo a gonadal function evaluation, including a semen analysis [60].
Recently, a concern was expressed about the possible negative effect of COVID-19 vaccination on male fertility. Regarding the safety of COVID-19 vaccination, the two mRNA vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), were studied regarding their impact on semen parameters. In one study, semen parameters were assessed before and after the COVID-19 vaccination in 45 healthy volunteers (age range 18–50 years) [61]. No decrease was observed in any semen parameter after two doses of COVID-19 mRNA vaccine. Similarly, another study including 43 men after vaccination with BNT162b2 showed no decrease in semen parameters [46]. It was suggested that the mRNA vaccine, which does not contain the live virus, would be unlikely to affect semen parameters. According to a statement from the Society for Male Reproduction and Urology in 2021, there is no definitive data to support this association [61]. A study designed to assess the semen parameters of infertile men demonstrated no impact of vaccinations on semen parameters or reproductive outcomes after assisted reproduction technology (ART) [62], regardless the vaccination type (mRNA vaccines or viral vector). Similar results were reported in studies involving healthy volunteers [63] or fertile men [64].
3. Possible Mediators of SARS-CoV-2 Impact on Male Infertility
3.1. Oxidative Stress
The pathophysiology of respiratory virus infections (including SARS-CoV-2) involves inflammation, cytokine production and cell death, with the latter associated with reactive oxygen species (ROS) overproduction and increased oxidative stress. ROS overproduction in SARS-CoV-2 triggers the nuclear factor kappa-light-chain-enhancer of the activated B-cell (NF-κB)-toll-like receptor pathway [65]. This mechanism further increases the release of cytokines, leading to inflammatory response exaggeration that may have a negative impact on male fertility [66]. Moreover, cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), have been associated with reduced semen characteristics [67]. The major role of oxidative stress in inducing male infertility is illustrated by the term “Male Oxidative Stress Infertility (MOSI)”, to be used when male infertility is caused by oxidative stress [68].
Recently, autopsy-based studies provided evidence that SARS-CoV-2 infection is associated with increased oxidative stress leading to testicular cell apoptosis, while severe COVID-19 illness was associated with decreased interstitial tissue volume and seminiferous tubuli length [69]. According to a prospective cohort study, including 84 patients with COVID-19 and 105 healthy controls, the COVID-19 group had higher ROS concentrations in semen, which were over 1000 relative light units higher (RLU/s/106 sperm) than the control group (400 RLU) throughout the study (p < 0.05). However, there was a decrease in ROS concentrations in the 60-day follow-up in the COVID-19 group (from >1000 to 700 RLU, p < 0.05); no change was observed in the control group [70]. Furthermore, ROS concentrations in semen samples diminished by 55.5% (p < 0.001) at 4 months after COVID-19 diagnosis compared with samples collected at the 14th day of infection [71].
The renin–angiotensin–aldosterone system (RAAS) plays a critical role in the pathogenesis of vascular disease and COVID-19. The SARS-CoV-2 enters the host cells through the ACE2 receptor cleavage by transmembrane protease, serine-2 (TMPRSS2). In addition, angiotensin II increase thrombin generation, possibly via a direct impact on tissue factors [72]. Interestingly, the testes have high concentrations of ACE2 mRNA and protein compared with other tissues in humans [73,74] (Figure 2). There is evidence that angiotensin II augments the production of ROS [72]. This mechanism leads to the generation of a proinflammatory phenotype in endothelial and vascular smooth muscle cells by the upregulation of adhesion molecules, chemokines and cytokines.

Figure 2.
The distribution of angiotensin-converting enzyme-2 receptors (ACE2r) throughout the male body. Ιn red scale, the 5 organs (starting with small intestine and testes) with the widest distribution of ACE2r (Ref. [74]). A large presence of ACE2r is also found in adipose tissue, the breast and colon (not shown).
The severity of disease, quantified by leukocyte count and C-reactive protein (CRP) concentrations, does not correlate with the production of oxidant and antioxidant defenses (hydrogen peroxide, total antioxidant capacity, reduced oxidized glutathione) and oxidative damage (malondialdehyde, carbonyl, sulfhydryl). Severely ill patients with high serum leukocyte counts and CRP concentrations may not be a decisive factor for changes in the redox profile [75].
