Variables Determining Higher Home Care Effectiveness in Patients with Chronic Cardiovascular Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
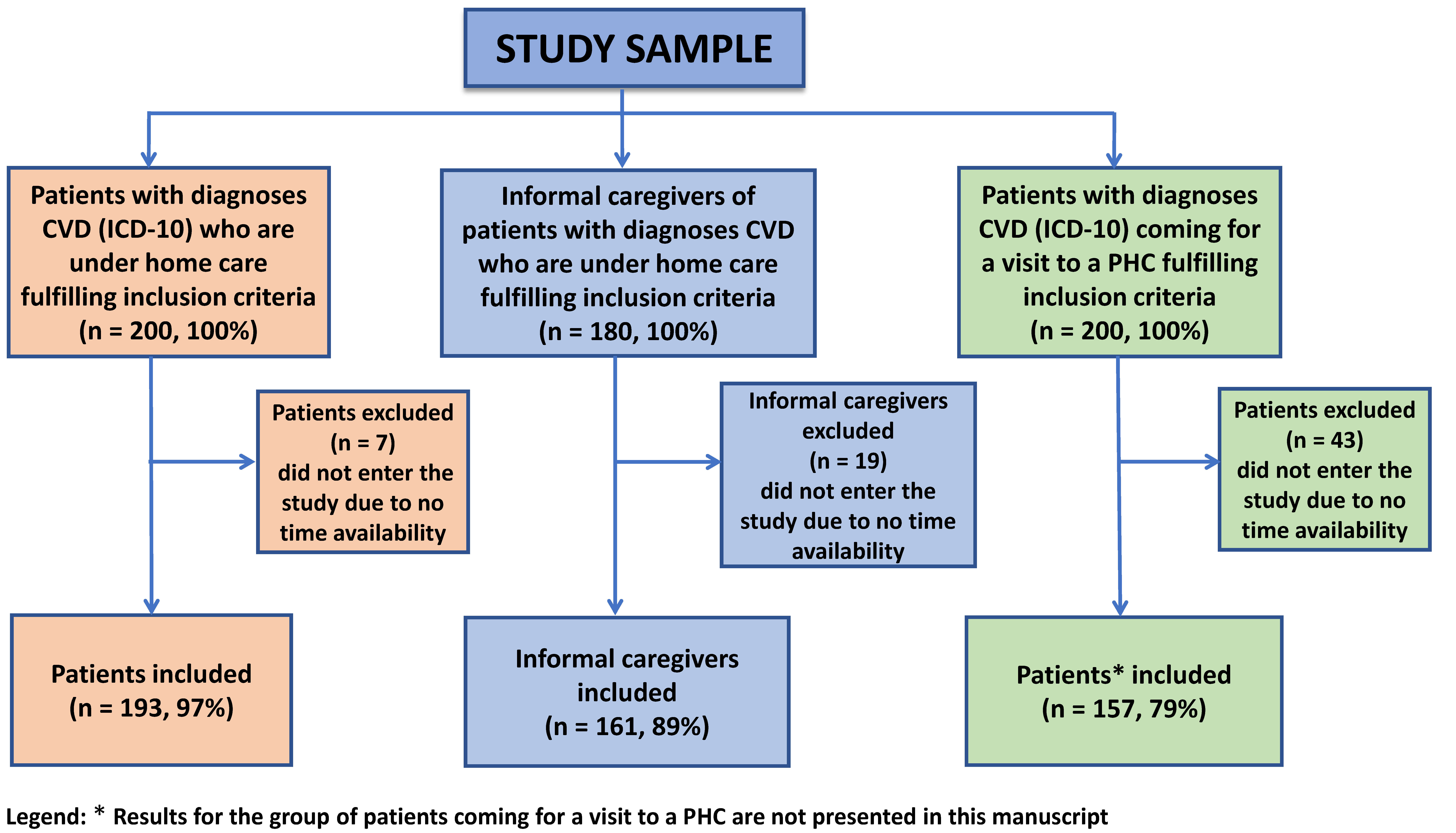
2.2. Participants
2.3. Variables and Data Collection
2.3.1. The WHOQOL-BREF Quality of Life Questionnaire
2.3.2. The Health Behavior Inventory Questionnaire
2.3.3. The Camberwell Assessment of Need Short Appraisal Schedule
2.3.4. The Hospital Anxiety and Depression Scale-Modified Version
2.3.5. Authors’ Self-Prepared Questionnaire
2.4. Ethical Aspects
2.5. Statistical Methods
3. Results
3.1. Sociodemographic Data of Patients with CVD
3.2. Clinical Data of CVD Patients with Worse (n = 84) and Better (n = 85) Health Care Effectiveness
3.3. Differences between Caregivers of Patients with Worse (n = 77) and Better (n = 57) Health Outcomes
3.4. Logistic Regression Analysis and Odds Ratio for the Effectiveness of Health Care in the Group of Patients and Their Caregivers
3.4.1. Logistic Regression Analysis and Odds Ratio—Model 1 (n = 130)
3.4.2. Logistic Regression Analysis and Odds Ratio—Model 2 (n = 130)
3.4.3. Logistic Regression Analysis and Odds Ratio—Model 3 (n = 120)
3.4.4. Logistic Regression Analysis and Odds Ratio—Model 4 (n = 124)
3.4.5. Logistic Regression Analysis and Odds Ratio—Model 5 (n = 124)
3.4.6. Logistic Regression Analysis and Odds Ratio—Model 6 (n = 123)
4. Discussion
4.1. Main Results
4.2. Sociodemographics of Patients with CVD vs. Health Care Effectiveness
4.3. Clinical Data from CVD Patients vs. Health Care Effectiveness
4.4. Variables Determining the Effectiveness of Health Care Compared to Caregivers of CVD Patients
4.5. Strengths of the Study
4.6. Limitations of the Study
4.7. Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. News. WHO Reveals Leading Causes of Death and Disability Worldwide: 2000–2019. Available online: https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 14 January 2022).
