Evaluation of Household Preparedness and Risk Factors for Cutaneous Leishmaniasis (CL) Using the Community Assessment for Public Health Emergency Response (CASPER) Method in Pakistan
Abstract
:1. Introduction
2. Material and Methods
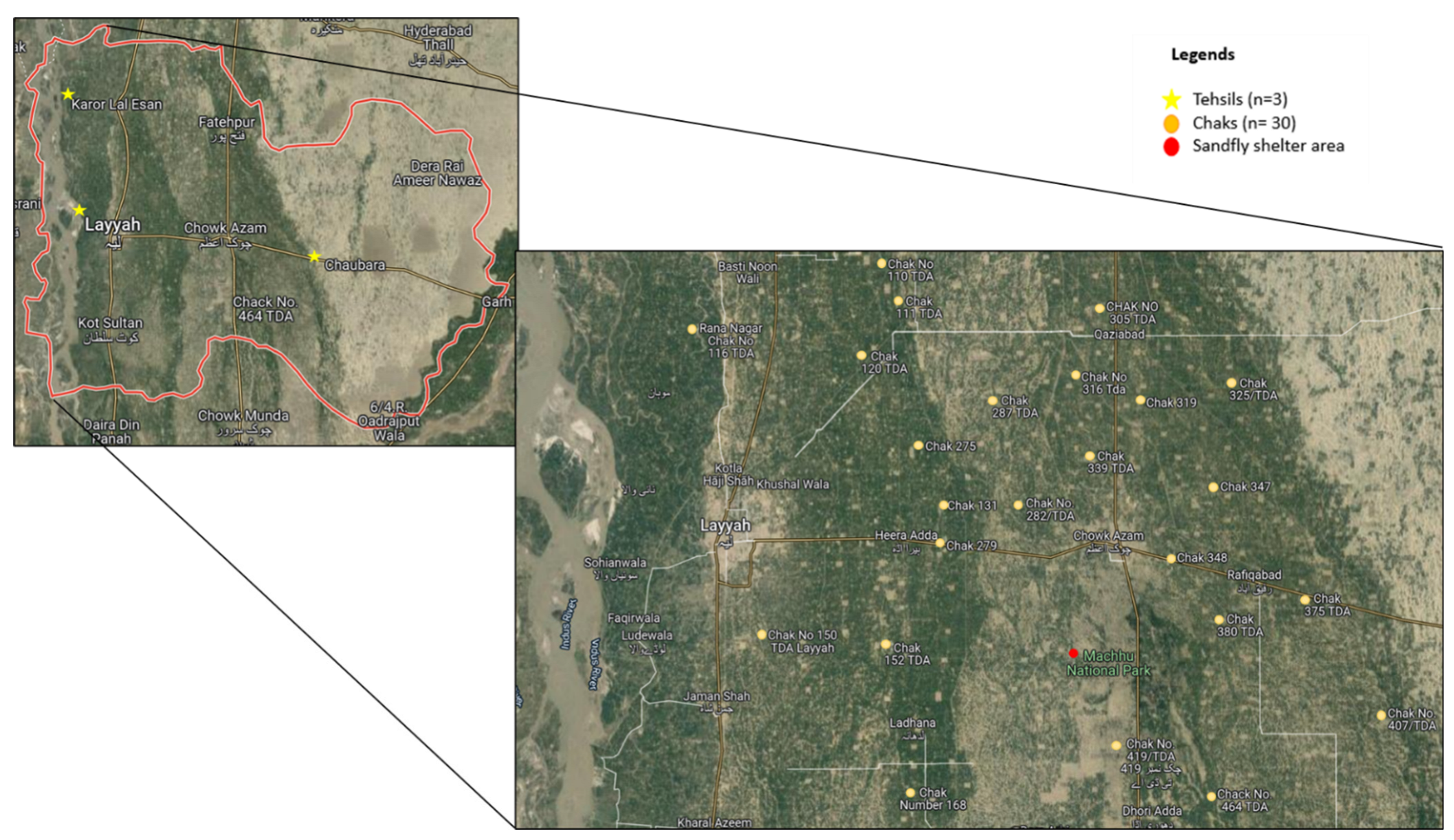
2.1. Study Area
2.2. Ethical Approval
2.3. Study Design
2.4. CASPER Survey
2.5. Participation and Population Size
2.6. Data Analysis
3. Results
3.1. Socio-Demographic Characteristics of the Households
3.2. Disease Concerns of Households
3.3. Preparedness of Households
3.4. Practices and Environmental Characteristics of Households
3.5. Preventive Behavior of Households
3.6. Association of Sociodemographic Characteristics with Disease Concern, Preparedness, Practices, and Preventive Behavior of Households
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elmahallawy, E.K.; Martínez, A.S.; Rodriguez-Granger, J.; Hoyos-Mallecot, Y.; Agil, A.; Mari, J.M.N.; Fernández, J.G. Diagnosis of leishmaniasis. J. Infect. Dev. Ctries 2014, 8, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, S.P.; Makaritsis, K.P.; Dalekos, G.N. Leishmaniasis revisited: Current aspects on epidemiology, diagnosis and treatment. J. Transl. Med. 2015, 3, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Lin, Q.; Ran, J.; Musa, S.S.; Yang, G.; Wang, W.; He, D. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019,to 2020: A data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020, 92, 214–217. [Google Scholar] [CrossRef] [Green Version]
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Ghatee, M.A.; Taylor, W.R.; Karamian, M. The geographical distribution of cutaneous leishmaniasis causative agents in Iran and its neighboring countries, a review. Front. Public Health 2020, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- de Souza, A.; Marins, D.S.S.; Mathias, S.L.; Monteiro, L.M.; Yukuyama, M.N.; Scarim, C.B.; Bou-Chacra, N.A. Promising nanotherapy in treating leishmaniasis. Int. J. Pharm. 2018, 547, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 1–15. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Team, W.L.C. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Dickie, E.A.; Ronin, C.; Sá, M.; Ciesielski, F.; Trouche, N.; Tavares, J.; MacDougall, J. Towards chemical validation of Leishmania infantum ribose 5-phosphate isomerase as a drug target. Antimicrob. Agents Chemother. 2021, 65, e01892-20. [Google Scholar] [CrossRef]
- Akram, A.; Khan, H.A.A.; Qadir, A.; Sabir, A.M. A cross-sectional survey of knowledge, attitude and practices related to cutaneous leishmaniasis and sand flies in Punjab, Pakistan. PLoS ONE 2015, 10, e0130929. [Google Scholar] [CrossRef]
