An Update on the Implications of New Psychoactive Substances in Public Health
Abstract
1. Brief Introduction
2. Classification
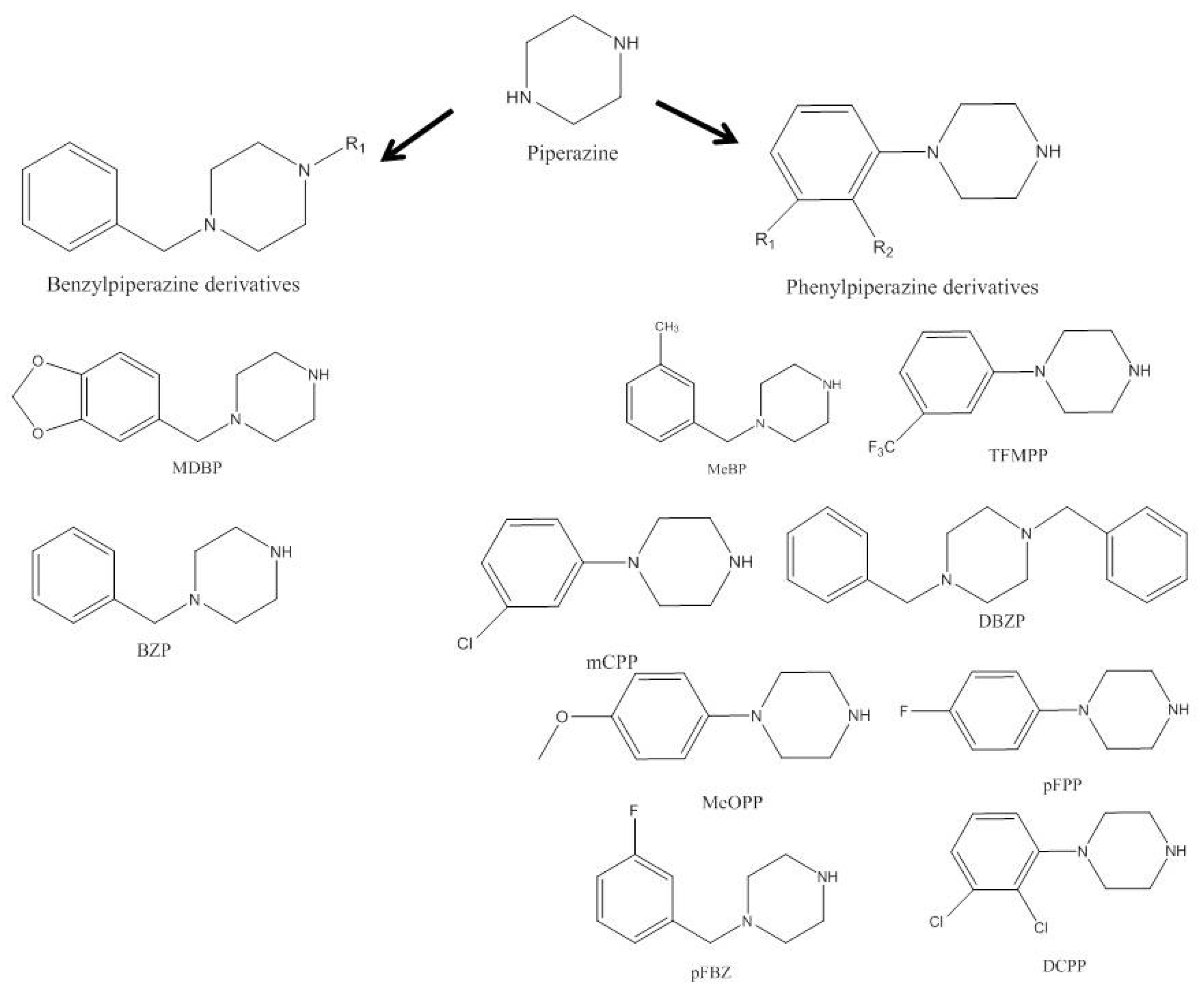
2.1. Piperazines
2.2. Aminoindanes
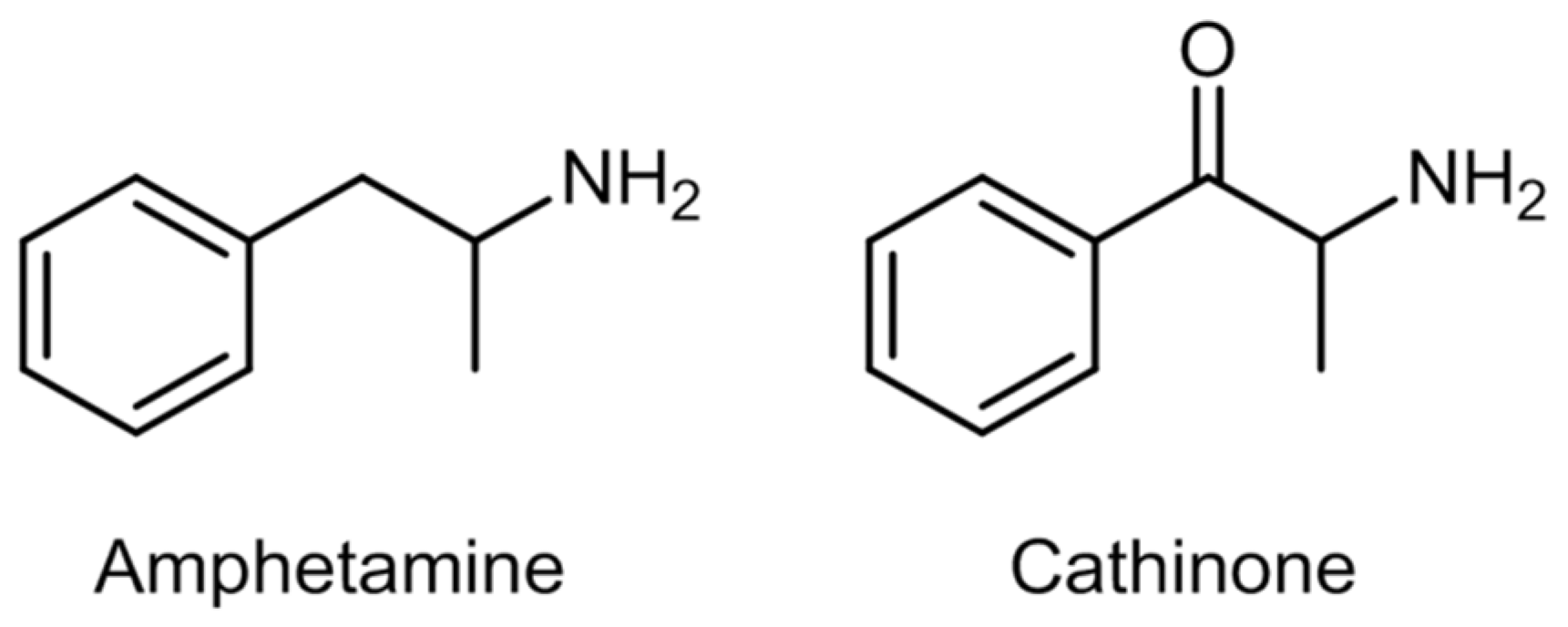
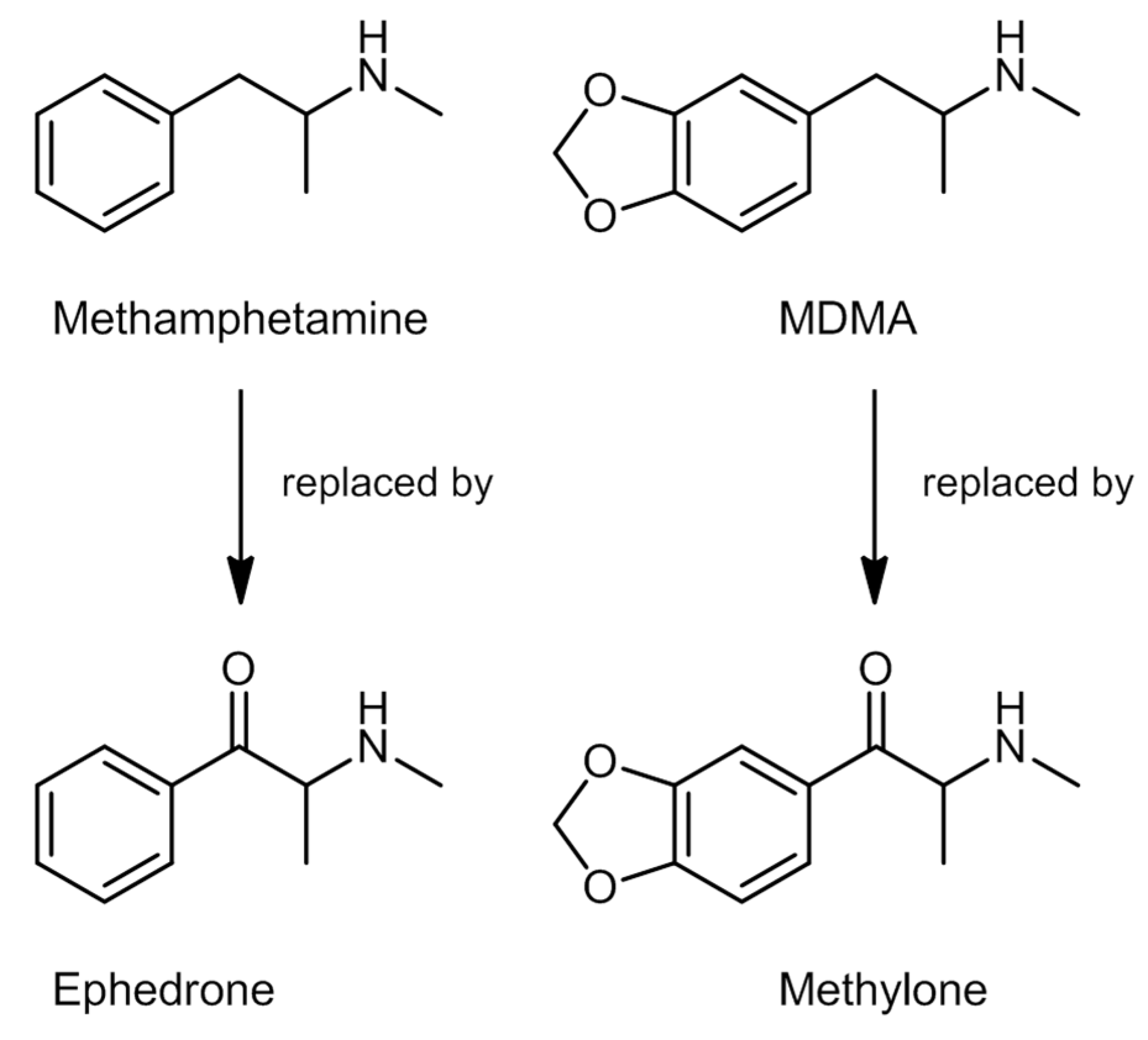
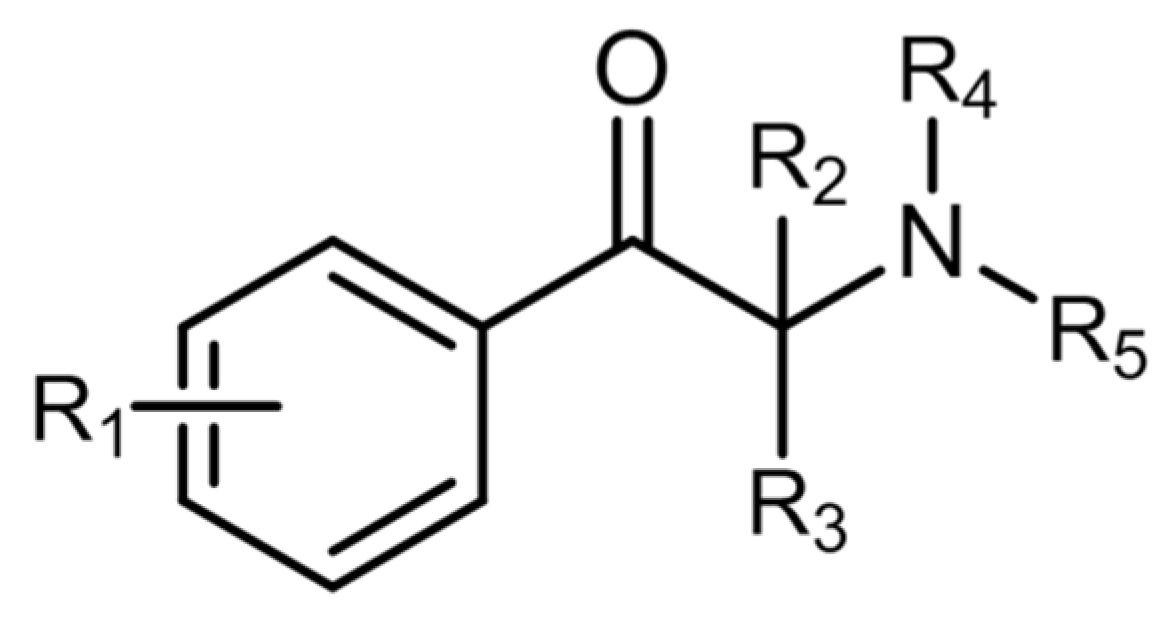
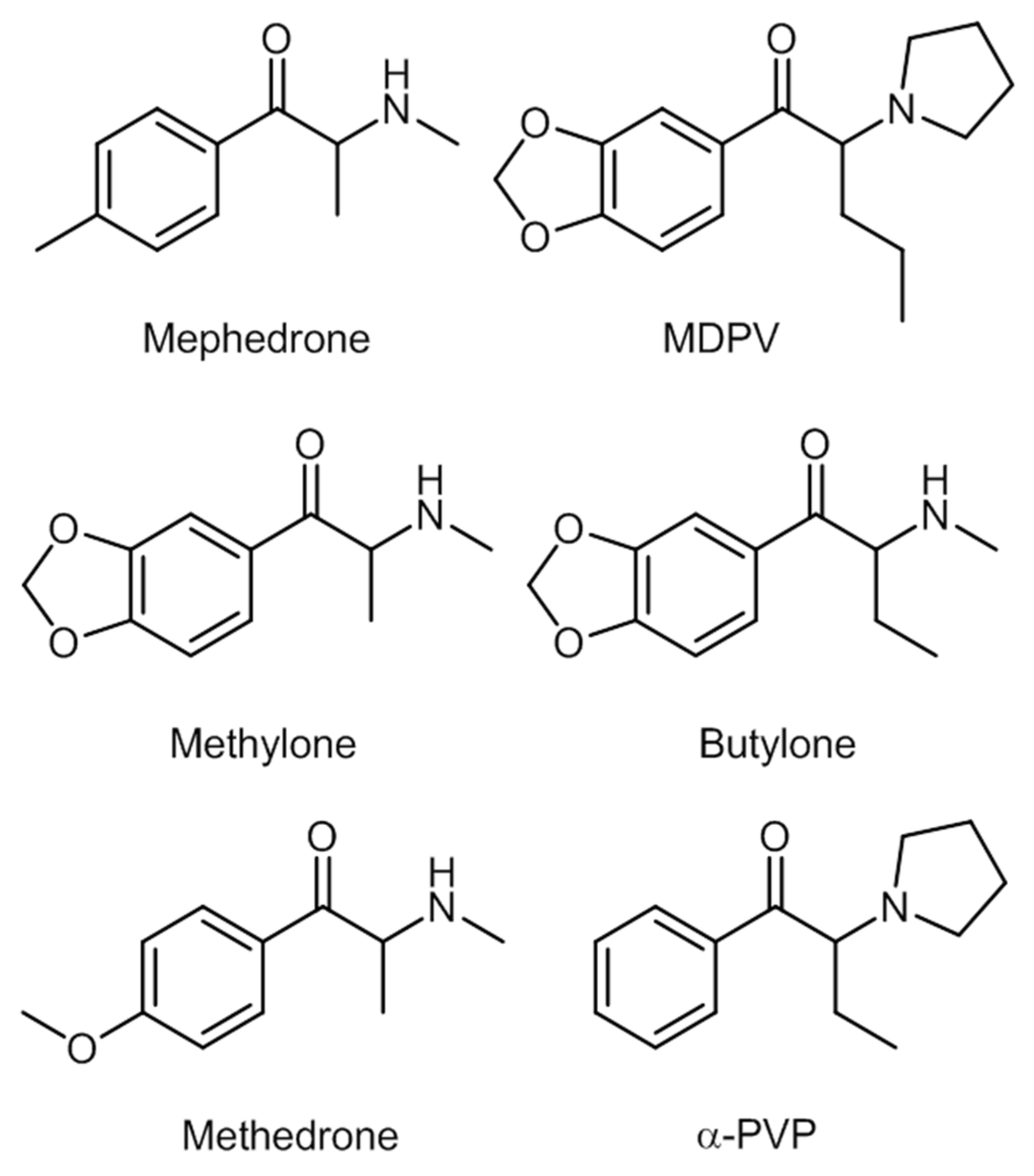
2.3. Synthetic Cathinones
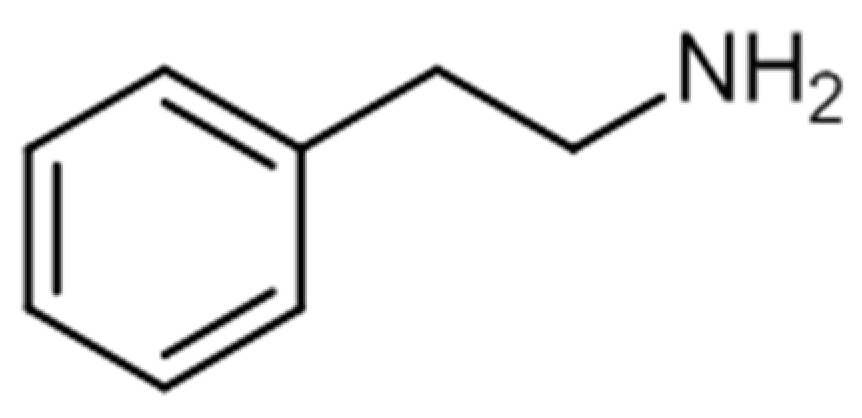
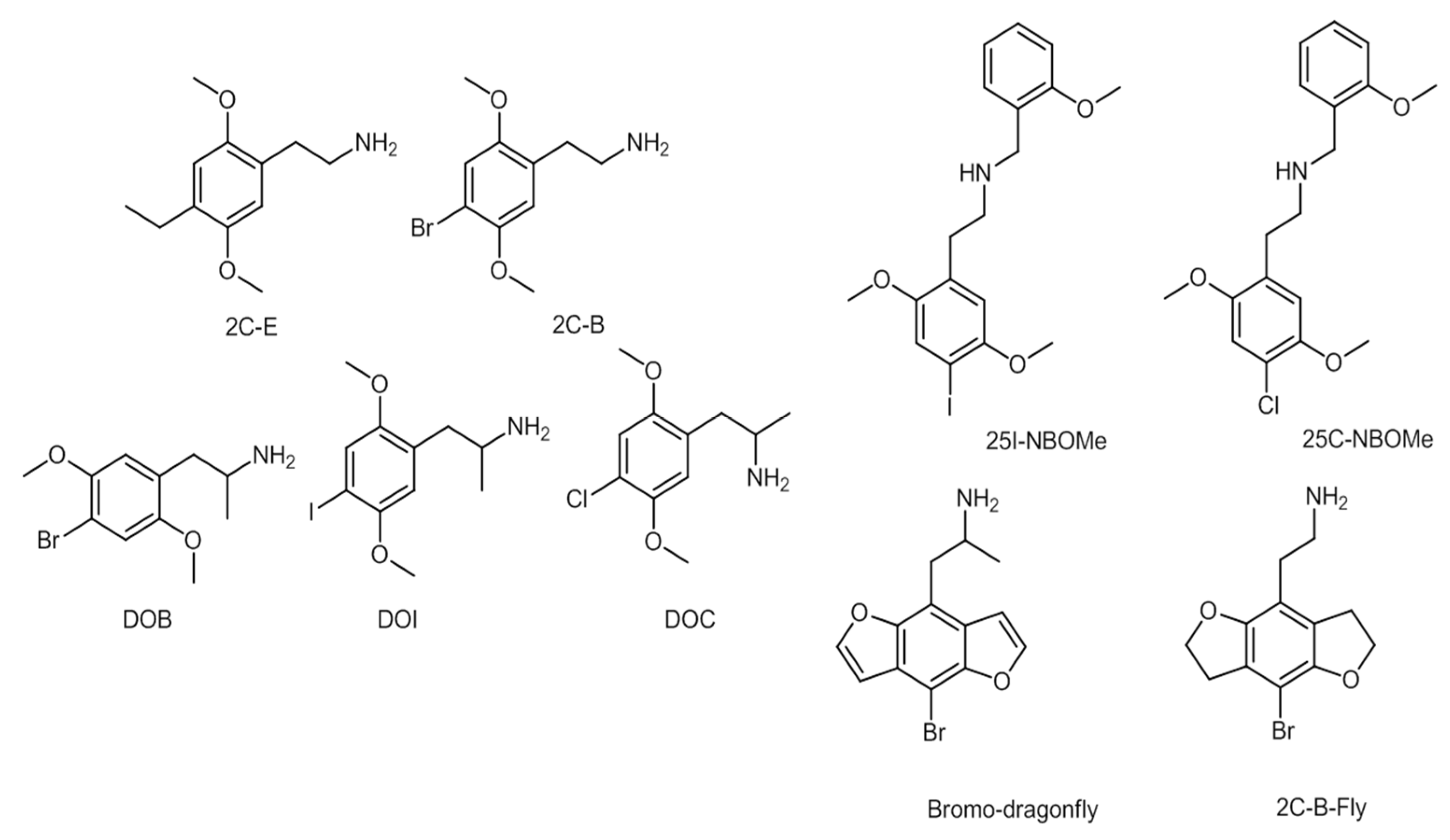
2.4. Phenylethylamines
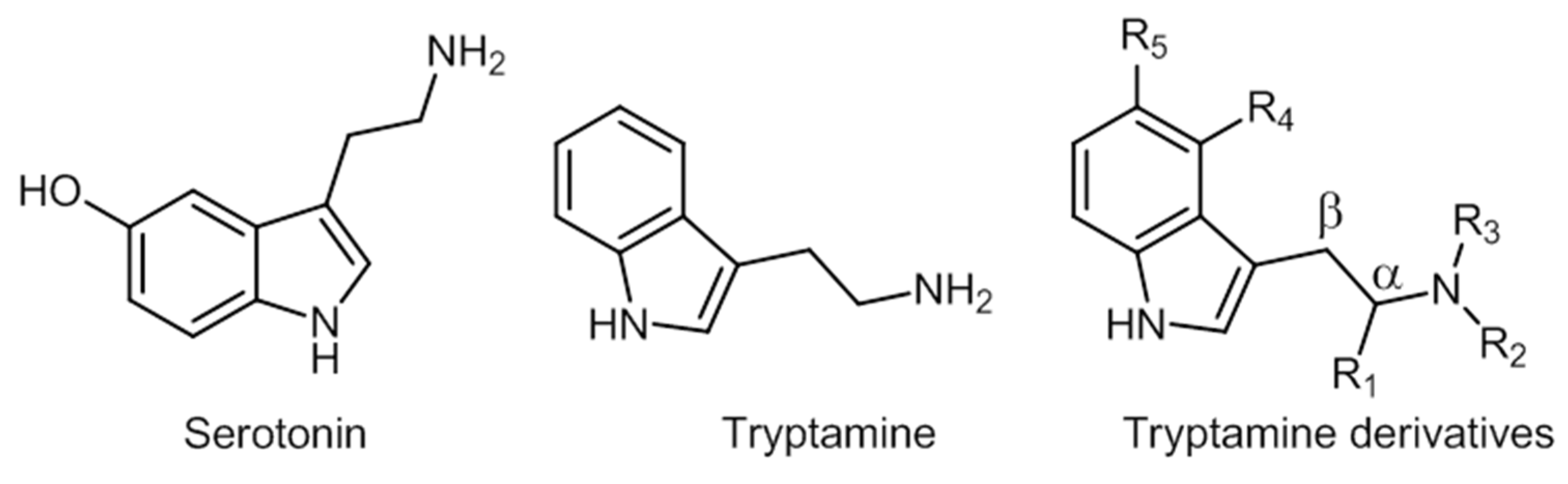
2.5. Tryptamines
2.6. Synthetic Cannabinoids
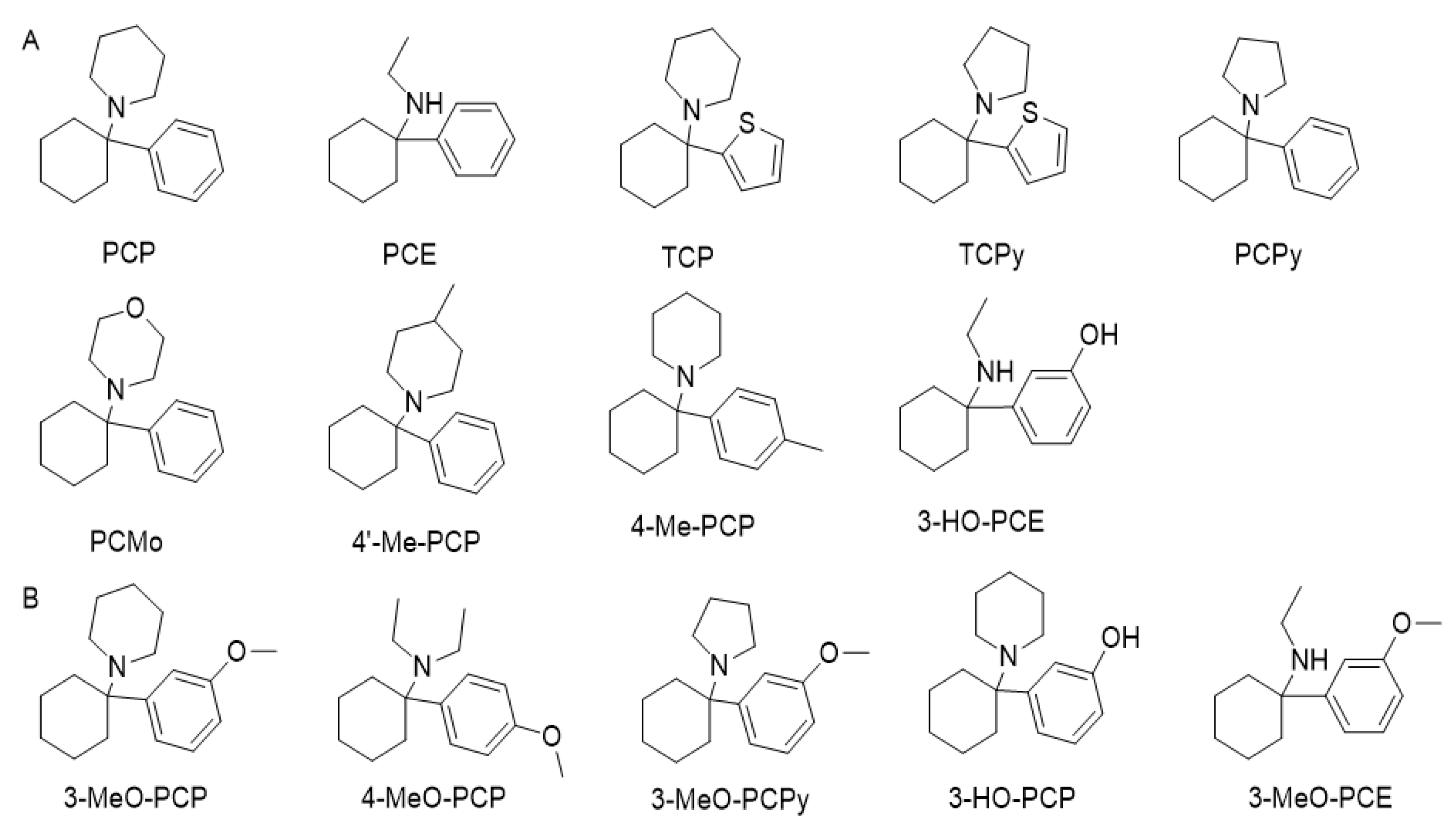
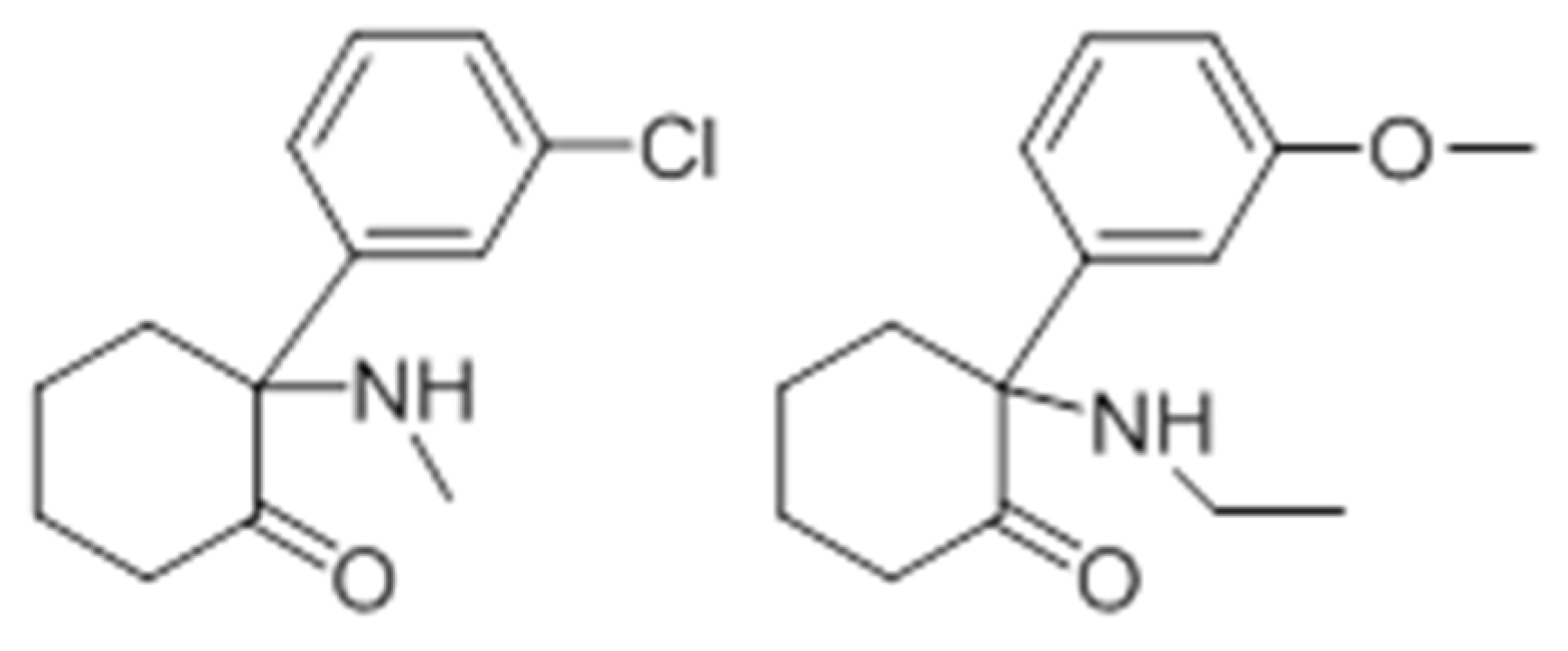
2.7. Phencyclidine Analogs
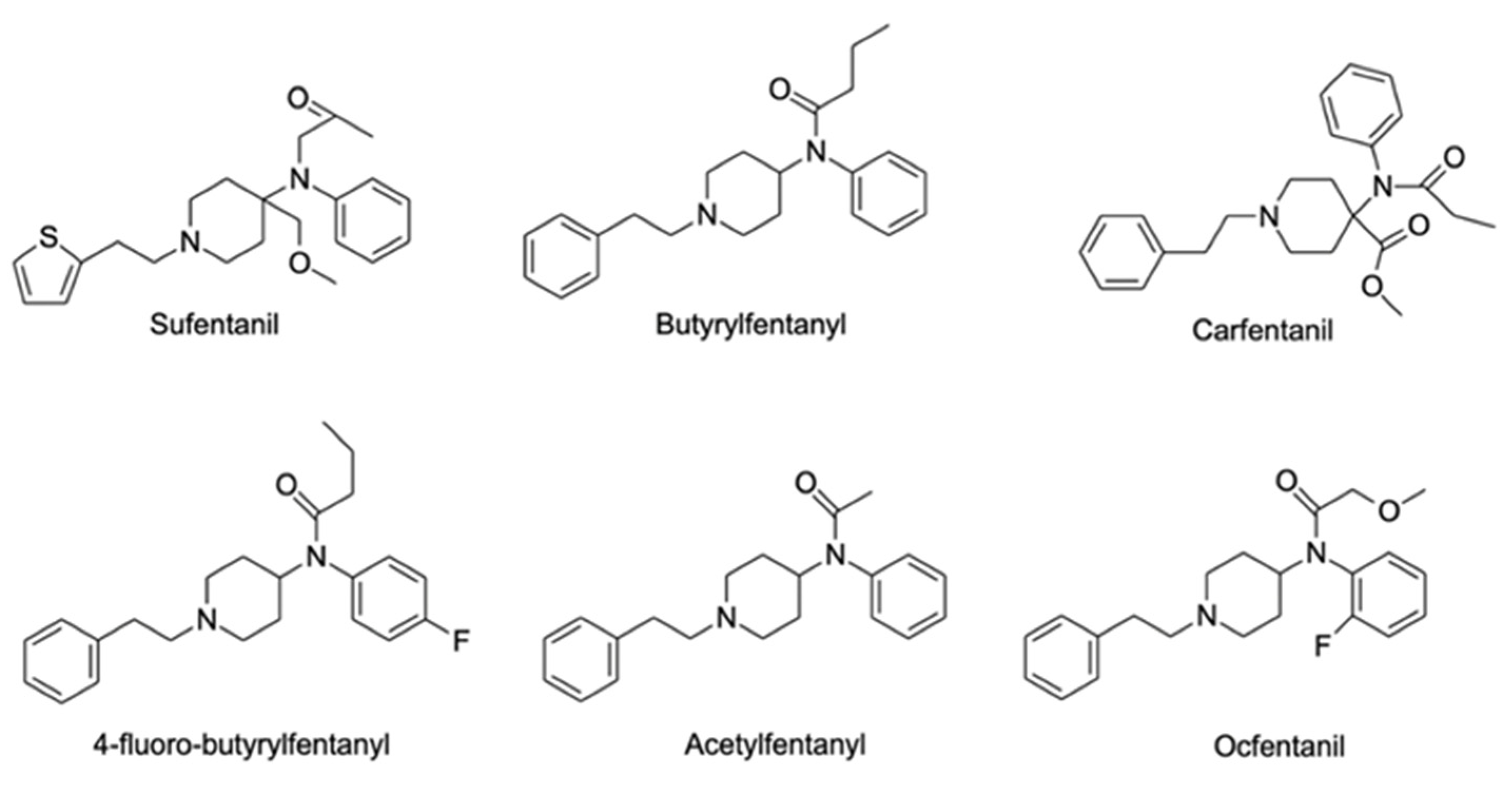
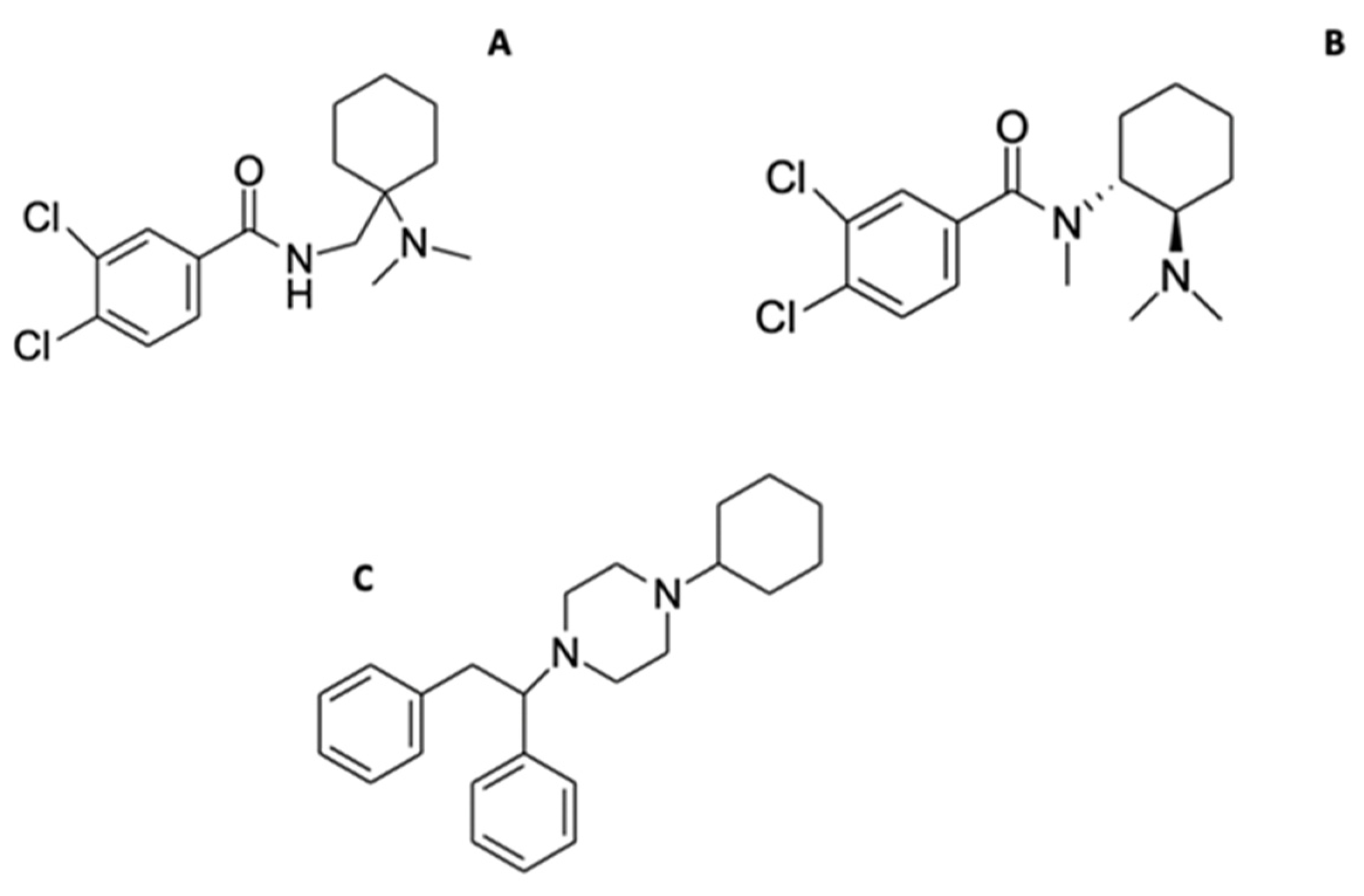
2.8. Synthetic Opioids
2.9. Plant-Based NPS
