Current Situation of Palytoxins and Cyclic Imines in Asia-Pacific Countries: Causative Phytoplankton Species and Seafood Poisoning
Abstract
:1. Introduction
1.1. Harmful Algal Blooms (HABs) and Shellfish Poisoning
1.2. Marine Biotoxins
2. Phytoplankton-Derived Toxins
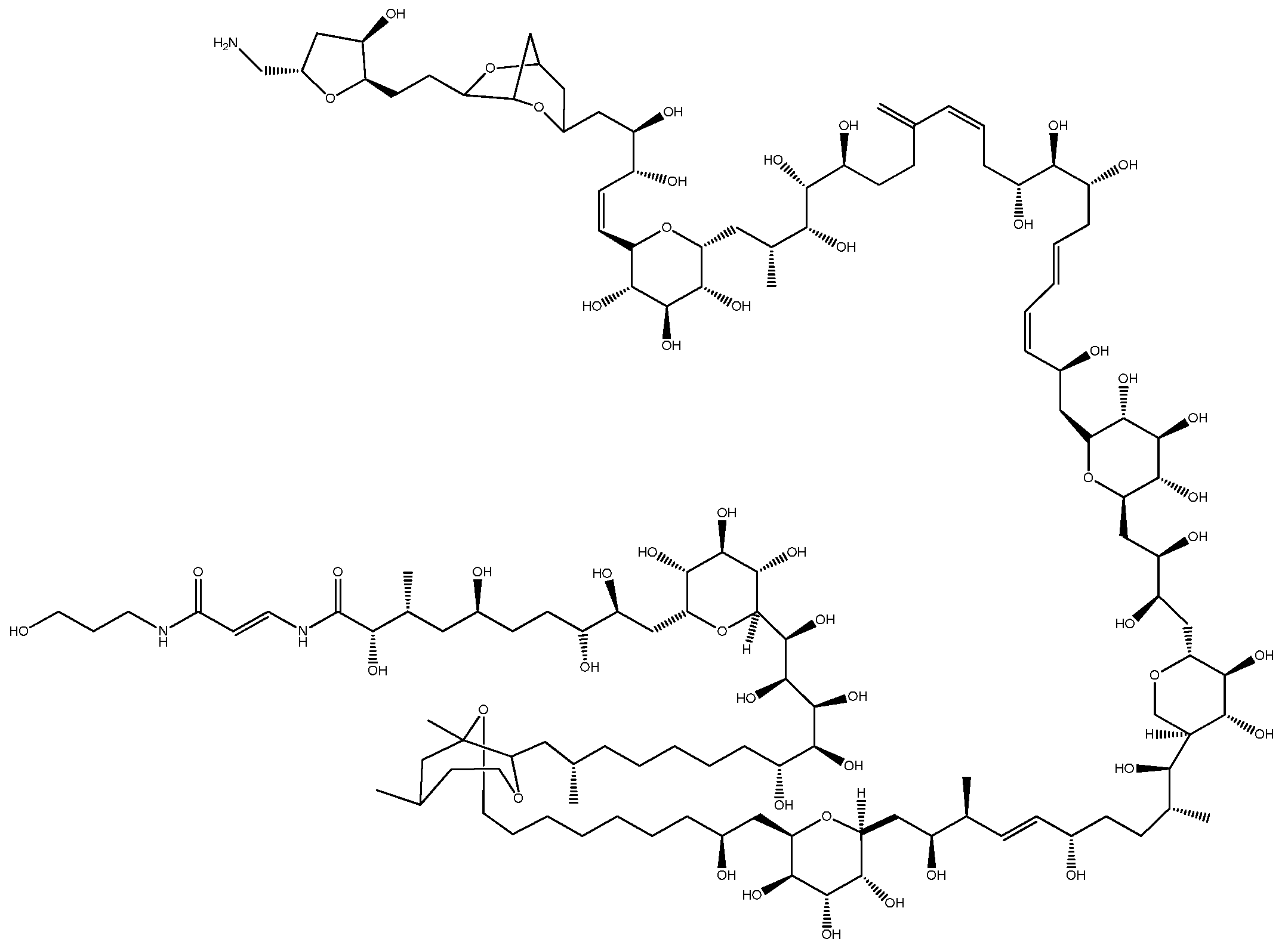
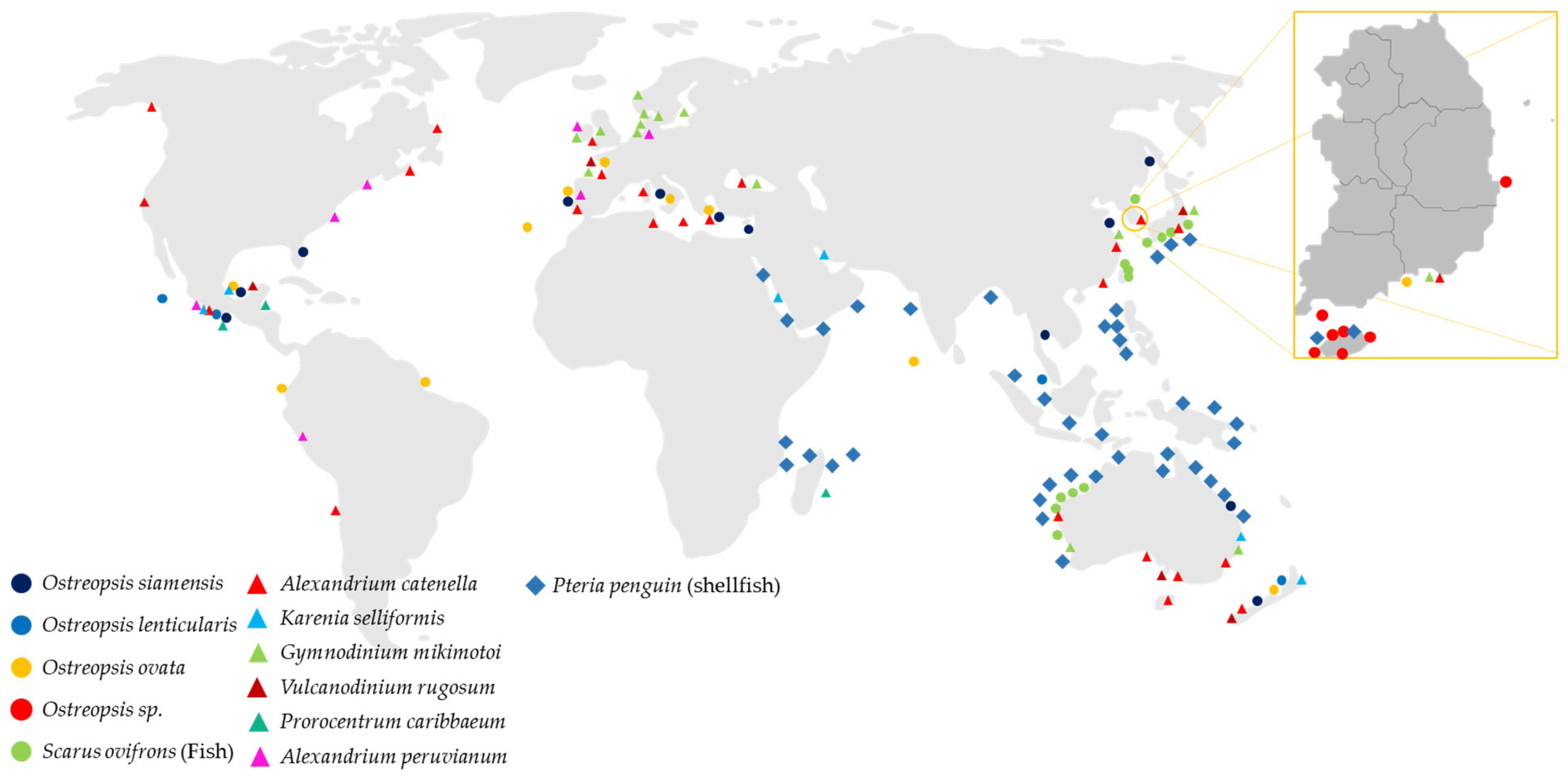
2.1. Ostreopsis—PlTX and Its Analogs
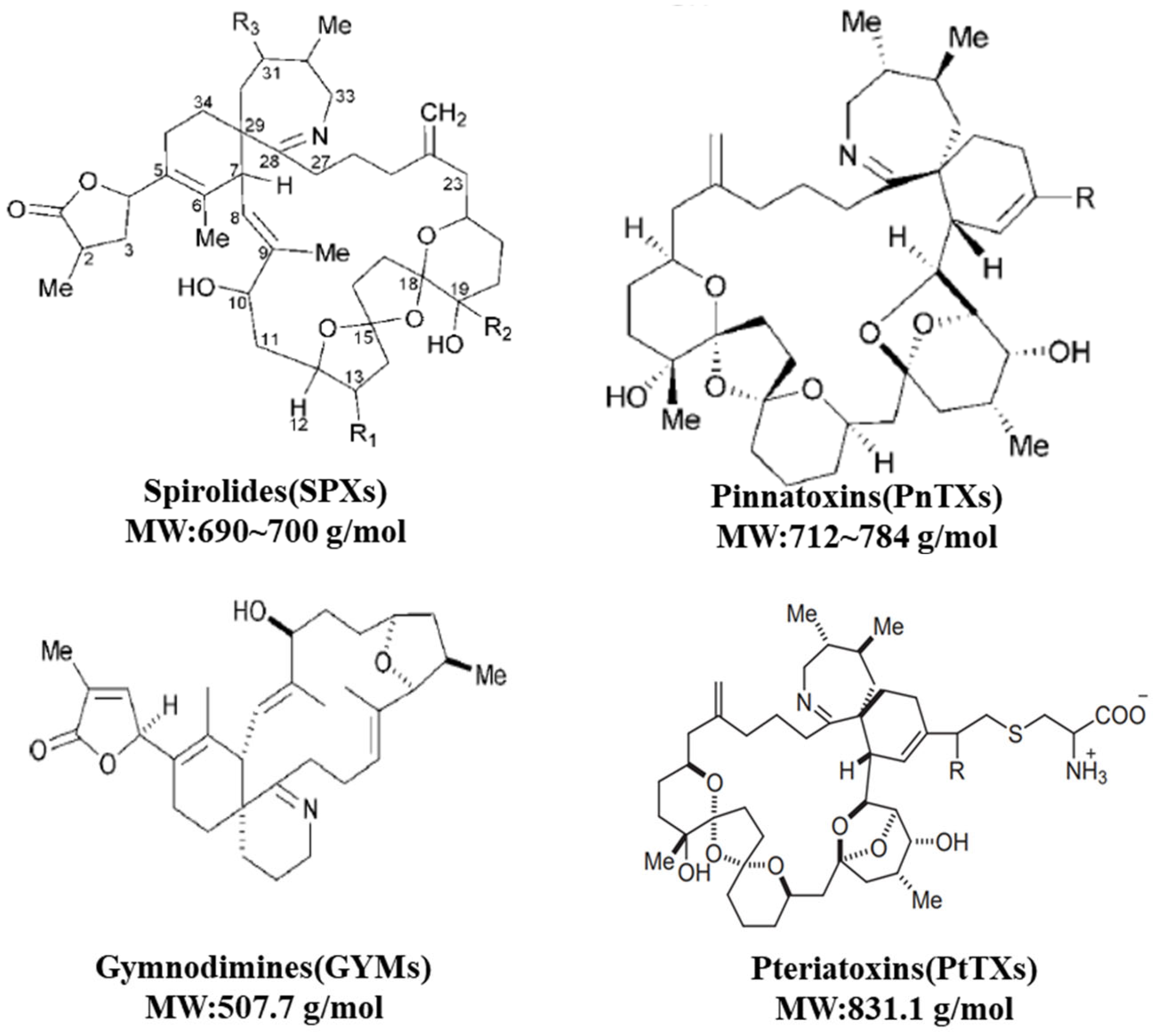
2.2. CIs: SPXs, PnTXs, GYMs and PtTXs
2.2.1. A. ostenfeldii—SPX and GYM
2.2.2. Vulcanodinium rugosum—PnTXs
2.2.3. P. penguin—PtTX
2.2.4. Karenia spp.—GYM
3. Analytical Protocols for Novel Marine Biotoxins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Roser, M.; Ritchie, H. Seafood Production. Available online: https://ourworldindata.org/seafood-production (accessed on 15 December 2021).
- Dolah, F.M.V. Marine algal toxins: Origins, health effects, and their increased occurrence. Environ. Health Perspect. 2000, 108, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Todd, E. Seafood-associated diseases and control in Canada. Rev. Sci. Tech. 1997, 16, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, I. Keeping shellfish safe to eat: A brief review of shellfish toxins, and methods for their detection. Trends Food Sci. Technol. 2000, 11, 235–244. [Google Scholar] [CrossRef]
- Mos, L. Domoic acid: A fascinating marine toxin. Environ. Toxicol. Pharmacol. 2001, 9, 79–85. [Google Scholar] [CrossRef]
- Whittle, K.; Gallacher, S. Marine toxins. Br. Med. Bull. 2000, 56, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [Green Version]
- Griffith, A.W.; Doherty, O.M.; Gobler, C.J. Ocean warming along temperate western boundaries of the Northern Hemisphere promotes an expansion of Cochlodinium polykrikoides blooms. Proc. R. Soc. B 2019, 286, 20190340. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, K.M.; Velthuis, M.; Van de Waal, D.B. Meta-analysis reveals enhanced growth of marine harmful algae from temperate regions with warming and elevated CO2 levels. Glob. Chang. Biol. 2019, 25, 2607–2618. [Google Scholar] [CrossRef] [Green Version]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef] [Green Version]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge1. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- NOAA; CSCOR; COP. Economic Impacts. The Harmful Algae Page. Available online: https://www.whoi.edu/redtide/impacts/economic (accessed on 20 December 2020).
