Sleep Satisfaction May Modify the Association between Metabolic Syndrome and BMI, Respectively, and Occupational Stress in Japanese Office Workers
Abstract
:1. Introduction
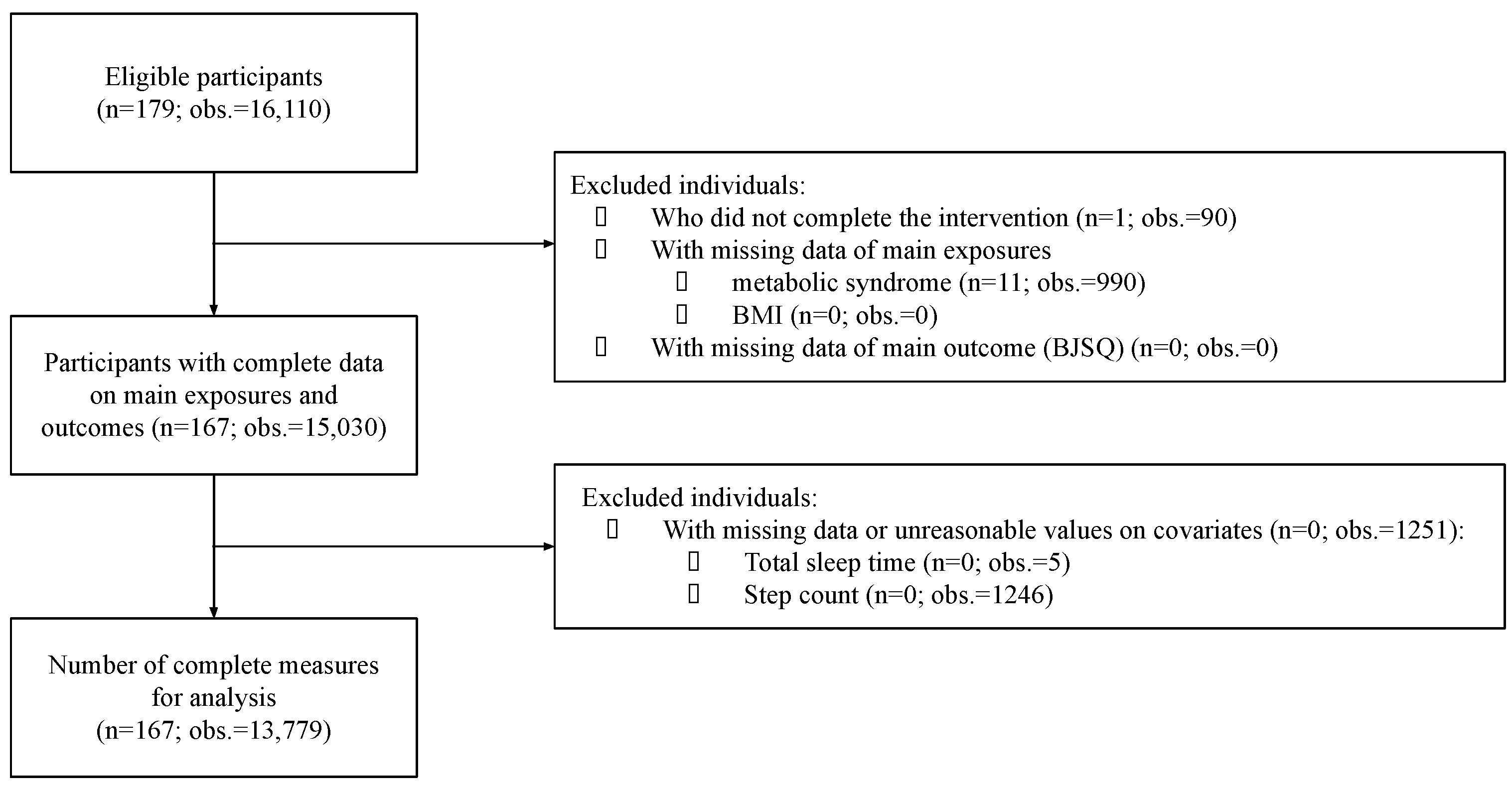
2. Materials and Methods
2.1. Study Population
2.2. Exposure Variables
2.3. Outcome Variable
2.4. Covariates
2.5. Statistical Analyses
2.6. Ethical Considerations
3. Results
3.1. Baseline Characteristics
3.2. Main Analyses
3.3. Sensitivity Analyses
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Gupta, V. Metabolic syndrome: What are the risks for humans? Biosci. Trends 2010, 4, 204–212. [Google Scholar] [PubMed]
- Grundy, S.M. Metabolic Syndrome Pandemic. Arter. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isomaa, B.; Almgren, P.; Tuomi, T.; Forsén, B.; Lahti, K.; Nissén, M.; Taskinen, M.-R.; Groop, L. Cardiovascular Morbidity and Mortality Associated With the Metabolic Syndrome. Diabetes Care 2001, 24, 683–689. [Google Scholar] [CrossRef] [Green Version]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Health Labour and Welfare. Overview of Vital Statistics. 2020. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei20/index.html (accessed on 25 November 2021).
- OECD. OECD Reviews of Public Health: Japan; OECD: Tokyo, Japan, 2019. [Google Scholar] [CrossRef]
- Cheng, Y.; Park, J.; Kim, Y.; Kawakami, N. The recognition of occupational diseases attributed to heavy workloads: Experiences in Japan, Korea, and Taiwan. Int. Arch. Occup. Environ. Health 2012, 85, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Kachi, Y.; Inoue, A.; Eguchi, H.; Kawakami, N.; Shimazu, A.; Tsutsumi, A. Occupational stress and the risk of turnover: A large prospective cohort study of employees in Japan. BMC Public Health 2020, 20, 174–178. [Google Scholar] [CrossRef]
- Tsutsumi, A.; Shimazu, A.; Eguchi, H.; Inoue, A.; Kawakami, N. A Japanese Stress Check Program screening tool predicts employee long-term sickness absence: A prospective study. J. Occup. Health 2018, 60, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, S. Hours of work and health in Japan. Ann. Epidemiol. 2019, 33, 64–71. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yoshikawa, T.; Takamoto, M.; Sasaki, T.; Matsumoto, S.; Kayashima, K.; Takeshima, T.; Takahashi, M. Overwork-related disorders in Japan: Recent trends and development of a national policy to promote preventive measures. Ind. Health 2017, 55, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Imamura, K.; Asai, Y.; Watanabe, K.; Tsutsumi, A.; Shimazu, A.; Inoue, A.; Hiro, H.; Odagiri, Y.; Yoshikawa, T.; Yoshikawa, E.; et al. Effect of the National Stress Check Program on mental health among workers in Japan: A 1-year retrospective cohort study. J. Occup. Health 2018, 60, 298–306. [Google Scholar] [CrossRef]