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The European Parliament and the Council of the European Union Directive (EU) 2017/2103 of the European Parliament and of the Council of 15 November 2017 Amending Council Framework Decision 2004/757/JHA in order to Include New Psychoactive Substances in the Definition of ‘Drug’ and Repealing Council Decision 2005/387. Available online: http://data.europa.eu/eli/dir/2017/2103/oj (accessed on 19 November 2021).
- Varì, M.R.; Mannocchi, G.; Tittarelli, R.; Campanozzi, L.L.; Nittari, G.; Feola, A.; Ronchi, F.U.; Ricci, G. New Psychoactive Substances: Evolution in the Exchange of Information and Innovative Legal Responses in the European Union. Int. J. Environ. Res. Public Health 2020, 17, 8704. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E.; Bryant, S.M.; Aks, S.E. Emerging Drugs of Abuse. Emerg. Med. Clin. N. Am. 2014, 32, 1–28. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime What are NPS? Available online: https://www.unodc.org/LSS/Page/NPS (accessed on 13 November 2021).
- United Nations Office on Drugs and Crime. Current NPS Threats Volume II; UNODC: Vienna, Austria, 2020. [Google Scholar]
- United Nations Office on Drugs and Crime. Early Warning Advisory on New Psychoactive Substances: NPS Substance Groups. Available online: https://www.unodc.org/LSS/SubstanceGroup/GroupsDashboard?testType=NPS (accessed on 17 November 2021).
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 204512532096719. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime. Early Warning Advisory on New Psychoactive Substances: Synthetic Cathinones. Available online: https://www.unodc.org/LSS/SubstanceGroup/Details/67b1ba69-1253-4ae9-bd93-fed1ae8e6802 (accessed on 19 November 2021).