- WHO. Health Topics. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 14 January 2022).
- European Commission. Eurostat. Search. Eurostat Regional Yearbook—2021 Edition. Available online: https://ec.europa.eu/eurostat/web/main/search/-/search/estatsearchportlet_WAR_estatsearchportlet_INSTANCE_bHVzuvn1SZ8J?p_auth=PB7ulkKJ&text=Incidence+of+cardiovascular+diseases (accessed on 17 January 2022).
- Nessler, J.; Zalewski, J.; Kozierkiewicz, A.; Gackowski, A.; Uchmanowicz, I.; Witkowski, A.; Ponikowski, P. Projekt programu kompleksowej opieki nad chorymi z niewydolnością serca (KONS). Kardiol. Inwazyjna 2018, 13, 10–17. [Google Scholar]
- Barrio-Cortes, J.; Soria-Ruiz-Ogarrio, M.; Martínez-Cuevas, M.; Castaño-Reguillo, A.; Bandeira-de Oliveira, M.; Beca-Martínez, M.T.; López-Rodríguez, M.C.; Jaime-Sisó, M.Á. Use of primary and hospital care health services by chronic patients according to risk level by adjusted morbidity groups. BMC Health Serv. Res. 2021, 21, 1046. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Cangelosi, G.; Nittari, G.; Pantanetti, P.; Debernardi, G.; Scuri, S.; Sagaro, G.G.; Nguyen, C.T.T.; Grappasonni, I. Chronic Care Model in Italy: A narrative review of the literature. Prim. Health Care Res. Dev. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Torres-Ricartea, M.; Crusat-Abelloa, E.; Penuelas-Rodrigueza, S.; Zabaleta-del-Olmo, E. Nurse-led in Primary Health Care setting: A well-timed and promising organizational innovation. Enfer. Clin. 2015, 25, 87–91. [Google Scholar]
- Bitton, A.; Ratcliffe, H.L.; Veillard, J.H.; Kress, D.H.; Barkley, S.; Kimball, M.; Secci, F.; Wong, E.; Basu, L.; Taylor, C.; et al. Primary Health Care as a Foundation for Strengthening Health Systems in Low- and Middle-Income Countries. J. Gen. Intern. Med. 2017, 32, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Beltran, D.; Ruescas-Escolano, E.; Navarro-Palazón, A.I.; Cordero, A.; Gaubert-Tortosa, M.; Navarro-Perez, J.; Carratalá-Munuera, C.; Pertusa-Martínez, S.; Soler-Bahilo, E.; Brotons-Muntó, F.; et al. Effectiveness of a new health care organization model in primary care for chronic cardiovascular disease patients based on a multifactorial intervention: The PROPRESE randomized controlled trial. BMC Health Serv. Res. 2013, 13, 293. [Google Scholar] [CrossRef]
- Kurpas, D. Zadania podstawowej opieki zdrowotnej w zakresie opieki nad pacjentami z chorobami przewlekłymi. Zdr. Publiczne I Zarządzanie 2014, 12, 301–308. [Google Scholar]
- OECD iLibrary. Home. Books. OECD Health Policy Studies Realising the Potential of Primary Health Care Realising the Potential of Primary Health Care 2020. Available online: https://doi.org/10.1787/a92adee4-en (accessed on 17 January 2022). [CrossRef]
- Chow, S.K.; Wong, F.K.; Chan, T.M.; Chung, L.Y.; Chang, K.K.; Lee, R.P. Community nursing services for postdischarge chronically ill patients. J. Clin. Nurs. 2008, 17, 260–271. [Google Scholar] [CrossRef][Green Version]
- Kurpas, D. Paradygmat Opieki Nad Chorymi Przewlekle w Ramach Podstawowej Opieki Zdrowotnej; Uniwersytet Medyczny im. Piastów Śl: Wrocław, Polska, 2013. [Google Scholar]
- Puska, P. From Framingham to North Karelia: From descriptive epidemiology to public health action. Prog. Cardiovasc. Dis. 2010, 53, 15–20. [Google Scholar] [CrossRef]