3.2. Metabolic Stress
SARS-CoV-2 infection can lead to increased metabolic stress, while, inversely, metabolic stress is associated with increased vulnerability to SARS-CoV-2 infection. SARS-CoV-2 infection results in earlier development or worsening of type 2 diabetes mellitus (T2DM) [76]. The infection is characterized by multiple immunological complications (e.g., redox storm, cytokine storm) that may lead to β-cell impairment and apoptosis, islet capillary rarefaction, hypoxia and abnormal remodeling [77]. In turn, T2DM may cause infertility and functional hypogonadism in males, along with sexual dysfunction, such as erectile and ejaculatory dysfunction, impaired semen parameters and delayed puberty. Similarly, obesity increases the risk of several disorders and diseases, including T2DM, sleep apnea, cardiovascular risk and thrombosis, contributing to increased metabolic stress. While obesity has been linked to abnormal semen parameters, its overall effect on hormone concentrations or sperm DNA integrity varies, probably due to different pathogenetic pathways involved in obesity and gonadal function [78]. Obesity, associated in the past with high risk for severe H1N1 influenza virus infection, is now regarded a risk factor for admission to intensive care units and mechanical ventilation in SARS-CoV-2-affected patients [79].
3.3. Psychological Stress
The prevalence of psychological stress and anxiety was increased even before the COVID-19 era [80]. SARS-CoV-2 infection is typically accompanied by severe psychological stress. A case–control study of 11,923,499 individuals in the UK verified that patients with a positive PCR test were presented with increased risk of psychiatric morbidity, fatigue and sleep problems in the following period [81]. A cross-sectional, single-centered study on infertile men coping with emotional reactions due to no access to fertility clinics due to COVID-19 revealed increased levels of stress, worry and frustration [81].
SARS-CoV-2 infection has a direct impact on cognitive function that can lead to “brain fog”, causing changes to the development and/or functioning of the central nervous system (CNS) [82]. This phenomenon is known as “silent” or “happy hypoxia” [83]. This is a form of hypoxia, usually without symptoms, which is due to a response to hyperventilation and dyspnea, which is inadequate for the increased CNS demands for oxygen. In this scenario, the impaired mitochondrial function fails to respond to the hypoxic microenvironment, and CNS changes are thus observed.
Psychological stress per se has been linked to several conditions and diseases, including hypertension, cardiovascular disease, obesity, depression, and mortality. In addition, it may adversely affect male fertility. Of note, psychological stress may lead to a cytokine storm that in turn can induce oxidative stress. In mouse models, chronic unpredictable stress induces disruptions in the blood–testis barrier and compromised semen characteristics [84]. Recently, male mice underwent chronic-restraint stress for 3 months in conical centrifuge tubes; during the stress period, mice were placed in boxes with attenuated sound and light. The application of stress to the father led to inherited reproductive disorders in the offspring, such as impaired semen parameters and infertility [85].
Psychological stress developed during the pandemic may have lead to a delay in attempting fertility. In an Italian study, one-third of infertile couples desiring fertility before the pandemic was found to have changed their decision during the lockdowns [86]. Precise numerical data on the overall impact of COVID-19 on fertility among various populations will not be available until some time has passed [87]. Anyway, quarantines and other restricting measures that were widely applied in several countries during the COVID-19 pandemic resulted in high rates of sexual dysfunction and reductions in sexual activity; the effect seems to be higher in women than men [88]. Sexual dysfunction could lead to male infertility. One out of six men of infertile couples has erectile dysfunction or premature ejaculation, and one out of ten has orgasmic dysfunction [89]. Furthermore, reduced intercourse frequency has a negative impact on couple fecundity [90]. It can be concluded that if a person’s psychological condition improves, the frequency of sexual intercourse is likely to increase, resulting in an increase in fertility.
4. Conclusions
There is increasing evidence of a possible adverse effect of SARS-CoV-2 infection on male fertility by simultaneously elevating metabolic, oxidative and psychological stress. If this hypothesis is confirmed, patients with underlying diseases, particularly those with T2DM and arterial hypertension, would be more likely to present with gonadal injury after SARS-CoV-2 and should be counseled accordingly. Most importantly, as RAAS is a key player in SARS-CoV-2 infection and its gonadal complications, an investigation into whether the use of RAAS blockers could result in a reduction in the SARS-CoV2 impact on male patients’ gonadal function would be of high clinical importance.