- Parente, E.B. Is body mass index still a good tool for obesity evaluation? Arch. Endocrinol. Metab. 2016, 60, 507–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelber, R.P.; Gaziano, J.M.; Orav, E.J.; Manson, J.E.; Buring, J.E.; Kurth, T. Measures of Obesity and Cardiovascular Risk among Men and Women. J. Am. Coll. Cardiol. 2008, 52, 605–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Qian, L. Association between lifetime stress and obesity in Canadians. Prev. Med. 2012, 55, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Isasi, C.R.; Parrinello, C.M.; Jung, M.M.; Carnethon, M.R.; Birnbaum-Weitzman, O.; Espinoza, R.A.; Penedo, F.J.; Perreira, K.M.; Schneiderman, N.; Sotres-Alvarez, D.; et al. Psychosocial stress is associated with obesity and diet quality in Hispanic/Latino adults. Ann. Epidemiol. 2015, 25, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Kouvonen, A.; Kivimäki, M.; Cox, S.J.; Cox, T.; Vahtera, J. Relationship Between Work Stress and Body Mass Index Among 45,810 Female and Male Employees. Psychosom. Med. 2005, 67, 577–583. [Google Scholar] [CrossRef]
- Moore, C.J.; Cunningham, S.A. Social Position, Psychological Stress, and Obesity: A Systematic Review. J. Acad. Nutr. Diet. 2012, 112, 518–526. [Google Scholar] [CrossRef]
- Riffer, F.; Sprung, M.; Münch, H.; Kaiser, E.; Streibl, L.; Heneis, K.; Kautzky-Willer, A. Relationship between psychological stress and metabolism in morbidly obese individuals. Wien. Klin. Wochenschr. 2020, 132, 139–149. [Google Scholar] [CrossRef]
- Solovieva, S.; Lallukka, T.; Virtanen, M.; Viikari-Juntura, E. Psychosocial factors at work, long work hours, and obesity: A systematic review. Scand. J. Work. Environ. Health 2013, 39, 241–258. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-Y.; Lee, S.E.; Kim, S.H.; Chung, H.W.; Kim, W.Y. Psychological Distress Is Associated with Inadequate Dietary Intake in Vietnamese Marriage Immigrant Women in Korea. J. Am. Diet. Assoc. 2010, 110, 779–785. [Google Scholar] [CrossRef]
- Tan, T.; Leung, C.W. Associations between perceived stress and BMI and waist circumference in Chinese adults: Data from the 2015 China Health and Nutrition Survey. Public Health Nutr. 2021, 24, 4965–4974. [Google Scholar] [CrossRef]
- Eftekhari, S.; Alipour, F.; Aminian, O.; Saraei, M. The association between job stress and metabolic syndrome among medical university staff. J. Diabetes Metab. Disord. 2021, 20, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Magnavita, N.; Fileni, A. Work stress and metabolic syndrome in radiologists: First evidence. Radiol. Med. 2014, 119, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, T.; Sasaki, J.; Ueshima, H.; Egusa, G.; Kinoshita, M.; Shimamoto, K.; Daida, H.; Biro, S.; Hirobe, K.; Funahashi, T.; et al. Metabolic Syndrome. J. Atheroscler. Thromb. 2008, 15, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Health Labour and Welfare. the National Health and Nutrition Survey. Available online: https://www.mhlw.go.jp/content/000681200.pdf (accessed on 12 March 2022). (In Japanese)