- Zeb, I.; Ali, A.; Nawab, J.; Khan, M.Q.; Kamil, A.; Tsai, K.H. Cutaneous leishmaniasis in male schoolchildren in the upper and lower Dir districts of Khyber Pakhtunkhwa, and a review of previous record in Pakistan. Acta Trop. 2020, 209, 105578. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.; Qureshi, N.A.; Qureshi, M.Z.; Fatima, H.; Afzal, M.; Alhewairini, S.S. Molecular epidemiological survey of cutaneous leishmaniasis from Azad Jammu and Kashmir, Pakistan. Acta Trop. 2020, 206, 105434. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.H.; ul Bari, A.; Hashim, R.; Khan, I.; Muneer, A.; Shah, A.; Sutherland, C.J. Cutaneous leishmaniasis in Khyber Pakhtunkhwa province of Pakistan: Clinical diversity and species-level diagnosis. Am. J. Trop. Med. 2016, 95, 1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuzaid, A.A.; Abdoon, A.M.; Aldahan, M.A.; Alzahrani, A.G.; Alhakeem, R.F.; Asiri, A.M.; Memish, Z.A. Cutaneous leishmaniasis in Saudi Arabia: A comprehensive overview. Vector-Borne Zoonotic Dis. 2017, 17, 673–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horney, J.; Goldberg, D.; Hammond, T.; Stone, K.; Smitherman, S. Assessing the prevalence of risk factors for neglected tropical diseases in Brazos County, Texas. PLoS Curr. 2017, 9. [Google Scholar] [CrossRef]
- Horney, J.; Davis, M.K.; Davis, S.E.; Fleischauer, A. An evaluation of community assessment for public health emergency response (CASPER) in North Carolina, 2003–2010. Prehospital. Disaster. Med. 2013, 28, 94–98. [Google Scholar] [CrossRef]
- Schnall, A.H.; Wolkin, A.F.; Roth, J.; Ellis, E.M. Community Assessments for Public Health Emergency Response (CASPERs)—US Virgin Islands, 2017–2018. Am. J. Public Health 2019, 109, S303–S308. [Google Scholar] [CrossRef]
- Kolaczinski, J.; Brooker, S.; Reyburn, H.; Rowland, M. Epidemiology of anthroponotic cutaneous leishmaniasis in Afghan refugee camps in northwest Pakistan. Trans. R Soc. Trop. Med. Hyg. 2004, 98, 373–378. [Google Scholar] [CrossRef]
- Mursalin, S.M.; Sheikh, A.; Crilly, J.; Bino, S. Leishmaniasis Gap Analysis Report and Action Plan: Strengthening the Epidemiologial Surveillance, Diagnosis and Treatment of Visceral and Cutaneous Leishmaniasis in Albania, Jordan and Pakistan; Connecting Organisations for Regional Disease Surveillance (CORDS): San Francisco, CA, USA, 2015. [Google Scholar]
- Ahmed, N.; Mahmood, A.; Mahmood, A.; Tahir, S.; Bano, A.; Malik, R.N.; Ishtiaq, M. Relative importance of indigenous medicinal plants from Layyah district, Punjab Province, Pakistan. J. Ethnopharmacol. 2014, 155, 509–523. [Google Scholar] [CrossRef]