- Nielsen, L.T.; Hansen, P.J.; Krock, B.; Vismann, B. Accumulation, transformation and breakdown of DSP toxins from the toxic dinoflagellate Dinophysis acuta in blue mussels, Mytilus edulis. Toxicon 2016, 117, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munday, R.; Reeve, J. Risk Assessment of Shellfish Toxins. Toxins 2013, 5, 2109–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, S.K.; Plattner, G.-K.; Nauels, A.; Xia, Y.; Stocker, T.F. Climate Change 2013: The Physical Science Basis. In An Overview of the Working Group 1 Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC); IPCC: Geneva, Switzerland, 2014; p. 3544. [Google Scholar]
- Lemke, P.; Ren, J.; Alley, R.B.; Allison, I.; Carrasco, J.; Flato, G.; Fujii, Y.; Kaser, G.; Ote, P.M.; Thomas, R.H.; et al. Observations: Changes in Snow, Ice and Frozen Ground. In Climate Change 2007: The Physical Science Basis Changes; Cambridge University Press: Cambridge, UK, 2007; pp. 387–432. [Google Scholar]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.B.; Tignor, M.; Miller, H.L. (Eds.) IPCC, 2007: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Core Writing Team. IPCC 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef]
- Poletti, R.; Milandri, A.; Pompei, M. Algal Biotoxins of Marine Origin: New Indications from the European Union. Vet. Res. Commun. 2003, 27, 173–182. [Google Scholar] [CrossRef]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- Gerssen, A.; Mulder, P.P.J.; McElhinney, M.A.; de Boer, J. Liquid chromatography–tandem mass spectrometry method for the detection of marine lipophilic toxins under alkaline conditions. J. Chromatogr. A 2009, 1216, 1421–1430. [Google Scholar] [CrossRef]
- Jiang, B. Review on Marine Biotoxins Toxicity and Detection Methods. In Proceedings of the 2020 10th International Conference on Biomedical Engineering and Technology, Tokyo, Japan, 15–18 September 2020; pp. 28–33. [Google Scholar]
- Brissard, C.; Hervé, F.; Sibat, M.; Séchet, V.; Hess, P.; Amzil, Z.; Herrenknecht, C. Characterization of ovatoxin-h, a new ovatoxin analog, and evaluation of chromatographic columns for ovatoxin analysis and purification. J. Chromatogr. A 2015, 1388, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Tartaglione, L.; Mazzeo, A.; Dell’Aversano, C.; Forino, M.; Giussani, V.; Capellacci, S.; Penna, A.; Asnaghi, V.; Faimali, M.; Chiantore, M. Chemical, molecular, and eco-toxicological investigation of Ostreopsis sp. from Cyprus Island: Structural insights into four new ovatoxins by LC-HRMS/MS. Anal. Bioanal. Chem. 2016, 408, 915–932. [Google Scholar] [CrossRef] [Green Version]
- Pelin, M.; Brovedani, V.; Sosa, S.; Tubaro, A. Palytoxin-containing aquarium soft corals as an emerging sanitary problem. Mar. Drugs 2016, 14, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, P.D.; Brimble, M.A. Synthesis of macrocyclic shellfish toxins containing spiroimine moieties. Nat. Prod. Rep. 2007, 24, 869–885. [Google Scholar] [CrossRef] [PubMed]
- Kita, M.; Uemura, D. Shellfish Poisons. In Molluscs: From Chemo-Ecological Study to Biotechnological Application; Cimino, G., Gavagnin, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 25–51. [Google Scholar]
- Beaumont, S.; Ilardi, E.A.; Tappin, N.D.C.; Zakarian, A. Marine Toxins with Spiroimine Rings: Total Synthesis of Pinnatoxin A. Eur. J. Org. Chem. 2010, 2010, 5743–5765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.Y.; Liang, Y.B. Cyclic imine toxin gymnodimine: A review. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2009, 20, 2308–2313. [Google Scholar]
- Hu, T.; Burton, I.W.; Cembella, A.D.; Curtis, J.M.; Quilliam, M.A.; Walter, J.A.; Wright, J.L. Characterization of spirolides a, c, and 13-desmethyl c, new marine toxins isolated from toxic plankton and contaminated shellfish. J. Nat. Prod. 2001, 64, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Satake, M.; Mackenzie, L.; Kaspar, H.F.; Yasumoto, T. Gymnodimine, a new marine toxin of unprecedented structure isolated from New Zealand oysters and the dinoflagellate, Gymnodinium sp. Tetrahedron Lett. 1995, 36, 7093–7096. [Google Scholar] [CrossRef]
- Biré, R.; Krys, S.; Frémy, J.M.; Dragacci, S.; Stirling, D.; Kharrat, R. First evidence on occurrence of gymnodimine in clams from Tunisia. J. Nat. Toxins 2002, 11, 269–275. [Google Scholar]
- Chou, T.; Osamu, K.; Uemura, D. Relative stereochemistry of pinnatoxin A, a potent shellfish poison from Pinna muricata. Tetrahedron Lett. 1996, 37, 4023–4026. [Google Scholar] [CrossRef]
- Chou, T.; Haino, T.; Kuramoto, M.; Uemura, D. Isolation and structure of pinnatoxin D, a new shellfish poison from the okinawan bivalve Pinna muricata. Tetrahedron Lett. 1996, 37, 4027–4030. [Google Scholar] [CrossRef]
- Selwood, A.I.; Miles, C.O.; Wilkins, A.L.; van Ginkel, R.; Munday, R.; Rise, F.; McNabb, P. Isolation, Structural Determination and Acute Toxicity of Pinnatoxins E, F and G. J. Agric. Food Chem. 2010, 58, 6532–6542. [Google Scholar] [CrossRef]
- Hao, J.; Matsuura, F.; Kishi, Y.; Kita, M.; Uemura, D.; Asai, N.; Iwashita, T. Stereochemistry of Pteriatoxins A, B, and C. J. Am. Chem. Soc. 2006, 128, 7742–7743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholin, C.A.; Gulland, F.; Doucette, G.J.; Benson, S.; Busman, M.; Chavez, F.P.; Cordaro, J.; DeLong, R.; De Vogelaere, A.; Harvey, J.; et al. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 2000, 403, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, S.F.; Carrera, C.; Vilariño, N.; Louzao, M.C.; Santamarina, G.; Cantalapiedra, A.G.; Botana, L.M. Acute Cardiotoxicity Evaluation of the Marine Biotoxins OA, DTX-1 and YTX. Toxins 2015, 7, 1030–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on Marine Biotoxins in Shellfish—Cyclic Imines (Spirolides, Gymnodimines, Pinnatoxins and Pteriatoxins); EFSA: Parma, Italy, 2010; p. 1628. [Google Scholar]
- Richter, I.; Fidler, A.E. Detection of marine microalgal biotoxins using bioassays based on functional expression of tunicate xenobiotic receptors in yeast. Toxicon 2015, 95, 13–22. [Google Scholar] [CrossRef]
- Turner, A.D.; Goya, A.B. Occurrence and profiles of lipophilic toxins in shellfish harvested from Argentina. Toxicon 2015, 102, 32–42. [Google Scholar] [CrossRef]
- Biré, R.; Trotereau, S.; Lemée, R.; Delpont, C.; Chabot, B.; Aumond, Y.; Krys, S. Occurrence of palytoxins in marine organisms from different trophic levels of the French Mediterranean coast harvested in 2009. Harmful Algae 2013, 28, 10–22. [Google Scholar] [CrossRef]