Author Contributions
Conceptualization: G.M., D.G.G. and L.H.D.; methodology: G.M., D.G.G. and L.H.D.; writing—original draft preparation: G.M. and S.V.; writing—review and editing: D.G.G. and L.H.D.; supervision: D.G.G. and L.H.D. All authors have read and agreed to the published version of the manuscript.
Funding
The implementation of this postdoctoral research/work is co-financed by Greece and the European Union (European Social Fund) through the Operational Program “Human Resources Development, Education and Lifelong Learning”, in the framework of the Act “Support of Postgraduate Researchers—B cycle” (MIS 5033021), implemented by the State Scholarships Foundation (IKY).
Institutional Review Board Statement
This article does not contain any studies with human participants performed by any of the authors.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsang, H.F.; Chan, L.W.C.; Cho, W.C.S.; Yu, A.C.S.; Yim, A.K.Y.; Chan, A.K.C.; Ng, L.P.W.; Wong, Y.K.E.; Pei, X.M.; Li, M.J.W.; et al. An update on COVID-19 pandemic: The epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev. Anti-Infect. Ther. 2021, 19, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Max, R. COVID-19 Cases throughout the World. 2022. Available online: https://ourworldindata.org/covid-cases (accessed on 1 March 2022).
- Tabatabaeizadeh, S.A. Airborne transmission of COVID-19 and the role of face mask to prevent it: A systematic review and meta-analysis. Eur. J. Med. Res. 2021, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Amirfakhryan, H.; Safari, F. Outbreak of SARS-CoV2: Pathogenesis of infection and cardiovascular involvement. Hell. J. Cardiol. 2021, 62, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Abrahao-Machado, L.F.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Gallo Marin, B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Bienvenu, L.A.; Noonan, J.; Wang, X.; Peter, K. Higher mortality of COVID-19 in males: Sex differences in immune response and cardiovascular comorbidities. Cardiovasc. Res. 2020, 116, 2197–2206. [Google Scholar] [CrossRef]
- Nimgaonkar, I.; Valeri, L.; Susser, E.; Hussain, S.; Sunderram, J.; Aviv, A. The age pattern of the male-to-female ratio in mortality from COVID-19 mirrors that of cardiovascular disease in the general population. Aging 2021, 13, 3190–3201. [Google Scholar] [CrossRef]
- Aitken, R.J. COVID-19 and male infertility: An update. Andrology 2022, 10, 8–10. [Google Scholar] [CrossRef]
- Evans, E.P.P.; Scholten, J.T.M.; Mzyk, A.; Reyes-San-Martin, C.; Llumbet, A.E.; Hamoh, T.; Arts, E.G.J.M.; Schirhagl, R.; Cantineau, A.E.P. Male subfertility and oxidative stress. Redox Biol. 2021, 46, 102071. [Google Scholar] [CrossRef]
- Castellini, C.; D’andrea, S.; Cordeschi, G.; Totaro, M.; Parisi, A.; Di Emidio, G.; Tatone, C.; Francavilla, S.; Barbonetti, A. Pathophysiology of mitochondrial dysfunction in human spermatozoa: Focus on energetic metabolism, oxidative stress and apoptosis. Antioxidants 2021, 10, 695. [Google Scholar] [CrossRef]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Perheentupa, A.; Huhtaniemi, I. Aging of the human ovary and testis. Mol. Cell. Endocrinol. 2009, 299, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, A.; DeFalco, T. Essential roles of interstitial cells in testicular development and function. Andrology 2020, 8, 903–914. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, M. Insights into the nature of human testicular peritubular cells. Ann. Anat.-Anat. Anz. 2009, 191, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.A. CHAP TER 5 Male Genital System. Jubb Kennedy Palmer’s Pathol. Domest. Anim. 2016, 3, 465–510.e1. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Nordkap, L.; Priskorn, L.; Hansen, Å.M.; Bang, A.K.; Holmboe, S.A.; Schmidt, L.; Jensen, T.K.; Jørgensen, N. Psychological stress, stressful life events, male factor infertility, and testicular function: A cross-sectional study. Fertil. Steril. 2020, 113, 865–875. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Faidah, H.; Alexiou, A.; Batiha, G.E.S. Testosterone in COVID-19: An Adversary Bane or Comrade Boon. Front. Cell. Infect. Microbiol. 2021, 11, 832. [Google Scholar] [CrossRef]
- Collins, A.B.; Zhao, L.; Zhu, Z.; Givens, N.T.; Bai, Q.; Wakefield, M.R.; Fang, Y. Impact of COVID-19 on Male Fertility. Urology 2022, in press. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Moulin, T.C.; Schiöth, H.B. Sex differences in COVID-19: The role of androgens in disease severity and progression. Endocrine 2021, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.A.; Leisegang, K.; Majzoub, A.; Finelli, R.; Selvam, M.K.P.; Henkel, R.; Mojgan, M.; Agarwal, A. Male fertility and the COVID-19 pandemic: Systematic review of the literature. World J. Men’s Health 2020, 38, 506. [Google Scholar] [CrossRef] [PubMed]
- Salonia, A.; Pontillo, M.; Capogrosso, P.; Gregori, S.; Tassara, M.; Boeri, L.; Carenzi, C.; Abbate, C.; Cignoli, D.; Ferrara, A.M.; et al. Severely low testosterone in males with COVID-19: A case-control study. Andrology 2021, 9, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Cinislioglu, A.E.; Cinislioglu, N.; Demirdogen, S.O.; Sam, E.; Akkas, F.; Altay, M.S.; Utlu, M.; Sen, I.A.; Yildirim, F.; Kartal, S.; et al. The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: A prospective study. Andrology 2022, 10, 24–33. [Google Scholar] [CrossRef]
- Camici, M.; Zuppi, P.; Lorenzini, P.; Scarnecchia, L.; Pinetti, C.; Cicallini, S.; Nicastry, E.; Petrosillo, N.; Palmieri, F.; D’Offizi, G.; et al. Role of testosterone in SARS-CoV-2 infection: A key pathogenic factor and a biomarker for severe pneumonia. Int. J. Infect. Dis. 2021, 108, 244–251. [Google Scholar] [CrossRef]
- Dhindsa, S.; Zhang, N.; McPhaul, M.J.; Wu, Z.; Ghoshal, A.K.; Erlich, E.C.; Mani, K.; Randolph, G.J.; Edwards, J.R.; Mudd, P.A.; et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Netw. Open 2021, 4, e2111398. [Google Scholar] [CrossRef]
- Lanser, L.; Burkert, F.R.; Thommes, L.; Egger, A.; Hoermann, G.; Kaser, S.; Pinggera, G.M.; Anliker, M.; Griesmacher, A.; Weiss, G.; et al. Testosterone Deficiency Is a Risk Factor for Severe COVID-19. Front. Endocrinol. 2021, 12, 731. [Google Scholar] [CrossRef]
- Rastrelli, G.; Di Stasi, V.; Inglese, F.; Beccaria, M.; Garuti, M.; Di Costanzo, D.; Spreafico, F.; Greco, G.F.; Cervi, G.; Pecoriello, A.; et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology 2021, 9, 88–98. [Google Scholar] [CrossRef]
- Salonia, A.; Corona, G.; Giwercman, A.; Maggi, M.; Minhas, S.; Nappi, R.E.; Sofikitis, N.; Vignozzi, L. SARS-CoV-2, testosterone and frailty in males (PROTEGGIMI): A multidimensional research project. Andrology 2021, 9, 19–22. [Google Scholar] [CrossRef]
- Al-Lami, R.A.; Urban, R.J.; Volpi, E.; Algburi, A.M.A.; Baillargeon, J. Sex Hormones and Novel Corona Virus Infectious Disease (COVID-19). Mayo Clin. Proc. 2020, 95, 1710–1714. [Google Scholar] [CrossRef]
- Kumar, N.; Zuo, Y.; Yalavarthi, S.; Hunker, K.L.; Knight, J.S.; Kanthi, Y.; Obi, A.T.; Ganesh, S.K. SARS-CoV-2 spike protein s1-mediated endothelial injury and pro-inflammatory state is amplified by dihydrotestosterone and prevented by mineralocorticoid antagonism. Viruses 2021, 13, 2209. [Google Scholar] [CrossRef] [PubMed]