- Hu, C.; Boota, M.W.; Soomro, M.H.A.A.; Jian, S.; Zafar, Z.; Li, X. Mapping flood extend and its impact on land use/land cover and settlements variations: A case study of Layyah District, Punjab, Pakistan. Acta Geophys. 2021, 69, 2291–2304. [Google Scholar]
- Frary, R.B. A Brief Guide to Questionnaire Development. Virginia Polytechnic Institute and State University. 2003. Available online: http://ericae.net/ft/tamu/vpiques3.htm (accessed on 10 January 2021).
- Khan, A.; Sajid, R.; Gul, S.; Hussain, A.; Zehri, M.T.; Naz, S.; Simsek, S.; Waseem, S.; Afzal, M.S.; Naqvi, S.K.U.H.; et al. Epidemiological and pathological characteristics of Cutaneous Leishmaniasis from Baluchistan Province of Pakistan. Parasitolo 2021, 148, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Amin, T.T.; Kaliyadan, F.; Al-Ajyan, M.; Al-Arfaj, A.; Al-mujhim, M.; Al-Harbi, S.; Al Mohammed, H. Public awareness and attitudes towards cutaneous leishmaniasis in an endemic region in Saudi Arabia. J. Eur. Acad. Dermatol. 2012, 26, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, K.; Rijal, S.; Koirala, S.; Koirala, S.; Vanlerberghe, V.; Van der Stuyft, P.; Boelaert, M. Visceral leishmaniasis in southeastern Nepal: A cross-sectional survey on Leishmania donovani infection and its risk factors. Trop. Med. Int. Health 2006, 11, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, M.; Sádlová, J.; Chang, K.P.; Volf, P. Distribution and feeding preference of the sand flies Phlebotomus sergenti and P. papatasi in a cutaneous leishmaniasis focus in Sanliurfa, Turkey. Am. J. Trop. Med. 2003, 68, 6–9. [Google Scholar] [CrossRef]
- Killick-Kendrick, R.; Killick-Kendrick, M.; Tang, Y. Anthroponotic cutaneous leishmaniasis in Kabul, Afghanistan: The high susceptibility of Phlebotomus sergenti to Leishmania tropica. Trans. R. Soc. 1995, 89, 477. [Google Scholar] [CrossRef]
- Klassen-Fischer, M.K.; Neafie, R.C.; Wear, D.J.; Meyers, W.M. Crytosporidiosis, Isosporiasis, Cyclosporiasis & Sarcocystosis; Inova Central Lab: Fairfax, VA, USA, 2011. [Google Scholar]
- Saberi, S.; Zamani, A.; Motamedi, N.; Nilforoushzadeh, M.A.; Jaffary, F.; Rahimi, E.; Hejazi, S.H. The knowledge, attitude, and prevention practices of students regarding cutaneous leishmaniasis in the hyperendemic region of the Shahid Babaie Airbase. Vector Borne Zoonotic Dis. 2012, 12, 306–309. [Google Scholar] [CrossRef]
- Koirala, S.; Parija, S.C.; Karki, P.; Das, M.L. Knowledge, attitudes, and practices about kala-azar and its sandfly vector in rural communities of Nepal. Bull. World Health Organ. 1998, 76, 485–490. [Google Scholar]