- Bruce, K.L.; Leterme, S.C.; Ellis, A.V.; Lenehan, C.E. Approaches for the detection of harmful algal blooms using oligonucleotide interactions. Anal. Bioanal. Chem. 2015, 407, 95–116. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, Y.; Li, C.; Wei, X.; Liu, Y. Impacts of alien species invasion on the South China Sea ecosystem and related control strategies. Chin. J. Ecol. 2013, 32, 2186–2193. [Google Scholar]
- LIU, Y.; WU HX, X. The ecology of invasions by marine exotic species. J. Biosaf. 2013, 22, 8–16. [Google Scholar]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Lee, B.; Park, M.G. Distribution and genetic diversity of the toxic benthic dinoflagellate genus Ostreopsis in Korea. Harmful Algae 2020, 96, 101820. [Google Scholar] [CrossRef] [PubMed]
- Sildever, S.; Jerney, J.; Kremp, A.; Oikawa, H.; Sakamoto, S.; Yamaguchi, M.; Baba, K.; Mori, A.; Fukui, T.; Nonomura, T.; et al. Genetic relatedness of a new Japanese isolates of Alexandrium ostenfeldii bloom population with global isolates. Harmful Algae 2019, 84, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.L.; Preskitt, L.B. A survey of epiphytic dinoflagellates from the coastal waters of the island of Hawai‘i. Harmful Algae 2007, 6, 658–669. [Google Scholar] [CrossRef]
- Pin, L.C.; Teen, L.P.; Ahmad, A.; Usup, G. Genetic Diversity of Ostreopsis ovata (Dinophyceae) from Malaysia. Mar. Biotechnol. 2001, 3, 246–255. [Google Scholar] [CrossRef]
- Parsons, M.L.; Aligizaki, K.; Bottein, M.-Y.D.; Fraga, S.; Morton, S.L.; Penna, A.; Rhodes, L. Gambierdiscus and Ostreopsis: Reassessment of the state of knowledge of their taxonomy, geography, ecophysiology, and toxicology. Harmful Algae 2012, 14, 107–129. [Google Scholar] [CrossRef]
- Granéli, E.; Vidyarathna, N.K.; Funari, E.; Cumaranatunga, P.R.T.; Scenati, R. Can increases in temperature stimulate blooms of the toxic benthic dinoflagellate Ostreopsis ovata? Harmful Algae 2011, 10, 165–172. [Google Scholar] [CrossRef]
- Scalco, E.; Brunet, C.; Marino, F.; Rossi, R.; Soprano, V.; Zingone, A.; Montresor, M. Growth and toxicity responses of Mediterranean Ostreopsis cf. ovata to seasonal irradiance and temperature conditions. Harmful Algae 2012, 17, 25–34. [Google Scholar] [CrossRef]
- Pezzolesi, L.; Guerrini, F.; Ciminiello, P.; Dell’Aversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Pistocchi, R. Influence of temperature and salinity on Ostreopsis cf. ovata growth and evaluation of toxin content through HR LC-MS and biological assays. Water Res. 2012, 46, 82–92. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Yoshimatsu, T.; Tanimoto, Y.; Sato, S.; Nishimura, T.; Uehara, K.; Adachi, M. Effects of temperature, salinity and their interaction on growth of the benthic dinoflagellate Ostreopsis cf. ovata (Dinophyceae) from Japanese coastal waters. Phycol. Res. 2012, 60, 297–304. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Magno, G.S.; Tartaglione, L.; Grillo, C.; Melchiorre, N. The Genoa 2005 Outbreak. Determination of Putative Palytoxin in Mediterranean Ostreopsis ovata by a New Liquid Chromatography Tandem Mass Spectrometry Method. Anal. Chem. 2006, 78, 6153–6159. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Grillo, C.; Melchiorre, N. Putative Palytoxin and Its New Analogue, Ovatoxin-a, in Ostreopsis ovata Collected Along the Ligurian Coasts During the 2006 Toxic Outbreak. J. Am. Soc. Mass Spectrom. 2008, 19, 111–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciminiello, P.; Dell’Aversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pistocchi, R. Complex palytoxin-like profile of Ostreopsis ovata. Identification of four new ovatoxins by high-resolution liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Battocchi, C.; Crinelli, R.; Carloni, E.; Magnani, M.; et al. Unique Toxin Profile of a Mediterranean Ostreopsis cf. ovata Strain: HR LC-MSn Characterization of Ovatoxin-f, a New Palytoxin Congener. Chem. Res. Toxicol. 2012, 25, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- García-Altares, M.; Tartaglione, L.; Dell’Aversano, C.; Carnicer, O.; de la Iglesia, P.; Forino, M.; Diogène, J.; Ciminiello, P. The novel ovatoxin-g and isobaric palytoxin (so far referred to as putative palytoxin) from Ostreopsis cf. ovata (NW Mediterranean Sea): Structural insights by LC-high resolution MSn. Anal. Bioanal. Chem. 2015, 407, 1191–1204. [Google Scholar] [CrossRef]
- Aligizaki, K.; Katikou, P.; Nikolaidis, G.; Panou, A. First episode of shellfish contamination by palytoxin-like compounds from Ostreopsis species (Aegean Sea, Greece). Toxicon 2008, 51, 418–427. [Google Scholar] [CrossRef]
- Aligizaki, K.; Katikou, P.; Milandri, A.; Diogène, J. Occurrence of palytoxin-group toxins in seafood and future strategies to complement the present state of the art. Toxicon 2011, 57, 390–399. [Google Scholar] [CrossRef]
- Amzil, Z.; Sibat, M.; Chomerat, N.; Grossel, H.; Marco-Miralles, F.; Lemee, R.; Nezan, E.; Sechet, V. Ovatoxin-a and Palytoxin Accumulation in Seafood in Relation to Ostreopsis cf. ovata Blooms on the French Mediterranean Coast. Mar. Drugs 2012, 10, 477–496. [Google Scholar] [CrossRef]
- Biré, R.; Trotereau, S.; Lemée, R.; Oregioni, D.; Delpont, C.; Krys, S.; Guérin, T. Hunt for Palytoxins in a Wide Variety of Marine Organisms Harvested in 2010 on the French Mediterranean Coast. Mar. Drugs 2015, 13, 5425–5446. [Google Scholar] [CrossRef] [Green Version]
- Okano, H.; Masuoka, H.; Kamei, S.; Seko, T.; Koyabu, S.; Tsuneoka, K.; Tamai, T.; Ueda, K.; Nakazawa, S.; Sugawa, M.; et al. Rhabdomyolysis and myocardial damage induced by palytoxin, a toxin of blue humphead parrotfish. Intern. Med. 1998, 37, 330–333. [Google Scholar] [CrossRef] [Green Version]
- Deeds, J.R.; Handy, S.M.; White, K.D.; Reimer, J.D. Palytoxin found in Palythoa sp. zoanthids (Anthozoa, Hexacorallia) sold in the home aquarium trade. PLoS ONE 2011, 6, e18235. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, S.; Arakawa, O.; Terada, M.; Nishio, S.; Takatani, T.; Mahmud, Y.; Noguchi, T. Ostreopsis sp., a possible origin of palytoxin (PTX) in parrotfish Scarus ovifrons. Toxicon 2003, 42, 29–33. [Google Scholar] [CrossRef]
- Taniyama, S.; Mahmud, Y.; Terada, M.; Takatani, T.; Arakawa, O.; Noguchi, T. Occurrence of a food poisoning incident by palytoxin from a serranid Epinephelus sp. in Japan. J. Nat. Toxins 2002, 11, 277–282. [Google Scholar] [PubMed]
- Noguchi, T. Palytoxin as the causative agent in the parrotfish poisoning. Prog. Venom Toxin Res. 1988, 26, 34. [Google Scholar]
- Onuma, Y.; Satake, M.; Ukena, T.; Roux, J.; Chanteau, S.; Rasolofonirina, N.; Ratsimaloto, M.; Naoki, H.; Yasumoto, T. Identification of putative palytoxin as the cause of clupeotoxism. Toxicon Off. J. Int. Soc. Toxinol. 1999, 37, 55–65. [Google Scholar] [CrossRef]