- Jaarsma, T.; Brons, M.; Kraai, I.; Luttik, M.L.; Stromberg, A. Components of heart failure management in home care; a literature review. Eur. J. Cardiovasc. Nurs. 2013, 12, 230–241. [Google Scholar] [CrossRef]
- Van Spall, H.G.C.; Rahman, T.; Mytton, O.; Ramasundarahettige, C.; Ibrahim, Q.; Kabali, C.; Coppens, M.; Brian Haynes, R.; Connolly, S. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: A systematic review and network meta-analysis. Eur. J. Hear. Fail. 2017, 19, 1427–1443. [Google Scholar] [CrossRef] [PubMed]
- Ugur, H.G.; Erci, B. The Effect of Home Care for Stroke Patients and Education of Caregivers on the Caregiver Burden and Quality of Life. Acta Clin. Croat. 2019, 58, 321–332. [Google Scholar] [PubMed]
- Ballo, P.; Francesco Profili, F.; Policardo, L.; Roti, L.; Francesconi, P.; Zuppirol, A. Opposite trends in hospitalization and mortality after implementation of a chronic care mod-el-based regional program for the management of patients with heart failure in primary care. BMC Health Serv. Res. 2018, 18, 388. [Google Scholar] [CrossRef] [PubMed]
- Narodowy Instytut Zdrowia Publicznego Państwowy Zakład Higieny—Państwowy Instytut Badawczy (NIZP PZH-PIB). Sytuacja Zdrowotna Ludności Polski i jej Uwarunkowania—Raport za 2020 rok. Available online: https://www.pzh.gov.pl/sytuacja-zdrowotna-ludnosci-polski-i-jej-uwarunkowania-raport-za-2020-rok (accessed on 28 January 2022).
- Szlenk-Czyczerska, E.; Guzek, M.; Bielska, D.E.; Ławnik, A.; Polański, P.; Kurpas, D. Needs, Aggravation, and Degree of Burnout in Informal Caregivers of Patients with Chronic Cardiovascular Disease. Int. J. Environ. Res. Public Health 2020, 17, 6427. [Google Scholar] [CrossRef] [PubMed]
- Szlenk-Czyczerska, E.; Guzek, M.; Bielska, D.E.; Ławnik, A.; Polański, P.; Kurpas, D. Factors Differentiating Rural and Urban Population in Determining Anxiety and Depression in Patients with Chronic Cardiovascular Disease: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 3231. [Google Scholar] [CrossRef] [PubMed]
- Szlenk-Czyczerska, E.; Guzek, M.; Bielska, D.E.; Ławnik, A.; Polański, P.; Kurpas, D. The Analysis of the Relationship between the Quality of Life Level and Expectations of Patients with Cardiovascular Diseases under the Home Care of Primary Care Nurses. Int. J. Environ. Res. Public Health 2022, 19, 3300. [Google Scholar] [CrossRef] [PubMed]
- Sevilla-Cazes, J.; Ahmad, F.S.; Bowles, K.H.; Jaskowiak, A.; Gallagher, T.; Goldberg, L.R.; Kangovi, S.; Alexander, M.; Riegel, B.; Barg, F.K.; et al. Heart Failure Home Management Challenges and Reasons for Readmission: A Qualitative Study to Understand the Patient’s Perspective. J. Gen. Intern. Med. 2018, 33, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.C.; Smith, K.; Wingham, J.; Eyre, V.; Greaves, C.J.; Warren, F.C.; Green, C.; Jolly, K.; Davis, R.C.; Doherty, P.J.; et al. REACH-HF investigators,. A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejec-tion fraction and their caregivers: The REACH-HFpEF Pilot Study. BMJ Open. 2018, 8, e019649. [Google Scholar]
- WHO. Tools and Toolkits. WHOQOL. WHOQOL_BREF. Available online: https://www.who.int/tools/whoqol/whoqol-bref (accessed on 3 April 2022).