- Coulibaly, C.; Sissoko, I.; Traore, B.; Diallo, A.; Samake, S.; Traore, S.; Doumbia, S. Diversity of sand flies (Diptera: Psychodidae: Phlebotominae) in two different eco-climatic and endemic zones of cutaneous Leishmaniasis in Mali, West Africa. J. Med. Entomol. 2016, 53, 923–927. [Google Scholar] [CrossRef] [Green Version]
- Hejazi, S.; Hazavei, S.; Bidabadi, L.S.; Shademani, A.; Siadat, A.; Zolfaghari-Baghbaderani, A.; Hosseini, S. Evaluation of knowledge, attitude and performance of the mothers of children affected by cutaneous leishmaniasis. Infect. Dis. 2010, 3, S3786. [Google Scholar] [CrossRef]
- Nandha, B.; Srinivasan, R.; Jambulingam, P. Cutaneous leishmaniasis: Knowledge, attitude and practices of the inhabitants of the Kani forest tribal settlements of Tiruvananthapuram district, Kerala, India. Health Educ. Res. 2014, 29, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Sarkari, B.; Qasem, A.; Shafaf, M.R. Knowledge, attitude, and practices related to cutaneous leishmaniasis in an endemic focus of cutaneous leishmaniasis, Southern Iran. Asian Pac. J. Trop. Biomed. 2014, 4, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Irum, S.; Aftab, M.; Khan, A.; Naz, S.; Simsek, S.; Habib, A.; Ahmed, H. Cutaneous Leishmaniasis (CL): A cross-sectional community-based survey on knowledge, attitude and practices in a highly endemic area of Waziristan (KPK Province), Pakistan. Acta Trop. 2021, 213, 105746. [Google Scholar] [CrossRef] [PubMed]
| Sr. No | Socio-Demographic Characteristics | Frequency (n) | Percentage (%) |
|---|---|---|---|
| 1 | Gender Male Female | 403 97 | 80.6 19.4 |
| 2 | Age 18–28 29–38 ≥39 | 168 175 157 | 33.6 35 31.4 |
| 3 | Household head Male Female | 363 137 | 72.6 27.4 |
| 4 | Household members 2–4 5–8 >8 | 73 324 103 | 14.6 64.8 20.6 |
| 5 | No of children in the household 1–3 4–6 >6 | 253 219 28 | 50.6 43.8 5.6 |
| 6 | Ethnicity Punjabi Saraiki Pathan | 181 251 68 | 36.2 50.2 13.6 |
| 7 | Activities followed by children Indoor Outdoor | 160 340 | 32 68 |
| 8 | Education College/University High School Secondary Primary Illiterate | 42 81 77 153 147 | 8.4 16.2 15.4 30.6 29.4 |
| 9 | Family structure Nuclear family Extended family | 364 136 | 72.8 27.2 |
| 10 | Monthly income (PKR) <10,000 10,000–25,000 25,000–50,000 >50,000 | 45 156 198 101 | 9 31.2 39.6 20.2 |
| Variables | Frequency (n) | Percentage (%) |
|---|---|---|
| Disease Concern | ||
|
Have you ever heard about Zoonotic disease? Yes No | 218 282 | 43.6 56.4 |
|
Do you know about cutaneous leishmaniasis (CL)? Yes No | 165 335 | 33 67 |
|
Did you ever see a leishmaniasis patient or have a history of leishmaniasis? Yes No | 56 444 | 11.2 88.8 |
|
Do you think cutaneous leishmaniasis is a lethal disease? Yes No | 31 469 | 6.2 93.8 |
|
Do you know about sandflies? Yes No | 235 265 | 47 53 |