- Suzuki, T.; Watanabe, R.; Matsushima, R.; Ishihara, K.; Uchida, H.; Kikutsugi, S.; Harada, T.; Nagai, H.; Adachi, M.; Yasumoto, T.; et al. LC-MS/MS analysis of palytoxin analogues in blue humphead parrotfish Scarus ovifrons causing human poisoning in Japan. Food Addit. Contam. Part A 2013, 30, 1358–1364. [Google Scholar] [CrossRef]
- Moore, R.E.; Bartolini, G. Structure of palytoxin. J. Am. Chem. Soc. 1981, 103, 2491–2494. [Google Scholar] [CrossRef]
- Rossini, G.P.; Bigiani, A. Palytoxin action on the Na+,K+-ATPase and the disruption of ion equilibria in biological systems. Toxicon 2011, 57, 429–439. [Google Scholar] [CrossRef]
- Wu, C.H. Palytoxin: Membrane mechanisms of action. Toxicon 2009, 54, 1183–1189. [Google Scholar] [CrossRef]
- Chain, E.P.o.C.i.t.F. Scientific Opinion on marine biotoxins in shellfish—Palytoxin group. EFSA J. 2009, 7, 1393. [Google Scholar] [CrossRef]
- Hu, T.; Curtis, J.M.; Walter, J.A.; Wright, J.L.C. Characterization of biologically inactive spirolides E and F: Identification of the spirolide pharmacophore. Tetrahedron Lett. 1996, 37, 7671–7674. [Google Scholar] [CrossRef]
- Gill, S.; Murphy, M.; Clausen, J.; Richard, D.; Quilliam, M.; MacKinnon, S.; LaBlanc, P.; Mueller, R.; Pulido, O. Neural Injury Biomarkers of Novel Shellfish Toxins, Spirolides: A Pilot Study Using Immunochemical and Transcriptional Analysis. NeuroToxicology 2003, 24, 593–604. [Google Scholar] [CrossRef]
- Sleno, L.; Chalmers, M.J.; Volmer, D.A. Structural study of spirolide marine toxins by mass spectrometry. Anal. Bioanal. Chem. 2004, 378, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; Sosa, S.; Hungerford, J. Chapter 69—Toxicology and diversity of marine toxins. In Veterinary Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2012; pp. 896–934. [Google Scholar]
- Don Richard, E.A.; Cembella, A.; Quilliam, M. Investigations into the toxicology and pharmacology of spirolides, a novel group of shellfish toxins. In Proceedings of the Ninth International Conference on Harmful Algal Blooms, Hobart, Australia, 7–11 February 2000; pp. 383–386. [Google Scholar]
- Takada, N.; Umemura, N.; Suenaga, K.; Chou, T.; Nagatsu, A.; Haino, T.; Yamada, K.; Uemura, D. Pinnatoxins B and C, the most toxic components in the pinnatoxin series from the Okinawan bivalve Pinna muricata. Tetrahedron Lett. 2001, 42, 3491–3494. [Google Scholar] [CrossRef]
- Takada, N.; Umemura, N.; Suenaga, K.; Uemura, D. Structural determination of pteriatoxins A, B and C, extremely potent toxins from the bivalve Pteria penguin. Tetrahedron Lett. 2001, 42, 3495–3497. [Google Scholar] [CrossRef]
- Southgate, P.C. Pearl oyster culture. Pearl Oyster 2008, 231–272. [Google Scholar] [CrossRef]
- Munday, R.; Towers, N.R.; Mackenzie, L.; Beuzenberg, V.; Holland, P.T.; Miles, C.O. Acute toxicity of gymnodimine to mice. Toxicon 2004, 44, 173–178. [Google Scholar] [CrossRef]
- Brand, L.E.; Campbell, L.; Bresnan, E. Karenia: The biology and ecology of a toxic genus. Harmful Algae 2012, 14, 156–178. [Google Scholar] [CrossRef]
- Oda, M. Gymnodinium mikimotoi Miyake et Kominami n. sp.(MS.) no akashiwo to ryusando no koka (the red tide of Gymnodinium mikimotoi Miyake et Kominami n. sp.(MS) and the influence of copper sulfate on the red tide). Zool. Mag 1935, 47, 35–48. [Google Scholar]
- Davis, C.C. Gymnodinium brevis sp. nov., a cause of discolored water and animal mortality in the Gulf of Mexico. Bot. Gaz. 1948, 109, 358–360. [Google Scholar] [CrossRef]
- Molgó, J.; Marchot, P.; Aráoz, R.; Benoit, E.; Iorga, B.I.; Zakarian, A.; Taylor, P.; Bourne, Y.; Servent, D. Cyclic imine toxins from dinoflagellates: A growing family of potent antagonists of the nicotinic acetylcholine receptors. J. Neurochem. 2017, 142, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Stivala, C.E.; Benoit, E.; Aráoz, R.; Servent, D.; Novikov, A.; Molgó, J.; Zakarian, A. Synthesis and biology of cyclic imine toxins, an emerging class of potent, globally distributed marine toxins. Nat. Prod. Rep. 2015, 32, 411–435. [Google Scholar] [CrossRef] [PubMed]
- Rundberget, T.; Aasen, J.A.B.; Selwood, A.I.; Miles, C.O. Pinnatoxins and spirolides in Norwegian blue mussels and seawater. Toxicon 2011, 58, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, U.; Kremp, A.; Tahvanainen, P.; Krock, B. Characterization of spirolide producing Alexandrium ostenfeldii (Dinophyceae) from the western Arctic. Harmful Algae 2014, 39, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Van Wagoner, R.M.; Misner, I.; Tomas, C.R.; Wright, J.L.C. Occurrence of 12-methylgymnodimine in a spirolide-producing dinoflagellate Alexandrium peruvianum and the biogenetic implications. Tetrahedron Lett. 2011, 52, 4243–4246. [Google Scholar] [CrossRef]
- Martens, H.; Tillmann, U.; Harju, K.; Dell’Aversano, C.; Tartaglione, L.; Krock, B. Toxin Variability Estimations of 68 Alexandrium ostenfeldii (Dinophyceae) Strains from The Netherlands Reveal a Novel Abundant Gymnodimine. Microorganisms 2017, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Van Wagoner, R.M.; Satake, M.; Wright, J.L.C. Polyketide biosynthesis in dinoflagellates: What makes it different? Nat. Prod. Rep. 2014, 31, 1101–1137. [Google Scholar] [CrossRef]
- Cembella, A.D.; Lewis, N.I.; Quilliam, M.A. The marine dinoflagellate Alexandrium ostenfeldii (Dinophyceae) as the causative organism of spirolide shellfish toxins. Phycologia 2000, 39, 67–74. [Google Scholar] [CrossRef]
- McNabb, P.; Rhodes, L.; Selwood, A. Results of analyses for brevetoxins and pinnatoxins in Rangaunu Harbour oysters, 1993–2008. Cawthron Rep. 2008, 1453, 18. [Google Scholar]
- McCarron, P.; Rourke, W.A.; Hardstaff, W.; Pooley, B.; Quilliam, M.A. Identification of Pinnatoxins and Discovery of Their Fatty Acid Ester Metabolites in Mussels (Mytilus edulis) from Eastern Canada. J. Agric. Food Chem. 2012, 60, 1437–1446. [Google Scholar] [CrossRef]
- Van de Waal, D.B.; Tillmann, U.; Martens, H.; Krock, B.; van Scheppingen, Y.; John, U. Characterization of multiple isolates from an Alexandrium ostenfeldii bloom in The Netherlands. Harmful Algae 2015, 49, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Suikkanen, S.; Kremp, A.; Hautala, H.; Krock, B. Paralytic shellfish toxins or spirolides? The role of environmental and genetic factors in toxin production of the Alexandrium ostenfeldii complex. Harmful Algae 2013, 26, 52–59. [Google Scholar] [CrossRef]