- Ministry of Health Labour and Welfare. Implementation manual of Stress Check Program Based on the Industrial Safety and Health Act. Available online: https://www.mhlw.go.jp/content/000533925.pdf (accessed on 25 November 2021). (In Japanese)
- Ministry of Health Labour and Welfare. Occupational Stress Check as a Measure of Mental Health and Overwork in the Workplace. Available online: https://www.mhlw.go.jp/bunya/roudoukijun/anzeneisei12/ (accessed on 25 November 2021). (In Japanese)
- Ministry of Health Labour and Welfare. Health Japan 21 (Kenko Nippon 21) Alcohol Section. Available online: https://www.mhlw.go.jp/www1/topics/kenko21_11/pdf/b5.pdf (accessed on 26 November 2021). (In Japanese)
- Ellis, B.W.; Johns, M.W.; Lancaster, R.; Raptopoulos, P.; Angelopoulos, N.; Priest, R.G. The St. Mary’s Hospital sleep questionnaire: A study of reliability. Sleep 1981, 4, 93–97. [Google Scholar] [CrossRef]
- Bergmann, N.; Gyntelberg, F.; Faber, J. The appraisal of chronic stress and the development of the metabolic syndrome: A systematic review of prospective cohort studies. Endocr. Connect. 2014, 3, R55–R80. [Google Scholar] [CrossRef]
- Sterling, P. Principles of Allostasis: Optimal Design, Predictive Regulation, Pathophysiology, and Rational Therapeutics. In Allostasis, Homeostasis, and the Costs of Physiological Adaptation; Schulkin, J., Ed.; Cambridge University Press: Cambridge, UK, 2004; pp. 17–64. [Google Scholar]
- Nielsen, K.S.; Bauer, J.M.; Hofmann, W. Examining the relationship between trait self-control and stress: Evidence on generalizability and outcome variability. J. Res. Pers. 2020, 84, 103901. [Google Scholar] [CrossRef]
- Baron, R.M.; Kenny, D.A. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986, 51, 1173. [Google Scholar] [CrossRef]
- Cabinet Office. Individuals and Corporates Survey on Work-Life Balance. Available online: http://wwwa.cao.go.jp/wlb/research/wlb_h2511/9_insatsu.pdf (accessed on 14 December 2021). (In Japanese)
- The Japanese Institute for Labour Policy and Training. Databook of International Labour Statistics. Available online: https://www.jil.go.jp/kokunai/statistics/databook/2019/documents/Databook2019.pdf (accessed on 14 December 2021). (In Japanese)