|
Can you identify/differentiate sandflies from common houseflies/mosquitoes? Yes No | 210 290 | 42 58 |
|
Do you think sandflies carry this disease? Yes No | 103 397 | 20.6 79.4 |
|
What is the peak incidence time? Morning Evening Dawn to dusk Night Do not Know | 87 101 170 65 77 | 17.4 20.2 34 13 15.4 |
|
Do you know about the life cycle of sandflies? Yes No | 59 441 | 11.8 88.2 |
|
In your opinion, CL is more common among which gender? Male Female Both Don’t Know | 19 36 326 119 | 3.8 7.2 65.2 23.8 |
|
Who is the at most at risk from CL? Pregnant women Women of childbearing age 15–44 Disable people. Adolescence 15–24 Children Older Adult Everybody Do not Know | 16 2 37 7 89 2 254 93 | 3.2 0.4 7.4 1.4 17.8 0.4 50.8 18.6 |
|
What are the risk factors for cutaneous leishmaniasis? Poor hygiene House architecture Walking barefoot Sleeping in an open area All above Don’t Know | 138 12 19 51 179 101 | 27.6 2.4 3.8 10.2 35.8 20.2 |
|
Where do you and members of your household typically get bitten by sandfly/mosquitoes? Home Work School Park Others | 282 103 49 58 8 | 56.4 20.6 9.8 11.6 1.6 |
|
Does camping in the desert⁄ farm (in an open area) increase the risk for disease? Yes No | 149 351 | 29.8 70.2 |
|
Can this disease be cured? Yes No | 148 352 | 29.6 70.4 |
| Variables | Frequency (n) | Percentage (%) |
|---|---|---|
| Preparedness | ||
|
CL is associated with dusty areas Agree Disagree | 202 298 | 40.4 59.6 |
|
CL is a serious health problem in this community Agree Disagree | 59 441 | 11.8 88.2 |
|
CL is treated by traditional and herbal preparations Agree Disagree | 189 311 | 37.8 62.2 |
|
Does your household have adequate drinking water? Yes No | 500 0 | 100 0 |
|
Do you or members of your family hear about this survey prior to us talking to you today? If yes, how did you or your HH member hear about it Social media Website Press release Family/friends No | 37 39 0 91 333 | 7.4 7.8 0 18.2 66.6 |
|
What actions do you believe the health department should take to prevent Cutaneous Leishmaniasis disease? Education/Awareness Inspection for waste management Spraying for sandflies | 184 173 143 | 36.8 34.6 28.6 |
| Variables | Frequency (n) | Percentage (%) |
|---|---|---|
| Practices and Environmental Characteristics | ||
|
How your family disposes waste or garbage every day? Throw garbage on the street Throw in bins Burn it properly | 325 95 80 | 65 19 16 |
|
How do you maintain good hygiene at home? Proper floor cleaning Spray at home Keeping house dust free All above None | 148 5 49 168 130 | 29.6 0.1 9.8 33.6 26 |
|
Do you walk barefoot at home? Yes No | 466 34 | 93.2 6.8 |
|
Do you have access to the forest? Yes No | 282 218 | 56.4 43.6 |