- Wołowicka, L.; Jaracz, K. Polska Wersja WHOQOL 100 i WHOQOL Bref. W.; Wołowicka, L., Ed.; Jakość życia w naukach medycznych; Wydawnictwo Uczelniane Akademii Medycznej w Poznaniu: Poznań, Poland, 2001. [Google Scholar]
- Jaracz, K.; Kalfoss, M.; Górna, K.; Bączyk, G. Quality of life in Polish: Psychometric properties of the Polish WHOQoL-Bref. Scand J. Caring Sci. 2006, 20, 251–260. [Google Scholar] [CrossRef]
- Juczyński, Z. Narzędzia Pomiaru w Promocji i Psychologii Zdrowia. Pracownia Testów Psychologicznych; Wydanie II: Warszawa, Poland, 2012. [Google Scholar]
- Stachowiak-Andrysiak, M.O.; Stelcer, B.O.; Mikstacki, A.D.; Kuliński, D.A.; Tamowicz, B.A. Ocena stanu psychicznego pacjentów z przewlekłą niewydolnością nerek (PNN) i ich adaptacji do stresu spowodowanego chorobą. Now. Lek. 2012, 81, 636–640. [Google Scholar]
- Kozera, E. Zależność między akceptacją choroby a poziomem lęku i depresji u pacjentek z nowotworem gruczołu piersiowego. Współcz. Pielęg. Ochr. Zdr. 2015, 4, 85–88. [Google Scholar]
- Schultz, W.M.; Kelli, H.M.; Lisko, J.C.; Varghese, T.; Shen, J.; Sandesara, P.; Quyyumi, A.A.; Taylor, H.A.; Gulati, M.; Harold, J.G.; et al. Socioeconomic Status and Cardiovascular Outcomes: Challenges and Interventions. Circulation 2018, 137, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.J.; Manns, B.J.; Weaver, R.G.; Hemmelgarn, B.R.; King-Shier, K.M.; Sanmartin, C. Financial barriers and adverse clinical outcomes among patients with cardiovascular-related chronic diseases: A cohort study. BMC Med. 2017, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Suligowska, K.; Gajewska, M.; Stokwiszewski, J.; Gaciong, Z.; Bandosz, P.; Wojtyniak, B.; Rutkowski, A.; Cianciara, D.; Wyrzykowski, B.; Zdrojewski, T. Niedostateczna wiedza Polaków na temat kryteriów nadciśnienia tętniczego i jego powikłań —wyniki badania NATPOL 2011. Nadciśn Tętn 2014, 18, 9–18. [Google Scholar]
- Raat, W.; Smeets, M.; Janssens, S.; Bert Vaes, B. Impact of primary care involvement and setting on multidisciplinary heart failure management: A systematic review and meta-analysis. ESC Heart Fail. 2021, 8, 802–818. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.F.S.; Thompson, D.R.; TF Lee, D.T.F. Disease management programmes for older people with heart failure: Crucial characteristics which improve post-discharge outcomes. Eur. Heart J. 2006, 27, 596–612. [Google Scholar] [CrossRef]
- Ryder, M.; Travers, B.; Ledwidge, M.; McDonald, K. Multidisciplinary care of heart failure: What have we learned and where can we improve. (Editorial). Eur. J. Cardiovasc. Nurs. 2003, 2, 247–249. [Google Scholar] [CrossRef]
- Newman, A.B.; Arnold, A.M.; Naydeck, B.L.; Fried, L.P.; Burke, G.L.; Enright, P.; Gottdiener, J.; Hirsch, C.; O’Leary, D.; Tracy, R. Successful aging: Effect of subclinical cardiovascular disease. Arch. Intern. Med. 2003, 163, 2315–2322. [Google Scholar] [CrossRef]
- Orzechowska, A.; Florkowski, A.; Gruszczyński, W.; Zboralski, K.; Wysokiński, A.; Gałecki, P.; Talarowska, M. Status socjoekonomiczny a zachowania agresywne i style radzenia sobie ze stresem. Psychiatr. Pol. 2009, 1, 53–63. Available online: http://psychiatriapolska.pl/uploads/images/PP_1_2009/Orzechowska%20s53_Psychiatria%20Polska%201_2009.pdf (accessed on 14 February 2022).