- Kohro, T.; Furui, Y.; Mitsutake, N.; Fujii, R.; Morita, H.; Oku, S.; Ohe, K.; Nagai, R. The Japanese National Health Screening and Intervention Program Aimed at Preventing Worsening of the Metabolic Syndrome. Int. Heart J. 2008, 49, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Tsushita, K.; Hosler, A.S.; Miura, K.; Ito, Y.; Fukuda, T.; Kitamura, A.; Tatara, K. Rationale and Descriptive Analysis of Specific Health Guidance: The Nationwide Lifestyle Intervention Program Targeting Metabolic Syndrome in Japan. J. Atheroscler. Thromb. 2018, 25, 308–322. [Google Scholar] [CrossRef] [Green Version]
- Lian, Y.; Yuan, Q.; Wang, G.; Tang, F. Association between sleep quality and metabolic syndrome: A systematic review and meta-analysis. Psychiatry Res. 2019, 274, 66–74. [Google Scholar] [CrossRef]
- Beccuti, G.; Pannain, S. Sleep and obesity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 402–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S.; Karatsoreos, I.N. Sleep Deprivation and Circadian Disruption: Stress, Allostasis, and Allostatic Load. Sleep Med. Clin. 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Itani, O.; Kaneita, Y.; Tokiya, M.; Jike, M.; Murata, A.; Nakagome, S.; Otsuka, Y.; Ohida, T. Short sleep duration, shift work, and actual days taken off work are predictive life-style risk factors for new-onset metabolic syndrome: A seven-year cohort study of 40,000 male workers. Sleep Med. 2017, 39, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kalmbach, D.A.; Anderson, J.R.; Drake, C.L. The impact of stress on sleep: Pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J. Sleep Res. 2018, 27, e12710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyoshima, K.; Inoue, T.; Shimura, A.; Masuya, J.; Fujimura, Y.; Higashi, S.; Kusumi, I. The relationship among sleep reactivity, job-related stress, and subjective cognitive dysfunction: A cross-sectional study using path analysis. Ind. Health 2021, 59, 229–238. [Google Scholar] [CrossRef]
- Kim, E.-J.; Dimsdale, J.E. The Effect of Psychosocial Stress on Sleep: A Review of Polysomnographic Evidence. Behav. Sleep Med. 2007, 5, 256–278. [Google Scholar] [CrossRef] [Green Version]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Quantity and quality of sleep and incidence of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2010, 33, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Buysse, D.J. Sleep health: Can we define it? Does it matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Drager, L.F.; Lopes, H.F.; Maki-Nunes, C.; Trombetta, I.C.; Toschi-Dias, E.; Alves, M.J.N.N.; Fraga, R.F.; Jun, J.C.; Negrão, C.E.; Krieger, E.M.; et al. The Impact of Obstructive Sleep Apnea on Metabolic and Inflammatory Markers in Consecutive Patients with Metabolic Syndrome. PLoS ONE 2010, 5, e12065. [Google Scholar] [CrossRef]
- Balbo, M.; Leproult, R.; Van Cauter, E. Impact of Sleep and Its Disturbances on Hypothalamo-Pituitary-Adrenal Axis Activity. Int. J. Endocrinol. 2010, 2010, 759234. [Google Scholar] [CrossRef] [Green Version]