|
Do you prefer spraying your houses and animal shelters? Yes No | 37 463 | 7.4 92.6 |
|
Do you prefer the use of nets treated with insecticides while sleeping? Yes No | 97 403 | 19.4 80.6 |
|
From what type of material your house is made up of Wood Bricks Stones Mud | 1 399 3 97 | 0.2 79.8 0.6 19.4 |
|
The floor of your house made up of Bricks Sand Stone | 115 172 213 | 23 34.4 42.6 |
|
Does the waste present around your house? Yes No | 376 124 | 75.2 24.8 |
|
Do you avoid areas of mosquito exposure? Yes No | 130 370 | 26 74 |
|
Do you have pets? Yes No | 361 139 | 72.2 27.8 |
|
How do you keep your pets? Free range Tied | 219 142 | 60.6 39.4 |
|
Do you use skin repellent? Yes No | 80 420 | 16 84 |
|
What, if any, are barriers to using mosquito repellent? Too expensive Product not available Prefer natural remedies Rashes or irritated skin All above | 110 86 223 78 3 | 22 17.2 44.6 15.6 0.6 |
|
What is your HH current source of drinking water? Unfiltered tap Filtered tap water | 430 70 | 86 14 |
| Variables | Frequency (n) | Percentage (%) |
|---|---|---|
| Preventive Behavior | ||
| Do you take any actions to protect yourselves? Yes No | 98 402 | 19.6 80.4 |
| Do you wear protective clothing? Yes No | 74 426 | 14.8 85.2 |
| Do you avoid being outside at peak times? Yes No | 45 455 | 0.9 91 |
| Do you use burn mosquito coils or candles? Yes No | 62 438 | 12.4 87.6 |
| After knowing this disease, what preventive measures will you take to prevent yourself from this disease? Bath daily Maintain good hygiene Stay away from animals Wear full sleeves Avoiding going outdoor during dusk and dawn All above None | 34 124 5 8 31 282 16 | 6.8 24.8 0.1 1.6 6.2 56.4 3.2 |
| Socio-Demographic Variables | Disease Concern | p-Value | |
|---|---|---|---|
| Adequate n (%) | Poor n (%) | ||
| Gender Male Female | 89 (22.1) 314 (77.9) | 43 (44.3) 54 (55.7) | p < 0.05 |
| Age 18–28 29–38 ≥39 | 49 (29.2) 46 (26.3) 37 (23.6) | 119 (70.8) 129 (73.7) 120 (76.4) | 0.51 |
| Household head Male Female | 95 (26.2) 37 (27) | 268 (73.8) 100 (73) | 0.85 |
| Household members 2–4 5–8 >8 | 39 (53.4) 79 (24.4) 14 (13.6) | 34 (46.6) 245 (75.6) 89 (86.4) | p < 0.05 |
| No of children in the household 1–3 4–6 >6 | 93 (36.8) 38 (17.4) 1 (3.6) | 160 (63.2) 181 (82.6) 27 (96.4) | p < 0.05 |
| Ethnicity Punjabi Saraiki Pathan | 51 (28.2) 77 (30.7) 4 (5.9) | 130 (71.8) 174 (69.3) 64 (94.1) | p < 0.05 |
| Activities followed by children Indoor Outdoor | 74 (46.3) 58 (17.1) | 86 (53.8) 282 (82.9) | p < 0.05 |
| Education College/University High School Secondary Primary Illiterate | 80 (54.4) 35 (22.9) 7 (9.1) 6 (7.4) 4 (9.5) | 67 (45.6) 118 (77.1) 70 (90.9) 75 (92.6) 38 (90.5) | p < 0.05 |