- Nitsche, M.P.; Bitran, M.; Pedrals, N.; Echeverria, G.; Rigotti, A. Positive psychosocial factors and cardiovascular health. Rev. Med. Chil 2014, 142, 1316–1323. [Google Scholar] [CrossRef]
- Kronish, I.M.; Moise, N. In Search of a „Magic Pill” for Medication Nonadherence. JAMA Intern. Med. 2017, 177, 631–632. [Google Scholar] [CrossRef] [PubMed]
- Buck, H.G.; Dickson, V.V.; Fida, R.; Riegel, B.; D’Agostino, F.; Alvaro, R.; Vellone, E. Predictors of hospitalization and quality of life in heart failure: A model of comorbidity, self-efficacy and self-care. Int. J. Nurs. Stud. 2015, 52, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Paterniani, A.; Sperati, F.; Esposito, G.; Cognetti, G.; Pulimeno, A.M.L.; Rocco, G.; Diamanti, P.; Bertini, L.; Baldeschi, G.C.; Varrassi, G.; et al. Quality of life and disability of chronic non-cancer pain in adults patients attending pain clinics: A prospective, multicenter, observational study. Appl. Nurs. Res. 2020, 56, 151332. [Google Scholar] [CrossRef] [PubMed]
- Comín-Colet, J.; Lorenzo, T.M.; González-Domínguez, A.; Oliva, J.; Merino, S.J. Impact of non-cardiovascular comorbidities on the quality of life of patients with chronic heart failure: A scoping review. Health Qual. Life Outcomes 2020, 18, 329. [Google Scholar] [CrossRef]
- Day, C.B.; Bierhals, C.C.B.K.; Santos, N.O.D.; Mocellin, D.; Predebon, M.L.; Dal Pizzol, F.L.F.; Paskulin, L.M.G. Nursing home care educational intervention for family caregivers of older adults post stroke (SHARE): Study protocol for a randomised trial. Trials 2018, 19, 96. [Google Scholar] [CrossRef]
- Skrzypek-Czerko, M.; Kozera, G.; Chwojnicki, K.; Nyka, W.M.; Roszmann, A. Znaczenie wsparcia społecznego dla poziomu lęku i depresji oraz obciążenia opiekunów pacjentów po udarze mózgu—wyniki wstępne. Pielęgn Neurol Neurochir 2013, 2, 18–26. [Google Scholar]
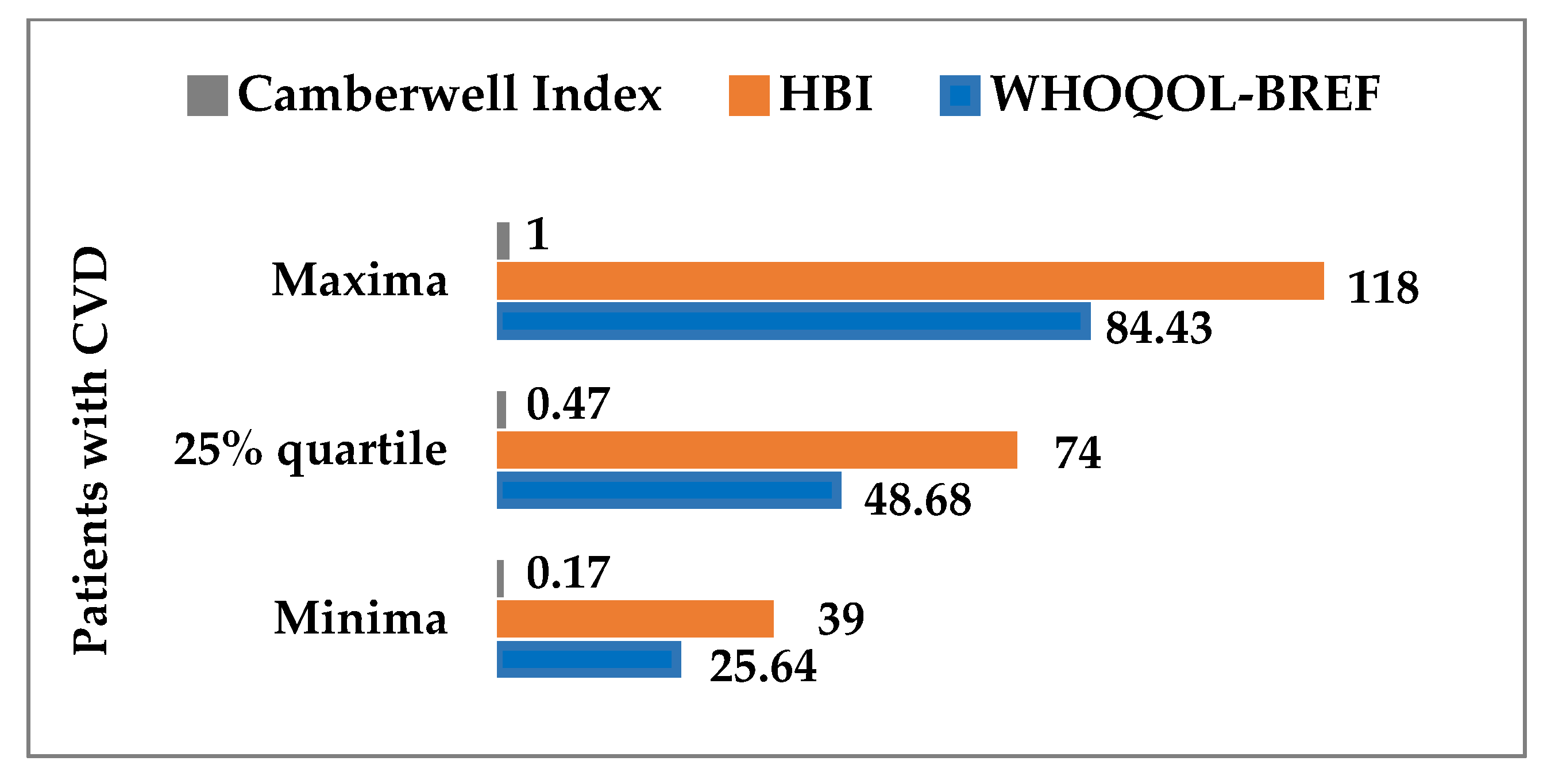
| Variable | ↓ LEHC | ↑ HEHC | Wilcoxon Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Me | q1 | q3 | n | Me | q1 | q3 | W | p | ||
| Duration of CVD (in years) | 81 | 10 | 5 | 15 | 83 | 10 | 5 | 15 | 3471.5 | 0.718 | |
| Number of in the last 12 months | visits to PHC | 84 | 5 | 1 | 10 | 85 | 5 | 3 | 10 | 3363.5 | 0.515 |
| cardiology clinic | 84 | 0.5 | 0 | 2 | 85 | 2 | 0 | 2 | 2744.5 | 0.007 | |
| home visits | 84 | 2 | 0 | 4 | 85 | 0 | 0 | 3 | 3998 | 0.154 | |
| telephone consultations | 84 | 2 | 0 | 5.25 | 85 | 0 | 0 | 3 | 4111.5 | 0.068 | |
| family nurse practitioner interventions | 84 | 9.5 | 1 | 20.75 | 85 | 5 | 0 | 12 | 4013.5 | 0.16 | |
| Health care services (how many times/12 months) | medical interview | 84 | 2 | 1 | 11.25 | 85 | 2 | 1 | 4 | 3781.5 | 0.501 |
| physical examination | 84 | 4 | 0 | 12 | 85 | 3 | 0 | 6 | 3990 | 0.181 | |