- Swerdloff, R.S.; Dudley, R.E.; Page, S.T.; Wang, C.; Salameh, W.A. Dihydrotestosterone: Biochemistry, physiology, and clinical implications of elevated blood levels. Endocr. Rev. 2017, 38, 220–254. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.P.; Horby, P.W. The breadth of viruses in human semen. Emerg. Infect. Dis. 2017, 23, 1922–1924. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhao, S.; Li, W.; Wang, Y.; Li, L.; Jiang, S.; Ren, W.; Yuan, Q.; Zhang, F.; Kong, F.; et al. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology 2021, 9, 42–47. [Google Scholar] [CrossRef]
- Pan, F.; Xiao, X.; Guo, J.; Song, Y.; Li, H.; Patel, D.; Spivak, A.; Alukal, J.; Zhang, X.; Xiong, C.; et al. No evidence of SARS-CoV-2 in semen of males recovering from COVID-19. Fertil Steril 2020, 113, 1135–1139. [Google Scholar] [CrossRef]
- Best, J.C.; Kuchakulla, M.; Khodamoradi, K.; Lima, T.F.N.; Frech, F.S.; Achua, J.; Rosete, O.; Mora, B.; Arora, H.; Ibrahim, E.; et al. Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: A prospective observational study. World J. Men’s Health 2021, 39, 489–495. [Google Scholar] [CrossRef]
- Burke, C.A.; Skytte, A.; Kasiri, S.; Howell, D.; Patel, Z.P.; Trolice, M.P.; Parekattil, S.J.; Michael, S.F.; Paul, L.M. A cohort study of men infected with COVID-19 for presence of SARS-CoV-2 virus in their semen. J. Assist. Reprod. Genet. 2021, 38, 785–789. [Google Scholar] [CrossRef]
- Sharma, A.P.; Sahoo, S.; Goyal, K.; Chandna, A.; Kirubanandhan, S.; Sharma, V.; Grover, S.; Singh, M.P.; Bhalla, A.; Singh, S.K. Absence of SARS-CoV-2 infection in the semen of men recovering from COVID-19 infection: An exploratory study and review of literature. Andrologia 2021, 53, e14136. [Google Scholar] [CrossRef]
- Ruan, Y.; Hu, B.; Liu, Z.; Liu, K.; Jiang, H.; Li, H.; Li, R.; Luan, Y.; Liu, X.; Yu, G.; et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: A perspective and urogenital evaluation. Andrology 2021, 9, 99–106. [Google Scholar] [CrossRef]
- Li, D.; Jin, M.; Bao, P.; Zhao, W.; Zhang, S. Clinical Characteristics and Results of Semen Tests Among Men With Coronavirus Disease 2019. JAMA Netw. Open 2020, 3, e208292. [Google Scholar] [CrossRef]
- Delaroche, L.; Bertine, M.; Oger, P.; Descamps, D.; Damond, F.; Genauzeau, E.; Meicler, P.; Le Hingrat, Q.; Lamazou, F.; Gschwind, R.; et al. Evaluation of SARS-CoV-2 in semen, seminal plasma, and spermatozoa pellet of COVID-19 patients in the acute stage of infection. PLoS ONE 2021, 16, e0260187. [Google Scholar] [CrossRef] [PubMed]
- Saylam, B.; Uguz, M.; Yarpuzlu, M.; Efesoy, O.; Akbay, E.; Çayan, S. The presence of SARS-CoV-2 virus in semen samples of patients with COVID-19 pneumonia. Andrologia 2021, 53, e14145. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yuan, X.; Wu, L.; Guo, N.; Yin, L.; Li, Y. COVID-19 and male reproduction: Current research and unknown factors. Andrology 2021, 9, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Mao, G.; Zheng, R.; Fang, M.; Yang, X.; Wang, L.; Qi, C. Testicular injury during SARS-CoV-2 infection may be neglected: An assessment from scRNA-seq profiling and protein detection of angiotensin-converting enzyme II. Exp. Ther. Med. 2021, 22, 1485. [Google Scholar] [CrossRef]
- Yang, M.; Chen, S.; Huang, B.; Jhong, J.M.; Su, H.; Chen, Y.J.; Cao, Q.; Ma, L.; He, J.; Li, X.F.; et al. Pathological Findings in the Testes of COVID-19 Patients: Clinical Implications. Eur. Urol. Focus 2020, 6, 1124–1129. [Google Scholar] [CrossRef]