| Family structure Nuclear family Extended family | 102 (28) 30 (22.1) | 262 (72) 106 (77.9) | 0.17 |
| Monthly income <10,000 10,000–25,000 25,000–50,000 >50,000 | 0 (0) 22 (14.1) 76 (38.4) 34 (33.7) | 45 (100) 134 (85.9) 122 (61.6) 67 (66.3) | p < 0.05 |
| Preparedness | |||
| Adequate n (%) | Poor n (%) | ||
| Gender Male Female | 77 (19.1) 38 (39.2) | 326 (80.9) 59 (60.8) | p < 0.05 |
| Household head Male Female | 88 (24.2) 27 (19.7) | 275 (75.8) 110 (80.3) | 0.28 |
| Household members 2–4 5–8 >8 | 39 (53.4) 65 (20.1) 11 (10.7) | 34 (46.6) 259 (79.9) 92 (89.3) | p < 0.05 |
| No of children in household 1–3 4–6 >6 | 76 (30) 36 (16.4) 3 (10.7) | 177 (70) 183 (83.6) 25 (89.3) | p < 0.05 |
| Ethnicity Punjabi Saraiki Pathan | 53 (29.3) 55 (21.9) 7 (10.3) | 128 (70.7) 196 (78.1) 61 (89.7) | p < 0.05 |
| Activities followed by children Indoor Outdoor | 56 (35) 59 (17.4) | 104 (65) 281 (82.6) | p < 0.05 |
| Education College/University High School Secondary Primary Illiterate | 64 (43.5) 29 (19) 9 (11.7) 9 (11.1) 4 (9.5) | 83 (56.5) 124 (81) 68 (88.3) 72 (88.9) 38 (90.5) | p < 0.05 |
| Family structure Nuclear family Extended family | 98 (26.9) 17 (12.5) | 266 (73.1) 119 (87.5) | p < 0.05 |
| Monthly income (PKR) <10,000 10,000–25,000 25,000–50,000 >50,000 | 2 (4.4) 21 (13.5) 64 (32.3) 28 (27.7) | 43 (95.6) 135 (86.5) 134 (67.7) 73 (72.3) | p < 0.05 |
| Practices | |||
| Adequate n (%) | Poor n (%) | ||
| Gender Male Female | 40 (9.9) 12 (12.4) | 363 (90.1) 85 (87.6) | 0.47 |
| Age 18–28 29–38 ≥39 | 23 (13.7) 23 (13.1) 6 (3.8) | 145 (86.3) 152 (86.9) 151 (96.2) | p < 0.05 |
| Household head Male Female | 39 (10.7) 13 (9.5) | 324 (89.3) 124 (90.5) | 0.68 |
| Household members 2–4 5–8 >8 | 13 (17.8) 31 (9.6) 8 (7.8) | 60 (82.2) 293 (90.4) 95 (92.2) | 0.07 |
| No. of children in household 1–3 4–6 >6 | 29 (11.5) 20 (9.1) 3 (10.7) | 224 (88.5) 199 (90.9) 25 (89.3) | 0.70 |
| Ethnicity Punjabi Saraiki Pathan | 26 (14.4) 24 (9.6) 2 (2.9) | 155 (85.6) 227 (90.4) 66 (97.1) | p < 0.05 |
| Activities followed by children Indoor Outdoor | 27 (16.9) 25 (7.4) | 133 (83.1) 315 (92.6) | p < 0.05 |
| Education College/University High School Secondary Primary Illiterate | 35 (23.8) 13 (8.5) 2 (2.6) 2 (2.5) 0 (0) | 112 (76.2) 140 (91.5) 75 (97.4) 79 (97.5) 42 (100) | p < 0.05 |
| Family structure Nuclear family Extended family | 35 (9.6) 17 (12.5) | 329 (90.4) 119 (87.5) | 0.34 |
| Monthly income <10,000 10,000–25,000 25,000–50,000 >50,000 | 0 (0) 3 (1.9) 31 (15.7) 18 (17.8) | 45 (100) 153 (98.1) 167 (84.3) 83 (82.2) | p < 0.05 |
| Preventive behavior | |||
| Adequate n (%) | Poor n (%) | ||