| blood pressure measurement | 84 | 12 | 6 | 24.5 | 85 | 12 | 5 | 23 | 3757.5 | 0.556 | |
| spirometry | 84 | 0 | 0 | 0 | 85 | 0 | 0 | 0 | 3547 | 0.916 | |
| diet control | 84 | 3 | 1 | 6.5 | 85 | 2 | 0 | 7 | 3889.5 | 0.31 | |
| BMI | 84 | 1 | 0 | 2.25 | 85 | 0 | 0 | 2 | 3908 | 0.26 | |
| pro-health education | 84 | 6.5 | 2 | 12 | 85 | 6 | 1 | 12 | 3763 | 0.542 | |
| others | 84 | 0 | 0 | 0 | 85 | 0 | 0 | 0 | 3154.5 | 0.032 | |
| Variable | Categories | n | % | n | % | Fisher test-p | |||||
| Current state of treatment (data provided by a nurse) | maintenance therapy | 83 | 98.8 | 81 | 96.4 | 0.8 | |||||
| others | 1 | 1.2 | 3 | 3.6 | |||||||
| total | 84 | 100 | 84 | 100 | |||||||
| Current state of treatment (data received from a patient) | maintenance therapy | 79 | 96.3 | 79 | 95.2 | 0.073 | |||||
| no treatment | 3 | 3.7 | 1 | 1.2 | |||||||
| others | 0 | 0 | 3 | 3.6 | |||||||
| total | 82 | 100 | 83 | 100 | |||||||
| Assessment of physical well-being | very bad | 10 | 11.9 | 0 | 0 | <0.001 | |||||
| bad | 40 | 47.6 | 10 | 11.8 | |||||||
| good | 19 | 22.6 | 45 | 52.9 | |||||||
| quite good | 12 | 14.3 | 27 | 31.8 | |||||||
| very good | 3 | 3.6 | 3 | 3.5 | |||||||
| total | 84 | 100 | 85 | 100 | |||||||
| Assessment of mental well-being | very bad | 6 | 7.2 | 0 | 0 | <0.001 | |||||
| bad | 35 | 42.2 | 7 | 8.3 | |||||||
| good | 20 | 24.1 | 50 | 59.5 | |||||||
| quite good | 20 | 24.1 | 18 | 21.4 | |||||||
| very good | 2 | 2.4 | 9 | 10.7 | |||||||
| total | 83 | 100 | 84 | 100 | |||||||
| Do you currently have any symptoms? | yes | 53 | 63.1 | 36 | 42.9 | 0.011 | |||||
| no | 31 | 36.9 | 48 | 57.1 | |||||||
| total | 84 | 100 | 84 | 100 | |||||||
| Adhere to the recommendations regarding lifestyle changes | yes | 43 | 51.2 | 48 | 56.5 | 0.539 | |||||
| no | 41 | 48.8 | 37 | 43.5 | |||||||
| total | 84 | 100 | 84 | 100 | |||||||
| Adhere to the recommendations regarding proper eating habits | yes | 35 | 41.7 | 42 | 49.4 | 0.355 | |||||
| no | 49 | 58.3 | 43 | 50.6 | |||||||
| total | 84 | 100 | 85 | 100 | |||||||
| Takes prescribed medications regularly | yes | 54 | 64.3 | 73 | 85.9 | 0.001 | |||||
| no | 30 | 35.7 | 12 | 14.1 | |||||||
| total | 84 | 100 | 85 | 100 | |||||||
| Models with 8 Explanatory Variables | Odds Ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 (n = 130) | Per Unit | Per Range | |||||||
| Var. | Chi2 = 66.66, df = 8, p < 0.001, pseudo R2 = 0.37 | bi | OR | 95% CI | 1/OR | OR | 95% CI | 1/OR | range |
| 1 | Age of patients (in years; 17–94) | −0.025 | 0.98 | 0.95–0.99 | 1.03 | 0.15 | 0.02–0.88 | 6.76 | 77 |
| 2 | Difficulties in nursing care: not taking prescribed medications regularly (1—no, 2—yes) | −1.405 | 0.25 | 0.07–0.72 | 4.08 | 0.25 | 0.07–0.72 | 4.08 | 1 |