- Hu, T.; Curtis, J.M.; Oshima, Y.; Quilliam, M.A.; Walter, J.A.; Watson-Wright, W.M.; Wright, J.L.C. Spirolides B and D, two novel macrocycles isolated from the digestive glands of shellfish. J. Chem. Soc. Chem. Commun. 1995, 2159–2161. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; Munday, R.; Suda, S.; Molenaar, S.; Hallegraeff, G. Dinoflagellate Vulcanodinium rugosum identified as the causative organism of pinnatoxins in Australia, New Zealand and Japan. Phycologia 2011, 50, 624–628. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, F.; Chen, S.; Tan, X.; Zuo, J.; Peng, J.; Xie, R. The isolation and bioactivities of pinnatoxin. Chin. J. Mar. Drugs 1990, 9, 33–35. [Google Scholar]
- Uemura, D.; Chou, T.; Haino, T.; Nagatsu, A.; Fukuzawa, S.; Zheng, S.-Z.; Chen, H.-S. Pinnatoxin A: A toxic amphoteric macrocycle from the Okinawan bivalve Pinna muricata. J. Am. Chem. Soc. 1995, 117, 1155–1156. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; Molenaar, S.; Munday, R.; Wilkinson, C.; Hallegraeff, G. Production of pinnatoxins E, F and G by scrippsielloid dinoflagellates isolated from Franklin Harbour, South Australia. N. Zealand J. Mar. Freshw. Res. 2011, 45, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; van Ginkel, R.; Holland, P.; Munday, R. Production of pinnatoxins by a peridinoid dinoflagellate isolated from Northland, New Zealand. Harmful Algae 2010, 9, 384–389. [Google Scholar] [CrossRef]
- Wunschel, D.S.; Valenzuela, B.R.; Kaiser, B.L.D.; Victry, K.; Woodruff, D. Method development for comprehensive extraction and analysis of marine toxins: Liquid-liquid extraction and tandem liquid chromatography separations coupled to electrospray tandem mass spectrometry. Talanta 2018, 187, 302–307. [Google Scholar] [CrossRef]
- Ninčević Gladan, Ž.; Arapov, J.; Casabianca, S.; Penna, A.; Honsell, G.; Brovedani, V.; Pelin, M.; Tartaglione, L.; Sosa, S.; Dell’Aversano, C.; et al. Massive Occurrence of the Harmful Benthic Dinoflagellate Ostreopsis cf. ovata in the Eastern Adriatic Sea. Toxins 2019, 11, 300. [Google Scholar] [CrossRef] [Green Version]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Forino, M.; Tartaglione, L. Liquid chromatography-high-resolution mass spectrometry for palytoxins in mussels. Anal. Bioanal. Chem. 2015, 407, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W.L.G.W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Mori, S.; Sugahara, K.; Maeda, M.; Nomoto, K.; Iwashita, T.; Yamagaki, T. Insecticidal activity guided isolation of palytoxin from a red alga, Chondria armata. Tetrahedron Lett. 2016, 57, 3612–3617. [Google Scholar] [CrossRef] [Green Version]
- Beress, L.; Zwick, J.; Kolkenbrock, H.; Kaul, P.; Wassermann, O. A method for the isolation of the caribbean palytoxin (C-PTX) from the coelenterate (zooanthid) Palythoa caribaeorum. Toxicon 1983, 21, 285–290. [Google Scholar] [CrossRef]
- Ukena, T.; Satake, M.; Usami, M.; OSHiMA, Y.; Naoki, H.; Fujita, T.; Kan, Y.; Yasumoto, T. Structure elucidation of ostreocin D, a palytoxin analog isolated from the dinoflagellate Ostreopsis siamensis. Biosci. Biotechnol. Biochem. 2001, 65, 2585–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, R.; Castellano, V.; Scalco, E.; Serpe, L.; Zingone, A.; Soprano, V. New palytoxin-like molecules in Mediterranean Ostreopsis cf. ovata (dinoflagellates) and in Palythoa tuberculosa detected by liquid chromatography-electrospray ionization time-of-flight mass spectrometry. Toxicon 2010, 56, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Soliño, L.; García-Altares, M.; Godinho, L.; Costa, P.R. Toxin profile of Ostreopsis cf. ovata from Portuguese continental coast and Selvagens Islands (Madeira, Portugal). Toxicon 2020, 181, 91–101. [Google Scholar] [CrossRef]
- Selwood, A.I.; van Ginkel, R.; Harwood, D.T.; McNabb, P.S.; Rhodes, L.R.; Holland, P.T. A sensitive assay for palytoxins, ovatoxins and ostreocins using LC-MS/MS analysis of cleavage fragments from micro-scale oxidation. Toxicon 2012, 60, 810–820. [Google Scholar] [CrossRef]
- Kvrgić, K.; Lešić, T.; Aysal, A.I.; Džafić, N.; Pleadin, J. Cyclic imines in shellfish and ascidians in the northern Adriatic Sea. Food Addit. Contaminants. Part B Surveill. 2021, 14, 12–22. [Google Scholar] [CrossRef]
- Moreiras, G.; Leão, J.M.; Gago-Martínez, A. Analysis of Cyclic Imines in Mussels (Mytilus galloprovincialis) from Galicia (NW Spain) by LC-MS/MS. Int. J. Environ. Res. Public Health 2020, 17, 281. [Google Scholar] [CrossRef] [Green Version]
- Hassoun, A.E.R.; Ujević, I.; Mahfouz, C.; Fakhri, M.; Roje-Busatto, R.; Jemaa, S.; Nazlić, N. Occurrence of domoic acid and cyclic imines in marine biota from Lebanon-Eastern Mediterranean Sea. Sci. Total Environ. 2021, 755, 142542. [Google Scholar] [CrossRef]
- Ji, Y.; Yan, G.; Wang, G.; Liu, J.; Tang, Z.; Yan, Y.; Qiu, J.; Zhang, L.; Pan, W.; Fu, Y.; et al. Prevalence and distribution of domoic acid and cyclic imines in bivalve mollusks from Beibu Gulf, China. J. Hazard. Mater. 2022, 423, 127078. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Rafuse, C.; Lewis, N.I.; Li, A.; Meng, F.; Beach, D.G.; McCarron, P. Screening of cyclic imine and paralytic shellfish toxins in isolates of the genus Alexandrium (Dinophyceae) from Atlantic Canada. Harmful Algae 2018, 77, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.J.; McCarron, P. A mussel tissue certified reference material for multiple phycotoxins. Part 5: Profiling by liquid chromatography–high-resolution mass spectrometry. Anal. Bioanal. Chem. 2021, 413, 2055–2069. [Google Scholar] [CrossRef] [PubMed]
- Mattarozzi, M.; Cavazza, A.; Calfapietra, A.; Cangini, M.; Pigozzi, S.; Bianchi, F.; Careri, M. Analytical screening of marine algal toxins for seafood safety assessment in a protected Mediterranean shallow water environment. Food Addit. Contam. Part A 2019, 36, 612–624. [Google Scholar] [CrossRef]
- Otero, P.; Vale, C.; Boente-Juncal, A.; Costas, C.; Louzao, M.C.; Botana, L.M. Detection of Cyclic Imine Toxins in Dietary Supplements of Green Lipped Mussels (Perna canaliculus) and in Shellfish Mytilus chilensis. Toxins 2020, 12, 613. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Rowland-Pilgrim, S.; Turner, L.M.; Rai, A.; Venugopal, M.N.; Karunasagar, I.; Godhe, A. Assessing the presence of marine toxins in bivalve molluscs from southwest India. Toxicon 2017, 140, 147–156. [Google Scholar] [CrossRef]
- Varriale, F.; Tartaglione, L.; Cinti, S.; Milandri, A.; Dall’Ara, S.; Calfapietra, A.; Dell’Aversano, C. Development of a data dependent acquisition-based approach for the identification of unknown fast-acting toxins and their ester metabolites. Talanta 2021, 224, 121842. [Google Scholar] [CrossRef]
- Rossignoli, A.E.; Mariño, C.; Martín, H.; Blanco, J. Development of a Fast Liquid Chromatography Coupled to Mass Spectrometry Method (LC-MS/MS) to Determine Fourteen Lipophilic Shellfish Toxins Based on Fused–Core Technology: In-House Validation. Mar. Drugs 2021, 19, 603. [Google Scholar] [CrossRef]