- Achua, J.K.; Chu, K.Y.; Ibrahim, E.; Khodamoradi, K.; Delma, K.S.; Iakymenko, O.A.; Kryvenko, O.N.; Arora, H.; Ramasamy, R. Histopathology and ultrastructural findings of fatal COVID-19 infections on testis. World J. Men’s Health 2020, 39, 65–74. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; Adeniji, A.A.; Cardona, W.D.; du Plessis, S.S. SARS-CoV-2 (COVID-19) and male fertility: Where are we? Reprod. Toxicol. 2021, 99, 65–70. [Google Scholar] [CrossRef]
- Radzikowska, U.; Ding, M.; Tan, G.; Zhakparov, D.; Peng, Y.; Wawrzyniak, P.; Wang, M.; Li, S.; Morita, H.; Altunbulakli, C.; et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy 2020, 75, 2829–2845. [Google Scholar] [CrossRef]
- Liu, S.; Han, L.; Li, J.; Li, H. Electrochemical detection of ACE2 as a biomarker for diagnosis of COVID-19 and potential male infertility. Biosens. Bioelectron. 2022, 198, 113788. [Google Scholar] [CrossRef]
- Shen, Q.; Xiao, X.; Aierken, A.; Yue, W.; Wu, X.; Liao, M.; Hua, J. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J. Cell. Mol. Med. 2020, 24, 9472–9477. [Google Scholar] [CrossRef]
- Donders, G.G.; Bosmans, E.; Reumers, J.; Donder, F.; Jonckheere, J.; Salembier, G.; Stern, N.; Jacqyemyn, Y.; Ombelet, W.; Depuydt, C.E. Sperm quality and absence of SARS-CoV-2 RNA in semen after COVID-19 infection: A prospective, observational study and validation of the SpermCOVID test. Fertil Steril 2020, 117, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Coppi, M.; Baldi, E.; Sebastianelli, A.; Zaccaro, C.; Morselli, S.; Pecoraro, A.; Manera, A.; Nicoletti, R.; Liaci, A.; et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum. Reprod. 2021, 36, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.; Abdelhamid, M.; Fellah, A.A.; Elmarghani, A. An Assessment of Men Semen Alterations in SARS-CoV-2: Is Fever the Principal Concern? Reprod. Sci. 2022. Published Online First. [Google Scholar] [CrossRef]
- Hayashi, T.; Miyata, A.; Yamada, T. The impact of commonly prescribed drugs on male fertility. Hum. Fertil 2008, 11, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Hamarat, M.B.; Ozkent, M.S.; Yilmaz, B.; Aksanyar, S.Y.; Karabacak, K. Effect of SARS-CoV-2 infection on semen parameters. Can. Urol. Assoc. J. 2021, 16, 173–177. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, J.; Ren, J.; Zhao, Y.; Chen, J.; Chen, X. Effect of COVID-19 on Male Reproductive System—A Systematic Review. Front. Endocrinol. 2021, 12, 677701. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A. Human semen quality as affected by SARS-CoV-2 infection: An up-to-date review. Andrologia 2021, 54, e14295. [Google Scholar] [CrossRef]
- Sengupta, P.; Leisegang, K.; Agarwal, A. The impact of COVID-19 on the male reproductive tract and fertility: A systematic review. Arab J. Urol. 2021, 19, 423–436. [Google Scholar] [CrossRef]
- Corona, G.; Baldi, E.; Isidori, A.M.; Paoli, D.; Pallotti, F.; De Santis, L.; Francavilla, F.; La Vignera, S.; Selice, R.; Caponecchia, L.; et al. SARS-CoV-2 infection, male fertility and sperm cryopreservation: A position statement of the Italian Society of Andrology and Sexual Medicine (SIAMS) (Società Italiana di Andrologia e Medicina della Sessualità). J. Endocrinol. Investig. 2020, 43, 1153–1157. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, M. COVID-19 Vaccine and Male Fertility. Urol. J. 2021, 18, 6897. [Google Scholar] [CrossRef]
- Reschini, M.; Pagliardini, L.; Boeri, L.; Piazzini, F.; Bandini, V.; Fornelli, G.; Dolci, C.; Cermisoni, G.C.; Viganò, P.; Somigliana, E.; et al. COVID-19 Vaccination Does Not Affect Reproductive Health Parameters in Men. Front. Public Health 2022, 10, 839967. [Google Scholar] [CrossRef]