- European Monitoring Centre for Drugs and Addiction. Spotlight on… Synthetic cannabinoids. Available online: https://www.emcdda.europa.eu/spotlights/synthetic-cannabinoids_en (accessed on 16 November 2021).
- Luethi, D.; Liechti, M.E. Designer drugs: Mechanism of action and adverse effects. Arch. Toxicol. 2020, 94, 1085. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. Early Warning Advisory on New Psychoactive Substances: Phenethylamines. Available online: https://www.unodc.org/LSS/SubstanceGroup/Details/275dd468-75a3-4609-9e96-cc5a2f0da467 (accessed on 21 November 2021).
- United Nations Office on Drugs and Crime. The challenge of New Psychoactive Substances. Available online: https://www.unodc.org/unodc/en/scientists/the-challenge-of-new-psychoactive-substances---global-smart-programme.html (accessed on 3 November 2021).
- Arbo, M.D.; Bastos, M.L.; Carmo, H.F. Piperazine compounds as drugs of abuse. Drug Alcohol Depend. 2012, 122, 174–185. [Google Scholar] [CrossRef]
- Pinterova, N.; Horsley, R.R.; Palenicek, T. Synthetic Aminoindanes: A Summary of Existing Knowledge. Front. Psychiatry 2017, 8, 236. [Google Scholar] [CrossRef]
- Prosser, J.M.; Nelson, L.S. The toxicology of bath salts: A review of synthetic cathinones. J. Med. Toxicol. 2012, 8, 33–42. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. Early Warning Advisory on New Psychoactive Substances: Tryptamines. Available online: https://www.unodc.org/LSS/SubstanceGroup/Details/68c027b6-0ed9-4c07-a139-7f1ca7ffce84 (accessed on 8 December 2021).
- United Nations Office on Drugs and Crime. Early Warning Advisory on New Psychoactive Substances: Synthetic Cannabinoids. Available online: https://www.unodc.org/LSS/SubstanceGroup/Details/ae45ce06-6d33-4f5f-916a-e873f07bde02 (accessed on 8 December 2021).
- United Nations Office on Drugs and Crime. Early Warning Advisory on New Psychoactive Substances: Phencyclidine-Type Substances. Available online: https://www.unodc.org/LSS/SubstanceGroup/Details/6bf165ed-82e7-47e0-9eaa-daacc42d99cd (accessed on 8 December 2021).
- United Nations Office on Drugs and Crime. Early Warning Advisory on New Psychoactive Substances: Other substances. Available online: https://www.unodc.org/LSS/SubstanceGroup/Details/01f2d3e0-91d1-4406-87db-e7129d40a371 (accessed on 8 December 2021).
- Suzuki, J.; El-Haddad, S. A review: Fentanyl and non-pharmaceutical fentanyls. Drug Alcohol Depend. 2017, 171, 107–116. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. New Benzodiazepines in Europe–A Review. Available online: https://www.emcdda.europa.eu/publications/rapid-communications/new-benzodiazepines-europe-review_en (accessed on 20 November 2021).