| Gender Male Female | 55 (13.6) 22 (22.7) | 348 (86.4) 75 (77.3) | p < 0.05 |
| Age 18–28 29–38 ≥39 | 41 (24.4) 25 (14.3) 11 (7) | 127 (75.6) 150 (85.7) 146 (93) | p < 0.05 |
| Household head Male Female | 58 (16) 19 (13.9) | 305 (84) 118 (86.1) | 0.56 |
| Household members 2–4 5–8 >8 | 19 (26) 54 (16.7) 4 (3.9) | 54 (74) 270 (83.3) 99 (96.1) | p < 0.05 |
| No of children in household 1–3 4–6 >6 | 45 (17.8) 29 (13.2) 3 (10.7) | 208 (82.2) 190 (86.8) 25 (89.3) | 0.30 |
| Ethnicity Punjabi Saraiki Pathan | 33 (18.2) 43 (17.1) 1 (1.5) | 148 (81.8) 208 (82.9) 67/98.5 | p < 0.05 |
| Activities followed by children Indoor Outdoor | 32 (20) 45 (13.2) | 128 (80) 295 (86.8) | p < 0.05 |
| Education College/University High School Secondary Primary Illiterate | 48 (32.7) 19 (12.4) 6 (7.8) 4 (4.9) 0 (0) | 99 (67.3) 134 (87.6) 71 (92.2) 77 (95.1) 42 (100) | p < 0.05 |
| Family structure Nuclear family Extended family | 60 (16.5) 17 (12.5) | 304 (83.5) 119 (87.5) | 0.27 |
| Monthly income <10,000 10,000–25,000 25,000–50,000 >50,000 | 0 (0) 12 (7.7) 42 (21.2) 23 (22.8) | 45 (100) 144 (92.3) 156 (78.8) 78 (77.2) | p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Numan, M.; Naz, S.; Gilani, R.; Minhas, A.; Ahmed, H.; Cao, J. Evaluation of Household Preparedness and Risk Factors for Cutaneous Leishmaniasis (CL) Using the Community Assessment for Public Health Emergency Response (CASPER) Method in Pakistan. Int. J. Environ. Res. Public Health 2022, 19, 5068. https://doi.org/10.3390/ijerph19095068
Numan M, Naz S, Gilani R, Minhas A, Ahmed H, Cao J. Evaluation of Household Preparedness and Risk Factors for Cutaneous Leishmaniasis (CL) Using the Community Assessment for Public Health Emergency Response (CASPER) Method in Pakistan. International Journal of Environmental Research and Public Health. 2022; 19(9):5068. https://doi.org/10.3390/ijerph19095068
Chicago/Turabian StyleNuman, Muhammad, Shumaila Naz, Rehama Gilani, Azhar Minhas, Haroon Ahmed, and Jianping Cao. 2022. "Evaluation of Household Preparedness and Risk Factors for Cutaneous Leishmaniasis (CL) Using the Community Assessment for Public Health Emergency Response (CASPER) Method in Pakistan" International Journal of Environmental Research and Public Health 19, no. 9: 5068. https://doi.org/10.3390/ijerph19095068
APA StyleNuman, M., Naz, S., Gilani, R., Minhas, A., Ahmed, H., & Cao, J. (2022). Evaluation of Household Preparedness and Risk Factors for Cutaneous Leishmaniasis (CL) Using the Community Assessment for Public Health Emergency Response (CASPER) Method in Pakistan. International Journal of Environmental Research and Public Health, 19(9), 5068. https://doi.org/10.3390/ijerph19095068