- Berry, S.D.; Johnson, K.S.; Myles, L.; Herndon, L.; Montoya, A.; Fashaw, S.; Gifford, D. Lessons learned from frontline skilled nursing facility staff regarding COVID-19 vaccine hesitancy. J. Am. Geriatr. Soc. 2021, 69, 1140–1146. [Google Scholar] [CrossRef]
- Lifshitz, D.; Haas, J.; Lebovitz, O.; Raviv, G.; Orvieto, R.; Aizer, A. Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reprod. Biomed. Online 2022, 44, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. SARS-CoV-2 and Male Infertility: Possible Multifaceted Pathology. Reprod. Sci. 2021, 28, 23–26. [Google Scholar] [CrossRef]
- Haghpanah, A.; Masjedi, F.; Alborzi, S.; Hosseinpour, A.; Dehghani, A.; Malekmakan, L.; Roozbeh, J. Potential mechanisms of SARS-CoV-2 action on male gonadal function and fertility: Current status and future prospects. Andrologia 2021, 53, e13883. [Google Scholar] [CrossRef] [PubMed]
- Morselli, S.; Sebastianelli, A.; Liaci, A.; Zaccaro, C.; Pecoraro, A.; Nicoletti, R.; Manera, A.; Bisegna, C.; Campi, R.; Pollini, S.; et al. Male reproductive system inflammation after healing from coronavirus disease 2019. Andrology 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gu, L.; Ren, X.; Liu, Y.; Qian, K.; Lan, R.; Wang, T.; Jin, L.; Yang, J.; Liu, J. Prediction model for clinical pregnancy for ICSI after surgical sperm retrieval in different types of azoospermia. Hum. Reprod. 2020, 35, 1972–1982. [Google Scholar] [CrossRef]
- Moghimi, N.; Eslami Farsani, B.; Ghadipasha, M.; Mahmoudiasl, G.R.; Piryaei, A.; Aliaghaei, A.; Abdi, S.; Abbaszadeh, H.A.; Abdollahifar, M.A.; Forozesh, M. COVID-19 disrupts spermatogenesis through the oxidative stress pathway following induction of apoptosis. Apoptosis 2021, 26, 415–430. [Google Scholar] [CrossRef]
- Maleki, B.H.; Tartibian, B. COVID-19 and male reproductive function: A prospective, longitudinal cohort study. Reproduction 2021, 161, 319–331. [Google Scholar] [CrossRef]
- Falahieh, F.M.; Zarabadipour, M.; Mirani, M.; Abdiyan, M.; DInparvar, M.; Alizadeh, H.; Paktinat, S.; Hosseinirad, H. Effects of moderate COVID-19 infection on semen oxidative status and parameters 14 and 120 days after diagnosis. Reprod. Fertil Dev. 2021, 33, 683–690. [Google Scholar] [CrossRef]
- Ekholm, M.; Kahan, T. The Impact of the Renin-Angiotensin-Aldosterone System on Inflammation, Coagulation, and Atherothrombotic Complications, and to Aggravated COVID-19. Front. Pharmacol. 2021, 12, 1534. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Dutta, S. Does SARS-CoV-2 infection cause sperm DNA fragmentation? Possible link with oxidative stress. Eur. J. Contracept. Reprod. Health Care 2020, 25, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, L.; Zhang, Y.; Wang, X. An Investigation of the Expression of 2019 Novel Coronavirus Cell Receptor Gene ACE2 in a Wide Variety of Human Tissues. Infect. Dis. Poverty 2020, 9, 1–7. [Google Scholar]
- Gadotti, A.C.; Lipinski, A.L.; Vasconcellos, F.T.; Marqueze, L.F.; Cunha, E.B.; Campos, A.C.; Oliveira, C.F.; Amaral, A.N.; Baena, C.P.; Telles, J.P.; et al. Susceptibility of the patients infected with SARS-CoV-2 to oxidative stress and possible interplay with severity of the disease. Free Radic. Biol. Med. 2021, 165, 184–190. [Google Scholar] [CrossRef]