- Ye, J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int. J. Obes. 2009, 33, 54–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogilvie, R.P.; Patel, S.R. The epidemiology of sleep and obesity. Sleep Health 2017, 3, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Kushida, C.A. Lung Biology in Health & Disease. Sleep Deprivation: Basic Science, Physiology and Behavior; Marcel Dekker: New York, NY, USA, 2004; Volume 192. [Google Scholar]
- Dinges, D.F.; Pack, F.; Williams, K.; Gillen, K.A.; Powell, J.W.; Ott, G.E.; Aptowicz, C.; Pack, A. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 1997, 20, 267–277. [Google Scholar] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; L’Hermite-Balériaux, M.; Copinschi, G.; Penev, P.D.; Van Cauter, E. Leptin Levels Are Dependent on Sleep Duration: Relationships with Sympathovagal Balance, Carbohydrate Regulation, Cortisol, and Thyrotropin. J. Clin. Endocrinol. Metab. 2004, 89, 5762–5771. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, K.; Iso, H. The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol. Health 2017, 39, e2017003. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | MetS n = 52 | Pre-MetS n = 63 | No MetS n = 52 | p Value a | Test Statistic |
|---|---|---|---|---|---|
| Men (%) | 94.23 | 100 | 82.69 | 0.001 | Chi2(2) = 13.02 |
| Age at screening [mean (years ± SD)] | 46.8 ± 6.6 | 43.3 ± 8.2 | 42.4 ± 7.3 | 0.008 | F2164 = 5.04 |
| Smoking status (%) | 0.389 | Chi2(6) = 6.31 | |||
| Non-smoker | 42.3 | 39.7 | 53.9 | ||
| Past smoker | 32.7 | 33.3 | 21.2 | ||
| Current smoker (<20 cigarettes/day) | 13.5 | 19.1 | 9.6 | ||
| Current smoker (≥20 cigarettes/day) | 11.5 | 8.0 | 15.4 | ||
| Alcohol consumption (%) | 0.198 | Chi2(2) = 3.24 | |||
| <20 g ethanol/day | 53.9 | 42.9 | 36.5 | ||
| ≥20 g ethanol/day | 46.2 | 57.1 | 63.5 | ||
| Sleep satisfaction (5-point scale) [mean ± SD] | 3.5 ± 0.4 | 3.4 ± 0.5 | 3.5 ± 0.4 | 0.157 | F2164 = 1.87 |
| Number of servings of staple foods [mean ± SD] | 4.3 ± 1.0 | 4.4 ± 0.8 | 4.1 ± 0.9 | 0.453 | F2164 = 0.79 |
| Number of servings of main dish [mean ± SD] | 4.2 ± 1.2 | 4.3 ± 1.0 | 4.3 ± 1.1 | 0.822 | F2164 = 0.20 |
| Number of servings of dairy product [mean ± SD] | 1.9 ± 0.7 | 1.9 ± 0.7 | 1.9 ± 0.5 | 0.915 | F2164 = 0.09 |
| Number of steps/day [mean ± SD] | 11,416 ± 3184 | 10,473 ± 2478 | 11,044 ± 1917 | 0.142 | F2164 = 1.97 |
| Total sleep (hours)/day [mean ± SD] | 5.5 ± 0.6 | 5.6 ± 0.5 | 5.7 ± 0.5 | 0.264 | F2164 = 1.34 |
| The BJSQ | Model 1 a | Model 2 b | Model 3 c |
|---|---|---|---|
| Explanatory variable | β (CI) | β (CI) | β (CI) |
| Metabolic syndrome | |||
| No MetS | Reference | Reference | Reference |