| 3 | Attitude towards the disease and methods of treatment applied (1—positive, 2—negative) | −1.696 | 0.18 | 0.06–0.48 | 5.45 | 0.18 | 0.06–0.48 | 5.45 | 1 |
| 4 | Financial status (1—very good, 5—very bad) | −0.836 | 0.43 | 0.21–0.83 | 2.31 | 0.08 | 0.01–0.58 | 12.26 | 3 |
| 5 | HADS–M Aggression–patient (0—no, 6—high) | 0.310 | 1.36 | 1.05–1.81 | 0.73 | 6.42 | 1.33–35.23 | 0.16 | 6 |
| 6 | Patient: endocrinological disorders (1—no, 2—yes) | 1.821 | 6.18 | 1.83–23.78 | 0.16 | 6.18 | 1.83–23.78 | 0.16 | 1 |
| 7 | Improvement of a caregiver mental well-being after a nursing visit: I am full of hope and strength (1—no, 2—yes) | 1.500 | 4.48 | 1.24–18.05 | 0.22 | 4.48 | 1.24–18.05 | 0.22 | 1 |
| 8 | WHOQOL-BREF Social relations domain–caregiver (4—weak, 20—strong) | 0.218 | 1.24 | 1.09–1.44 | 0.80 | 32.62 | 4.13–333.84 | 0.03 | 16 |
| Model 2 (n = 130) | Per unit | Per range | |||||||
| Var. | Chi2 = 67.79, df = 8, p < 0.00001, pseudo R2 = 0.38 | bi | OR | 95% CI | 1/OR | OR | 95% CI | 1/OR | range |
| 9 | Health care services (how many times/12 months) - others | 0.226 | 1.25 | 1.03–1.64 | 0.80 | 29.60 | 1.48–1597.17 | 0.03 | 15 |
| 10 | Education (1—primary, 7—post-secondary) | 0.368 | 1.45 | 1.05–2.02 | 0.69 | 9.12 | 1.37–68.87 | 0.11 | 6 |
| and | bi values for the remaining variables in the model: (1) −1.519, (2) −1.912, (5) −0.787, (6) 0.346, (7) 1.411, (8) 0.190 | ||||||||
| Model 3 (n = 120) | Per unit | Per range | |||||||
| Var. | Chi2 = 63.21, df = 8, p < 0.00001, pseudo R2 = 0.38 | bi | OR | 95% CI | 1/OR | OR | 95% CI | 1/OR | range |
| 11 | Diagnosis of ICD-10: I99 (0—no, 1—yes) | −1.859 | 0.16 | 0.02–0.90 | 6.42 | 0.16 | 0.02–0.90 | 6.42 | 1 |
| 12 | WHOQOL-BREF Psychological domain–caregiver (9.33—low, 18—high) | 0.519 | 1.68 | 1.25–2.37 | 0.60 | 89.55 | 6.89–1768.19 | 0.01 | 8.67 |
| 13 | HBI sten scale–caregiver (1—low, 10—high) | 0.470 | 1.60 | 1.06–2.56 | 0.62 | 68.94 | 1.70–4648.61 | 0.02 | 9 |
| 14 | HBI Proper mental attitudes–caregiver (2.33—weak, 5—strong) | −1.526 | 0.22 | 0.04–0.92 | 4.60 | 0.02 | 0.00–0.81 | 58.5 | 2.67 |
| and | bi values for the remaining variables in the model: (2) −1.764, (3) −2.914, (6) 1.494, (9) 0.573 | ||||||||
| Model 4 (n = 124) | Per unit | Per range | |||||||
| Var. | Chi2 = 69.85, df = 8, p < 0.00001, pseudo R2 = 0.39 | bi | OR | 95% CI | 1/OR | OR | 95% CI | 1/OR | range |
| 15 | Number of visits at cardiology clinic (within last 12 months) (0–24) | 0.221 | 1.25 | 1.02–1.59 | 0.80 | 198.49 | 1.63–70,383.3 | 0.01 | 24 |
| 16 | Carer’s expectations of higher manual skills while performing nursing duties towards a community nurse: (1—no, 2—yes) | 1.405 | 4.08 | 1.31–14.34 | 0.25 | 4.08 | 1.31–14.33 | 0.25 | 1 |