- Silva, N.D.J.; Ribeiro-Silva, R.D.C.; Ferreira, A.J.F.; Teixeira, C.S.S.; Rocha, A.S.; Alves, F.J.O.; Falcão, I.R.; Pinto, E.D.J.; Santos, C.A.D.S.T.; Fiaccone, R.L.; et al. Combined association of obesity and other cardiometabolic diseases with severe COVID-19 outcomes: A nationwide cross-sectional study of 21 773 Brazilian adult and elderly inpatients. BMJ Open 2021, 11, e050739. [Google Scholar] [CrossRef]
- Hayden, M.R. An Immediate and Long-Term Complication of COVID-19 May Be Type 2 Diabetes Mellitus: The Central Role of β-Cell Dysfunction, Apoptosis and Exploration of Possible Mechanisms. Cells 2020, 9, 2475. [Google Scholar] [CrossRef]
- Kahn, B.E.; Brannigan, R.E. Obesity and male infertility. Curr. Opin. Urol. 2017, 27, 441–445. [Google Scholar] [CrossRef]
- Dafallah Albashir, A.A. The potential impacts of obesity on COVID-19. Clin. Med. 2020, 20, E109–E113. [Google Scholar] [CrossRef]
- Lakhan, R.; Agrawal, A.; Sharma, M. Prevalence of Depression, Anxiety, and Stress during COVID-19 Pandemic. J. Neurosci. Rural Pract. 2020, 11, 519–525. [Google Scholar] [CrossRef]
- Abel, K.M.; Carr, M.J.; Ashcroft, D.M.; Chalder, T.; Chew-Graham, C.A.; Hope, H.; Kapur, N.; McManus, S.; Steeg, S.; Webb, R.T.; et al. Association of SARS-CoV-2 Infection with Psychological Distress, Psychotropic Prescribing, Fatigue, and Sleep Problems among UK Primary Care Patients. JAMA Netw. Open 2021, 4, e2134803. [Google Scholar] [CrossRef]
- Stefano, G.B.; Ptacek, R.; Ptackova, H.; Martin, A.; Kream, R.M. Selective neuronal mitochondrial targeting in SARS-CoV-2 infection affects cognitive processes to induce ‘Brain Fog’ and results in behavioral changes that favor viral survival. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e930886-1. [Google Scholar] [CrossRef] [PubMed]
- Gopal, A.B.; Chakraborty, S.; Padhan, P.K.; Barik, A.; Dixit, P.; Chakraborty, D.; Poirah, I.; Samal, S.; Sarkar, A.; Bhattacharyya, A. Silent hypoxia in COVID-19: A gut microbiota connection. Curr. Opin. Physiol. 2021, 23, 100456. [Google Scholar] [CrossRef] [PubMed]
- Kolbasi, B.; Bulbul, M.V.; Karabulut, S.; Altun, C.E.; Cakici, C.; Ulfer, G.; Mudok, T.; Keskin, I. Chronic unpredictable stress disturbs the blood–testis barrier affecting sperm parameters in mice. Reprod. Biomed. Online 2021, 42, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, Z.; Wang, G.; Wang, H.; Zhou, Y.; Zhao, X.; Cheng, C.Y.; Qiao, Y.; Sun, F. Sperm epigenetic alterations contribute to inter- and transgenerational effects of paternal exposure to long-term psychological stress via evading offspring embryonic reprogramming. Cell Discov. 2021, 7, 101. [Google Scholar] [CrossRef]
- Micelli, E.; Cito, G.; Cocci, A.; Polloni, G.; Russo, G.I.; Minervini, A.; Carini, M.; Natali, A.; Coccia, M.E. Desire for parenthood at the time of COVID-19 pandemic: An insight into the Italian situation. J. Psychosom. Obstet. Gynecol. 2020, 41, 183–190. [Google Scholar] [CrossRef]
- Madjunkov, M.; Dviri, M.; Librach, C. A comprehensive review of the impact of COVID-19 on human reproductive biology, assisted reproduction care and pregnancy: A Canadian perspective. J. Ovarian Res. 2020, 13, 140. [Google Scholar] [CrossRef]
- Masoudi, M.; Maasoumi, R.; Bragazzi, N.L. Effects of the COVID-19 pandemic on sexual functioning and activity: A systematic review and meta-analysis. BMC Public Health 2022, 22, 189. [Google Scholar] [CrossRef]
- Lotti, F.; Maggi, M. Sexual dysfunction and male infertility. Nat. Rev. Urol. 2018, 15, 287–307. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Sundaram, R.; Buck Louis, G.M.; Chavarro, J.E. Predictors of Sexual Intercourse Frequency Among Couples Trying to Conceive. J. Sex. Med. 2018, 15, 519–528. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).