| Pre-MetS | 10.80 ** (2.85, 18.82) | 9.02 ** (0.82, 17.22) | 7.84 * (0.17, 15.51) |
| MetS | 3.30 (−5.09, 11.69) | 0.71 (−7.98, 9.40) | 2.75 (−5.42, 10.91) |
| Intercept | 121.47 | 117.43 | 163.24 |
| The BJSQ | Model 1 a | Model 2 b | Model 3 c |
|---|---|---|---|
| Explanatory variable | β (CI) | β (CI) | β (CI) |
| BMI | 1.00 (−0.40, 2.40) | 0.92 (−0.50, 2.34) | 1.32 (−0.02, 2.65) |
| Intercept | 100.16 | 95.93 | 128.37 |
| The BJSQ | Model 1 a | Model 2 b | Model 3 c |
|---|---|---|---|
| Explanatory variable | β (CI) | β (CI) | β (CI) |
| Metabolic syndrome | |||
| No MetS | Reference | Reference | Reference |
| Pre-MetS | 16.36 ** (3.97, 28.75) | 14.29 * (1.20, 27.37) | 14.09 * (1.71, 26.48) |
| MetS | 12.42 (−0.74, 25.57) | 8.39 (−5.81, 22.59) | 14.72 * (0.93, 28.51) |
| Intercept | 126.50 | 126.18 | 148.48 |
| BMI | 2.12 (−0.18, 4.42) | 2.07 (−0.44, 4.58) | 2.52 * (0.05, 4.99) |
| Intercept | 76.22 | 78.58 | 77.07 |
| The BJSQ | Model 1 a | Model 2 b | Model 3 c |
|---|---|---|---|
| Explanatory variable | β (CI) | β (CI) | β (CI) |
| Metabolic syndrome | |||
| No MetS | Reference | Reference | Reference |
| Pre-MetS | 5.24 (−4.44, 14.93) | 3.13 (−6.90, 13.15) | 3.18 (−7.01, 13.37) |
| MetS | −6.98 (−17.1, 3.14) | −9.11 (−19.27, 1.05) | −8.14 (−18.72, 2.45) |
| Intercept | 107.59 | 102.26 | 145.62 |
| BMI | 0.37 (−1.28, 2.02) | 0.34 (−1.33, 2.01) | 0.63 (−1.11, 2.37) |
| Intercept | 102.52 | 96.92 | 147.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, H.; Svensson, T.; Chung, U.-i.; Svensson, A.K. Sleep Satisfaction May Modify the Association between Metabolic Syndrome and BMI, Respectively, and Occupational Stress in Japanese Office Workers. Int. J. Environ. Res. Public Health 2022, 19, 5095. https://doi.org/10.3390/ijerph19095095
Pham H, Svensson T, Chung U-i, Svensson AK. Sleep Satisfaction May Modify the Association between Metabolic Syndrome and BMI, Respectively, and Occupational Stress in Japanese Office Workers. International Journal of Environmental Research and Public Health. 2022; 19(9):5095. https://doi.org/10.3390/ijerph19095095
Chicago/Turabian StylePham, Helena, Thomas Svensson, Ung-il Chung, and Akiko Kishi Svensson. 2022. "Sleep Satisfaction May Modify the Association between Metabolic Syndrome and BMI, Respectively, and Occupational Stress in Japanese Office Workers" International Journal of Environmental Research and Public Health 19, no. 9: 5095. https://doi.org/10.3390/ijerph19095095
APA StylePham, H., Svensson, T., Chung, U.-i., & Svensson, A. K. (2022). Sleep Satisfaction May Modify the Association between Metabolic Syndrome and BMI, Respectively, and Occupational Stress in Japanese Office Workers. International Journal of Environmental Research and Public Health, 19(9), 5095. https://doi.org/10.3390/ijerph19095095