| 17 | WHOQOL-BREF Physical domain–caregiver (8.57—weak, 19.43—strong) | 0.213 | 1.24 | 1.05–1.49 | 0.81 | 10.09 | 1.66–76.09 | 0.10 | 10.86 |
| and | bi values for the remaining variables in the model: (2) −1.416, (3) −1.983, (4) −1.294, (5) 0.308, (7) 1.689 | ||||||||
| Models with 7 Explanatory Variables | Odds Ratio | ||||||||
| Model 5 (n = 124) | Per unit | Per range | |||||||
| Var. | Chi2 = 59.76, df = 7, p < 0.00001, pseudo R2 = 0.35 | bi | OR | 95% CI | 1/OR | OR | 95% CI | 1/OR | range |
| 18 | Self-assessment of patient’s current mental well-being (1—very bad, 5—very good) | 0.506 | 1.66 | 1.04–2.74 | 0.60 | 7.55 | 1.19–56.44 | 0.13 | 4 |
| 19 | Nursing: endocrinological disorders (1—no, 2—yes) | 1.240 | 3.46 | 1.08–12.35 | 0.29 | 3.46 | 1.08–12.35 | 0.29 | 1 |
| and | bi values for the remaining variables in the model: (3) −2.677, (4) −1.245, (11) −1.821, (17) 0.189, (18) 1.249 | ||||||||
| Model 6 (n = 123) | Per unit | Per range | |||||||
| Var. | Chi2 = 38.63, df = 7, p < 0.00001, pseudo R2 = 0.23 | bi | OR | 95% CI | 1/OR | OR | 95% CI | 1/OR | range |
| 19 | Self-assessment of patient’s physical well-being (1—very bad, 5—very good) | 0.556 | 1.74 | 1.12–2.82 | 0.57 | 9.24 | 1.59–63.30 | 0.11 | 4 |
| 20 | Nurse: urological disorders (1—no, 2—yes) | −1.824 | 0.16 | 0.04–0.55 | 6.20 | 0.16 | 0.04–0.55 | 6.20 | 1 |
| and | bi values for the remaining variables in the model: (7) 1.380, (11) −1.687, (13) 0.409, (14) −1.074, (15) 0.199 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szlenk-Czyczerska, E.; Guzek, M.; Bielska, D.E.; Ławnik, A.; Polański, P.; Kurpas, D. Variables Determining Higher Home Care Effectiveness in Patients with Chronic Cardiovascular Disease. Int. J. Environ. Res. Public Health 2022, 19, 5170. https://doi.org/10.3390/ijerph19095170
Szlenk-Czyczerska E, Guzek M, Bielska DE, Ławnik A, Polański P, Kurpas D. Variables Determining Higher Home Care Effectiveness in Patients with Chronic Cardiovascular Disease. International Journal of Environmental Research and Public Health. 2022; 19(9):5170. https://doi.org/10.3390/ijerph19095170
Chicago/Turabian StyleSzlenk-Czyczerska, Elżbieta, Marika Guzek, Dorota Emilia Bielska, Anna Ławnik, Piotr Polański, and Donata Kurpas. 2022. "Variables Determining Higher Home Care Effectiveness in Patients with Chronic Cardiovascular Disease" International Journal of Environmental Research and Public Health 19, no. 9: 5170. https://doi.org/10.3390/ijerph19095170
APA StyleSzlenk-Czyczerska, E., Guzek, M., Bielska, D. E., Ławnik, A., Polański, P., & Kurpas, D. (2022). Variables Determining Higher Home Care Effectiveness in Patients with Chronic Cardiovascular Disease. International Journal of Environmental Research and Public Health, 19(9), 5170. https://doi.org/10.3390/ijerph19095170