| Type of Poisoning | Marine Biotoxins | Source | Symptomatology |
|---|---|---|---|
| PSP | Saxitoxin | Alexandrium spp. Gymnodinium catenatum Pyrodinium bahamense | Gastrointestinal symptoms Paralytic phenomena Recovery or death |
| ASP | Domoic acid | Pseudo-nitzschia spp. Nitzschia spp. | Gastrointestinal and neurological symptoms Cardiac or respiratory problems Recovery or death |
| DSP | Okadaic acid | Prorocentrum lima Dinophysis spp. | Gastrointestinal symptoms Recovery within 3 days |
| Dinophysistoxins | Dinophysis acuminata D. caudata | nausea, vomiting, severe diarrhea | |
| NSP | Brevetoxin | Karenia brevis | Gastrointestinal and neurological symptoms, respiratory problems, Recovery or death |
| AZA | Azaspiracid | Amphidoma languida Azadinium spinosum | Gastrointestinal symptoms |
| YTX | Yessotoxins | Proroceratium reticulatum Lingulodinium polyedrum Gonyaulax spinifera | Lack of observations in humans |
| CFP | Ciguatoxin | Gambierdiscus spp. | Gastrointestinal symptoms, cardiovascular or neurological problems |
| CIs | Spirolides | Alexandrium spp. Karenia spp. Vulcanodinium spp. Prorocentrum spp. | Lack of observations in humans |
| Pinnatoxins | Vulcanodinium rugosum Pteria penguin Pinna muricata | ||
| Pteriatoxins | |||
| Gymnodimines | Karenia selliformis (Gymnodinium selliformis) Alexandrium peruvianum Alexandrium ostenfeldii | ||
| PlTXs | Palytoxin Palytoxin-b 42-hydroxy-palytoxin Homo-palytoxin Bis-homo-palytoxin Neo-palytoxin Deoxy-palytoxin Mascarenotoxin-a, Mascarenotoxin-b, Mascarenotoxin-c Ovatoxin-a, b, c, d, e, f, g, h, i, j, k Ostreocin-a, b, d, e | Palythoa caribaeorum Palythoa toxica Ostreopsis lenticularis Ostreopsis siamensis Ostreopsis ovata Ostreopsis mascarenensis | Gastrointestinal symptoms Muscle and cutaneous problems |
| Species | Localization | Toxins | References |
|---|---|---|---|
| Scarus ovifrons | Japan | Palytoxin-like toxins | [71] |
| Calotomus japonicus | Japan | Palytoxin-like toxins | [69] |
| Epinephelus sp. | Japan | Palytoxin-like toxins | [72] |
| Ypsiscarus ovifrons | Japan | Palytoxin | [73] |
| Herklotsichthys quadrimaculatus | Madagascar | Palytoxin and its analogue | [74] |
| Toxins | Route | Lethal Dose | Refs. |
|---|---|---|---|
| Palytoxins | i.p. | i.p. injection into mice, LD50:
| [77,78,79] |
| Spirolides | i.p. | i.p. injection into mice, LD50:
| [80,81,82,83,84] |
| Oral | Oral administration to mice, LD50 (μg/kg):
| ||
| Pinnatoxins | i.p. | i.p. injection into mice, LD99 (μg/kg):
| [39,85] |
| Oral | Oral administration to mice, LD50 (μg/kg):
| ||
| Pteriatoxins | i.p. | i.p. injection into mice (LD99):
| [86,87] |
| Gymnodimines | i.p. | i.p. injection into mice, LD50 (μg/kg)
| [88,89,90,91] |
| Oral | Oral administration to mice LD50 (μg/kg):
|
| Sources | Target Toxins | Extraction | Clean up or Purification Process | Instruments | Column | Mobile Phase | Refs. |
|---|---|---|---|---|---|---|---|
| Chondria armata | PlTX | 4 L of water below 10 °C | DEAE-cellulose column (10 × 5 cm, OH- form) → LiChroprep RP-18 column (5 × 5 cm) → TSK G3000S column (1 × 7.5 cm). | Orbitrap Elite FT mass spectrometer | Reverse-phased column, Develosil C30-UG-3 2.0 i.d. ×100 mm | A: 0.1% acetic acid B: 0.1% acetic acid containing acetonitrile Gradient: 10–100% B for 23 min, minutes 25–26 100–10% B, minutes 26–30 10% B | [114] |
| Mussel | PlTX | Methanol (MeOH)–H2O 8:2 (v/v) | SPE (Strata-X, Strata-XL, OASIS HLB LP 6 cc, PolyLC INC) 1. Load: MeOH–H2O 2:8, 1:9, 5:95 2. Wash: MeOH–H2O 1:1, 4:6, 3:7, 1:9, H2O 100% 3. Elute: MeOH 100%, MeOH–H2O 9:1, 8:2, 8:2 with 0.2% acetic acid, MeOH with 1% acetic acid, MeOH– H2O 8:2 with 0.1% trifluoroacetic acid, isoPrOH–H2O 8:2, isoPrOH–H2O–acetic acid 40:59:1, 70:29:1. | LC-HRMS | 1. Gemini C18, 3 μm, 2 × 150 mm 2. Kinetex C18, 2.6 μm, 2.10 × 100 mm 3. Poroshell 120 EC-C18, 2.7 μm, 2.1 × 100 mm | A: H2O, 30 mM acetic acid B: 95% MeCN–H2O, 30 mM acetic acid | [115] |
| Palythoa caribaeorum | PlTX | 50% EtOH | Charcoal | Gel filtration | 1. Sephadex G-S0 Column, 7 × 150 cm | 0.1 M acetic acid | [115] |
| Gel filtration | 2. QAE-Sephadex A-25 Column, 3 × 30 cm | 0.01 M Tris-HCl, pH 8 | |||||
| Gel filtration | 3. SP-Sephadex column C-25, 1 × 60 cm | 0.01 M Na-acetate solution, pH 4.5 | |||||
| Ion exchange chromatography | 4. CM-Cellulose column, 1.5 × 30 cm | 0.005 M NH4-acetate buffer | |||||
| Gel filtration | 5. Biogel P-6 column (200–400 mesh) | 0.1 M acetic acid | |||||
| Ostreopsis siamensis | Ostreocin-D | MeOH followed by MeOH-H2O-AcOH (50:50:0.1) | Partitioning with CHCl3 and using aqueous part for further purification | Column chromatography | 1. Develosil Lop ODS column | MeOH-H2O-AcOH (90:10:0.2) | [116] |
| 2. Develosil TMS-5 column | MeCN-H2O-AcOH from 22:78:0.1 (20 min) to 25:75:0.1 (30 min) | ||||||
| Palythoa, Protopalythoa, or Zoanthus spp. | PlTXs (hydrophilic toxins) | Water → homogenization → ultrasonication (320 W) | Add chloroform:MeOH (2:1) → separate aqueous layer (palytoxin) → SPE (1 mL C-18-T (Phenomenex, Torrance, CA, USA) | LC-MS/MS | 1. 50 × 2.1 mm Kinetex column (2.6 µm particle with C-18 from Phenomenex) 2. 50 × 2.1 mm HILIC column (3.5 µm particle from Millipore) | A: water (0.1% formic acid) B: acetonitrile (0.1% formic acid) Gradient: 20% B to 80% B for 25 min | [110] |
| Ostreopsis cf. ovata | Ovatoxins a to e and isobaric PlTX | MeOH:water (1:1) → sonication for 10 min | - | LC-HRMS | Poroshell 120 EC-C18 (2.7 μm, 2.1 × 100 mm) column | A: water (30 mM acetic acid) B: 95% acetonitrile (30 mM acetic acid) Gradient: 28–29% B for 5 min 29–30% for 10 min, 30–100% B for 1 min, held for 5 min | [111] |
| O. cf. ovata | PlTX, ovatoxins a–d, mascarenotoxins a and c | MeOH:water (1:1) | 0.22 μm pore size membrane filter | LC/time-of-flight (TOF)-MS | Phenomenex Luna HILIC 3μ 150 × 2.00 mm | A: water (0.1% formic acid) B: 95% acetonitrile (0.1% formic acid) Gradient: minutes 0–2 10% A minutes 2–5 50% A minutes 5–10 50% A then linear decrease from 50% A to 10% A (initial condition) | [117] |
| O. cf. ovata | PlTX | 50% MeOH with sonication for 4 min | - | LC-HRMS | Accucore C18 column (2.6 μm, 100 × 2.1 mm; Thermo Fisher) | A: water (0.1% formic acid) B: acetonitrile (0.1% formic acid) Gradient: 26–29% B for 15 min, 29–99% B for 4 min, 99–29% B for 0.5 min, and hold for 10.5 min | [118] |