- Gonçalves, J.; Luís, Â.; Gallardo, E.; Duarte, A.P. Psychoactive substances of natural origin: Toxicological aspects, therapeutic properties and analysis in biological samples. Molecules 2021, 26, 1397. [Google Scholar] [CrossRef]
- Lo Faro, A.F.; Di Trana, A.; La Maida, N.; Tagliabracci, A.; Giorgetti, R.; Busardò, F.P. Biomedical analysis of New Psychoactive Substances (NPS) of natural origin. J. Pharm. Biomed. Anal. 2020, 179, 112945. [Google Scholar] [CrossRef]
- Meireles, V.; Rosado, T.; Barroso, M.; Soares, S.; Gonçalves, J.; Luís, Â.; Caramelo, D.; Simão, A.Y.; Fernández, N.; Duarte, A.P.; et al. Mitragyna speciosa: Clinical, Toxicological Aspects and Analysis in Biological and Non-Biological Samples. Medicines 2019, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.Y.; Gonçalves, J.; Gradillas, A.; García, A.; Restolho, J.; Fernández, N.; Rodilla, J.M.; Barroso, M.; Duarte, A.P.; Cristóvão, A.C.; et al. Evaluation of the Cytotoxicity of Ayahuasca Beverages. Molecules 2020, 25, 5594. [Google Scholar] [CrossRef] [PubMed]
- Rosado, T.; Gonçalves, J.; Luís, Â.; Malaca, S.; Soares, S.; Vieira, D.N.; Barroso, M.; Gallardo, E. Synthetic cannabinoids in biological specimens: A review of current analytical methods and sample preparation techniques. Bioanalysis 2018, 10, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.L.; Alves, V.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. Synthetic cathinones: An evolving class of new psychoactive substances. Crit. Rev. Toxicol. 2019, 49, 549–566. [Google Scholar] [CrossRef]
- Tabarra, I.; Soares, S.; Rosado, T.; Gonçalves, J.; Luís, Â.; Malaca, S.; Barroso, M.; Keller, T.; Restolho, J.; Gallardo, E. Novel synthetic opioids–toxicological aspects and analysis. Forensic Sci. Res. 2019, 4, 111–140. [Google Scholar] [CrossRef]
- Guirguis, A. New psychoactive substances: A public health issue. Int. J. Pharm. Pract. 2017, 25, 323–325. [Google Scholar] [CrossRef]
- Sumnall, H.R.; Evans-Brown, M.; McVeigh, J. Social, policy, and public health perspectives on new psychoactive substances. Drug Test. Anal. 2011, 3, 515–523. [Google Scholar] [CrossRef]
- Souto, C.; Göethel, G.; Peruzzi, C.P.; Cestonaro, L.V.; Garcia, I.; Ávila, D.S.; Eifler-Lima, V.; Carmo, H.; Bastos, M.D.L.; Garcia, S.C.; et al. Piperazine designer drugs elicit toxicity in the alternative in vivo model Caenorhabditis elegans. J. Appl. Toxicol. 2020, 40, 363–372. [Google Scholar] [CrossRef]
- Welz, A.; Koba, M. Piperazine derivatives as dangerous abused compounds. Acta Pharm. 2020, 70, 423–441. [Google Scholar] [CrossRef]
- Zhang, R.H.; Guo, H.Y.; Deng, H.; Li, J.; Quan, Z.S. Piperazine skeleton in the structural modification of natural products: A review. J. Enzyme Inhib. Med. Chem. 2021, 36, 1165–1197. [Google Scholar] [CrossRef]
- Moreno, I.E.D.; da Fonseca, B.M.; Barroso, M.; Costa, S.; Queiroz, J.A.; Gallardo, E. Determination of piperazine-type stimulants in human urine by means of microextraction in packed sorbent and high performance liquid chromatography-diode array detection. J. Pharm. Biomed. Anal. 2012, 61, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S. Current awareness of piperazines: Pharmacology and toxicology. Drug Test. Anal. 2011, 3, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.; LeBlanc, R.; Botch-Jones, S. Stability of synthetic piperazines in human whole blood. J. Anal. Toxicol. 2018, 42, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Peters, F.T. Analytical toxicology of emerging drugs of abuse-An update. Ther. Drug Monit. 2012, 34, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Zapata, F.; Matey, J.M.; Montalvo, G.; García-Ruiz, C. Chemical classification of new psychoactive substances (NPS). Microchem. J. 2021, 163, 105877. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Benzylpiperazine (BZP) and other Piperazines Drug Profile. Available online: https://www.emcdda.europa.eu/publications/drug-profiles/bzp_en (accessed on 4 December 2021).
- Moreno, I.E.D.; da Fonseca, B.M.; Magalhães, A.R.; Geraldes, V.S.; Queiroz, J.A.; Barroso, M.; Costa, S.; Gallardo, E. Rapid determination of piperazine-type stimulants in human urine by microextraction in packed sorbent after method optimization using a multivariate approach. J. Chromatogr. A 2012, 1222, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Byrska, B.; Zuba, D.; Stanaszek, R. Determination of piperazine derivatives in “legal highs”. Probl. Forensic Sci. 2010, LXXXI, 101–113. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. New Psychoactive Substances in Prison. Available online: https://www.emcdda.europa.eu/system/files/publications/8869/nps-in-prison.pdf (accessed on 8 December 2021).
- Darke, S.; Duflou, J.; Peacock, A.; Farrell, M.; Lappin, J. Characteristics and circumstances of death related to new psychoactive stimulants and hallucinogens in Australia. Drug Alcohol Depend. 2019, 204, 107556. [Google Scholar] [CrossRef]
- Lee, H.; Wang, G.Y.; Curley, L.E.; Sollers, J.J.; Kydd, R.R.; Kirk, I.J.; Russell, B.R. Acute effects of BZP, TFMPP and the combination of BZP and TFMPP in comparison to dexamphetamine on an auditory oddball task using electroencephalography: A single-dose study. Psychopharmacology 2016, 233, 863–871. [Google Scholar] [CrossRef]
- Gaillard, Y.P.; Cuquel, A.C.; Boucher, A.; Romeuf, L.; Bevalot, F.; Prevosto, J.M.; Menard, J.M. A Fatality Following Ingestion of the Designer Drug Meta-Chlorophenylpiperazine (mCPP) in an Asthmatic-HPLC-MS/MS Detection in Biofluids and Hair. J. Forensic Sci. 2013, 58, 263–269. [Google Scholar] [CrossRef]
- Dias da Silva, D.; Silva, M.J.; Moreira, P.; Martins, M.J.; Valente, M.J.; Carvalho, F.; Bastos, M.L.; Carmo, H. In vitro hepatotoxicity of ‘Legal X’: The combination of 1-benzylpiperazine (BZP) and 1-(m-trifluoromethylphenyl)piperazine (TFMPP) triggers oxidative stress, mitochondrial impairment and apoptosis. Arch. Toxicol. 2017, 91, 1413–1430. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.M.Z.; Butler, R. BZP-party pills: A review of research on benzylpiperazine as a recreational drug. Int. J. Drug Policy 2011, 22, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Antia, U.; Tingle, M.D.; Russell, B.R. Validation of an LC-MS method for the detection and quantification of BZP and TFMPP and their hydroxylated metabolites in human plasma and its application to the pharmacokinetic study of TFMPP in humans. J. Forensic Sci. 2010, 55, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Antia, U.; Lee, H.S.; Kydd, R.R.; Tingle, M.D.; Russell, B.R. Pharmacokinetics of “party pill” drug N-benzylpiperazine (BZP) in healthy human participants. Forensic Sci. Int. 2009, 186, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Gijsman, H.J.; Van Gerven, J.M.A.; Tieleman, M.C.; Schoemaker, R.C.; Pieters, M.S.M.; Ferrari, M.D.; Cohen, A.F.; Van Kempen, G.M.J. Pharmacokinetic and pharmacodynamic profile of oral and intravenous meta-chlorophenylpiperazine in healthy volunteers. J. Clin. Psychopharmacol. 1998, 18, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kersten, B.P.; McLaughlin, M.E. Toxicology and Management of Novel Psychoactive Drugs. J. Pharm. Pract. 2015, 28, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hondebrink, L.; Zwartsen, A.; Westerink, R.H.S. Effect fingerprinting of new psychoactive substances (NPS): What can we learn from in vitro data? Pharmacol. Ther. 2018, 182, 193–224. [Google Scholar] [CrossRef]
- Gee, P.; Schep, L. 1-Benzylpiperazine and other Piperazine-based Derivatives. In Novel Psychoactive Substance; Dargan, P.I., Wood, D.M., Eds.; Academic Press: London, UK, 2013; pp. 179–209. [Google Scholar]
- Katz, D.P.; Deruiter, J.; Bhattacharya, D.; Ahuja, M.; Bhattacharya, S.; Clark, C.R.; Suppiramaniam, V.; Dhanasekaran, M. Benzylpiperazine: “A messy drug”. Drug Alcohol Depend. 2016, 164, 1–7. [Google Scholar] [CrossRef]
- Curley, L.E.; Kydd, R.R.; Kirk, I.J.; Russell, B.R. Differential responses to anticipation of reward after an acute dose of the designer drugs benzylpiperazine (BZP) and trifluoromethylphenylpiperazine (TFMPP) alone and in combination using functional magnetic resonance imaging (fMRI). Psychopharmacology 2013, 229, 673–685. [Google Scholar] [CrossRef]
- Simmler, L.D.; Rickli, A.; Schramm, Y.; Hoener, M.C.; Liechti, M.E. Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives. Biochem. Pharmacol. 2014, 88, 237–244. [Google Scholar] [CrossRef]
- Musselman, M.E.; Hampton, J.P. “Not for human consumption”: A review of emerging designer drugs. Pharmacotherapy 2014, 34, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Schifano, F.; Orsolini, L.; Duccio Papanti, G.; Corkery, J.M. Novel psychoactive substances of interest for psychiatry. World Psychiatry 2015, 14, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, C.; Sweetsur, P. The Impact of the Prohibition of Benzylpiperazine (BZP) ‘Legal Highs’ on the Prevalence of BZP, New Legal Highs and other Drug Use in New Zealand. Available online: https://khepri-node.dev.meta-infra.org/papers/the-impact-of-the-prohibition-of-benzylpiperazine/22819869 (accessed on 5 October 2021).