| O. cf. ovata | Isobaric PlTX, ovatoxins a–e, g | MeOH:water (80:20) | 0.22 μm syringe filter | hybrid linear ion trap LTQ Orbitrap XL™ Fourier transform mass spectrometer (FTMS) equipped with ESI ION MAX™ source (Thermo Fisher, San José, CA, USA) coupled to Agilent 1100 LC binary system | Poroshell 120 EC-C18, 2.7 μm, 100 × 2.10 mm column | A: water (30 mM acetic acid) B: 95% acetonitrile (30 mM acetic acid) Gradient: minutes 0–10 28–29% B minutes 10–20 30% B minutes 20–21 100% B and hold for 10 min | [64] |
| Greenshell mussel™ (Perna canaliculus), Pacific oyster (Crassostrea gigas), Parrot fish (Scarus ovifrons) | PlTX | MeOH:water (1:1) | 60 mg Strata-X SPE cartridge (Phenomenex, Torrance, CA, USA) Pre-conditioned: 3 mL of MeOH → 3 mL of water Wash: 2 mL of MeOH:water (2:3) → 2 mL of water | LC-MS/MS | Acquity C18 HSS 1.7 μm column 50 × 1 mm | A: water (0.1% formic acid) B: acetonitrile (0.1% formic acid) Gradient: 0% B for 0.5 min 60% B for 2.5 min 100% B for 2.5–3 min (flushing) | [119] |
| Mediterranean mussels, European oysters, queen scallops, and ascidians | GYM, PnTX-G, SPX-C | MeOH | - | Electrospray ionization (ESI)-MS/MS | Zorbax SB-C8RRHD 2.1 × 50 mm, 1.8 μm | A: water (3.66 mM ammonium formate + 53 mM formic acid) B: acetonitrile (3.66 mM ammonium formate + 53 mM formic acid) Gradient: 30–90% B for 0.5–8 min | [120] |
| Mussels (Mytilus galloprovincialis) | GYM-A, PnTX-G | MeOH | SPE, Strata-X cartridge, 30 mg/mL | LC-MS/MS | Agilent Zorbax SB-C8 Rapid Resolution HD (2.1 × 50 mm, 1.8 µm) | A: water (2 mM ammonium formate + 50 mM formic acid) B: 95% acetonitrile (2 mM ammonium formate + 50 mM formic acid) Gradient: minutes 0–5 20–50% B minutes 5–6 50–20% B | [121] |
| Patella rustica complex, Phorcus turbinatus, Spondylus spinosus | GYM-B, SPX | MeOH | - | LC-MS/MS (Agilent Technologies 6410) | 5 μm Poroshell C18, 50 × 2.1 mm Agilent column | A: water (2 mM ammonium formate) B: 95% acetonitrile (50 mM formic acid) | [122] |
| Shellfish | GYM-A, SPX | MeOH | LC-MS/MS (Thermo Ultimate 3000 HPLC system coupled to AB-Sciex Qtrap 4500 mass spectrometer) | Luna C18 column (50 mm × 2.1 mm Phenomenex) | A: water (2 mM ammonium formate + 50 mM formic acid) B: 95% acetonitrile (2 mM ammonium formate + 50 mM formic acid) Gradient: 5–50% B for 1 min, 50–100% B for 4 min, 100% B for 2 min. | [123] | |
| Mytilus edulis | GYM-A, SPX | MeOH | ESI-MS/MS | Thermo Finnegan BDS Hypersil C8 (50 mm × 2.1 mm, 3 μm) column | A: water (2 mM ammonium formate + 50 mM formic acid) B: 95% acetonitrile (2 mM ammonium formate + 50 mM formic acid) Gradient: 30% B to 90% B for 8 min, 90% B for 2.5 min, return for 0.5 min to 30% B | [24] | |
| Waters X-Bridge C18 (150 mm × 3 mm, 5 μm) column | A: water (6.7 mM ammonium hydroxide) B: 95% acetonitrile (6.7 mM ammonium hydroxide) Gradient: 10% B for 1 min and increase linearly to 90% B in 9 min, keep at 90% B for 3 min and return to 10% B in 2 min | ||||||
| Alexandrium ostenfeldii | 13-desmethyl SPX-C | 0.05% formic acid in MeOH | LC-MS | 50 × 2.1 mm i.d., 2.5 μm Luna C18 column (Phenomenex) | A: water (2 mM ammonium formate + 50 mM formic acid) B: 95% acetonitrile (2 mM ammonium formate + 50 mM formic acid) Gradient: 10–100% B for 6 min, 100% B for 2 min, return to 10% B for 0.5 min | [124] | |
| LC-HRMS | 2.7 μm Agilent Poroshell SB-C18 column | A: water (2 mM ammonium formate + 50 mM formic acid) B: 95% acetonitrile (2 mM ammonium formate + 50 mM formic acid) Gradient: 5–100% B for 20 min, 100% B for 5 min, return to 5% B for 1 min | |||||
| A. ostenfeldii | 13-desmethyl SPX-C | MeOH | SPE cartridge (Waters Oasis HLB) | LC-HRMS | Poroshell 120 SB C18 column (2.1 × 150 mm, 2.7 μm) | A: water (2 mM ammonium formate + 50 mM formic acid) B: 95% acetonitrile (2 mM ammonium formate + 50 mM formic acid) Gradient: 5–100% B for 25 min | [125] |
| Shellfishes | CIs | MeOH | LC-MS/MS | Poroshell 120 EC-C18 column (100 × 2.1 mm, 2.7 µm) | A: 2 mM ammonium acetate and 18 mM glacial acetic acid in 5.2% methanol B: 1 mM ammonium acetate in 100% methanol Gradient: 5% B until minute 1, 63% B until minute 2, 86% B until minute 4, 100% B until minute 11 | [126] | |
| Perna canaliculus (green-lipped mussel) Mytilus chilensis (shellfish) | 13-desmethyl SPX-C, PnTX-G | MeOH | UPLC-MS | Acquity UPLC BEH C18 (2.1 × 100 mm, 1.7 µm) | A: water (2 mM ammonium formate + 50 mM formic acid) B: 95% acetonitrile (2 mM ammonium formate + 50 mM formic acid) Gradient: 30–70% B until minute 3, 70% B until minute 4.5, 30% B until minute 4.6 | [127] | |
| Green mussels (Perna viridis), backwater oysters (Crassostrea madrasensis) | 13-desMeC SPX, 20-Me SPX-G, GYM | MeOH | SPE | LC-MS/MS | 1.7 μm, 2.1 × 50 mm Acquity BEH Amide UPLC column | - | [128] |
| Mytilus galloprovincialis and Ruditapes decussatus | 13-desMeC SPX, GYM-G, -H, -I, -J | MeOH | - | LC-HRMS | HyperClone BDS C8 column 50 × 2.0 mm, 13 Å, 3 μm | A: water (2 mM ammonium formate + 50 mM formic acid) B: 95% acetonitrile (2 mM ammonium formate + 50 mM formic acid) Gradient: 10–100% B for 10 min, 100% B for 15 min | [129] |
| mussels (Mytilus galloprovincialis), clams (Ruditapes philippinarum) and oysters (Ostrea edulis) | 13desmSPXC, GYMA, 13,19didesmSPXC, 20MethylSPXG and PnTXG And 9 other lipophilic toxins | MeOH | 0.22 μm syringe filter | LC-MS/MS | Phenomenex Kinetex EVO C18 “core–shell” column 50 mm × 2.1 mm, 2.6 µm | A: water (6.7 mM NH4OH (pH 11)) B: 90% acetonitrile (MeCN) (6.7 mM NH4OH (pH 11)) Gradient: 22% B for 0.1 min, 22–95% B for 1.8 min, maintaining until 2.90 min | [130] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; An, H.-J.; Kim, J.; Jeon, Y.-J. Current Situation of Palytoxins and Cyclic Imines in Asia-Pacific Countries: Causative Phytoplankton Species and Seafood Poisoning. Int. J. Environ. Res. Public Health 2022, 19, 4921. https://doi.org/10.3390/ijerph19084921
Kim Y-S, An H-J, Kim J, Jeon Y-J. Current Situation of Palytoxins and Cyclic Imines in Asia-Pacific Countries: Causative Phytoplankton Species and Seafood Poisoning. International Journal of Environmental Research and Public Health. 2022; 19(8):4921. https://doi.org/10.3390/ijerph19084921
Chicago/Turabian StyleKim, Young-Sang, Hyun-Joo An, Jaeseong Kim, and You-Jin Jeon. 2022. "Current Situation of Palytoxins and Cyclic Imines in Asia-Pacific Countries: Causative Phytoplankton Species and Seafood Poisoning" International Journal of Environmental Research and Public Health 19, no. 8: 4921. https://doi.org/10.3390/ijerph19084921
APA StyleKim, Y.-S., An, H.-J., Kim, J., & Jeon, Y.-J. (2022). Current Situation of Palytoxins and Cyclic Imines in Asia-Pacific Countries: Causative Phytoplankton Species and Seafood Poisoning. International Journal of Environmental Research and Public Health, 19(8), 4921. https://doi.org/10.3390/ijerph19084921