- Wood, D.M.; Button, J.; Lidder, S.; Ramsey, J.; Holt, D.W.; Dargan, P.I. Dissociative and sympathomimetic toxicity associated with recreational use of 1-(3-trifluoromethylphenyl) piperazine (TFMPP) and 1-benzylpiperzine (BZP). J. Med. Toxicol. 2008, 4, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Robert, S. “Designer drugs”: Update on the management of novel psychoactive substance misuse in the acute care setting. Clin. Med. J. R. Coll. Physicians Lond. 2014, 14, 409–415. [Google Scholar] [CrossRef]
- Costa de Souza e Escada, S. Métodos de Análise de Piperazinas em Fluidos Biológicos. Master’s Thesis, Univesidade de Aveiro, Aveiro, Portugal, 2007. [Google Scholar]
- Castaneto, M.S.; Barnes, A.J.; Concheiro, M.; Klette, K.L.; Martin, T.A.; Huestis, M.A. Biochip array technology immunoassay performance and quantitative confirmation of designer piperazines for urine workplace drug testing. Anal. Bioanal. Chem. 2015, 407, 4639–4648. [Google Scholar] [CrossRef]
- Arbo, M.D.; Silva, R.; Barbosa, D.J.; da Silva, D.D.; Rossato, L.G.; Bastos, M.D.L.; Carmo, H. Piperazine designer drugs induce toxicity in cardiomyoblast h9c2 cells through mitochondrial impairment. Toxicol. Lett. 2014, 229, 178–189. [Google Scholar] [CrossRef]
- Zwartsen, A.; de Korte, T.; Nacken, P.; de Lange, D.W.; Westerink, R.H.S.; Hondebrink, L. Cardiotoxicity screening of illicit drugs and new psychoactive substances (NPS) in human iPSC-derived cardiomyocytes using microelectrode array (MEA) recordings. J. Mol. Cell. Cardiol. 2019, 136, 102–112. [Google Scholar] [CrossRef]
- Boumrah, Y.; Rosset, M.; Lecompte, Y.; Bouanani, S.; Khimeche, K.; Dahmani, A. Development of a targeted GC/MS screening method and validation of an HPLC/DAD quantification method for piperazines-amphetamines mixtures in seized material. Egypt. J. Forensic Sci. 2014, 4, 90–99. [Google Scholar] [CrossRef]
- Thomas, J.M.; Dourish, C.T.; Tomlinson, J.; Hassan-Smith, Z.; Hansen, P.C.; Higgs, S. The 5-HT2C receptor agonist meta-chlorophenylpiperazine (mCPP) reduces palatable food consumption and BOLD fMRI responses to food images in healthy female volunteers. Psychopharmacology 2018, 235, 257–267. [Google Scholar] [CrossRef]
- Felsinga, D.E.; Canala, C.E.; Bootha, R.G. Ligand-directed serotonin 5-HT2C receptor desensitization and sensitization. Eur J Pharmacol 2019, 848, 131–139. [Google Scholar] [CrossRef]
- Scotton, W.J.; Hill, L.J.; Williams, A.C.; Barnes, N.M. Serotonin Syndrome: Pathophysiology, Clinical Features, Management, and Potential Future Directions. Int. J. Tryptophan Res. 2019, 12, 1178646919873925. [Google Scholar] [CrossRef] [PubMed]
- Dias-da-Silva, D.; Arbo, M.D.; Valente, M.J.; Bastos, M.L.; Carmo, H. Hepatotoxicity of piperazine designer drugs: Comparison of different in vitro models. Toxicol. Vitr. 2015, 29, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Persona, K.; Polus, A.; Góralska, J.; Gruca, A.; Dembin´ska, A.; Dembin´ska-Kiec´2, D.; Kiec´2, K.; Piekoszewski, W. An In Vitro Study of the Neurotoxic Effects of N-Benzylpiperazine: A Designer Drug of Abuse. Neurotox. Res. 2016, 29, 558–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parrott, A.C. Mood fluctuation and psychobiological instability: The same core functions are disrupted by novel psychoactive substances and established recreational drugs. Brain Sci. 2018, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Shelton, R.S. Secondary Beta Phenyl Propyl Amines and Pharmaceutical Compositions Thereof. U.S. Patent No. 2,298,630, 13 October 1942. [Google Scholar]
- Woodruff, E.H. Amino Compound. U.S. Patent No. 2,293,874, 25 August 1942. [Google Scholar]
- Manier, S.K.; Felske, C.; Eckstein, N.; Meyer, M. The metabolic fate of the two new psychoactive substances 2-aminoindane and N-methyl-2-aminoindane studied in vitro and in vivo to support drug testing. Drug Test. Anal. 2019, 12, 145–151. [Google Scholar] [CrossRef]
- Levin, N.; Graham, B.E.; Kolloff, H.G. Physiologically active indanamines. J. Org. Chem. 1944, 9, 380–391. [Google Scholar] [CrossRef]
- Witkin, L.B.; Heubner, C.F.; Galdi, F.; O’Keefe, E.; Spitaletta, P.; Plummer, A.J. Pharmacology of 2-amino-indane hydrochloride (Su-8629): A potent non-narcotic analgesic. J. Pharmacol. Exp. Ther. 1961, 133, 400–408. [Google Scholar]
- Solomons, E.; Sam, J. 2-Aminoindans of pharmacological interest. J. Med. Chem. 1973, 16, 1330–1333. [Google Scholar] [CrossRef]
- Martin, Y.C.; Jarboe, C.H.; Krause, R.A.; Lynn, K.R.; Dunnigan, D.; Holland, J.B. Potential anti-Parkinson drugs designed by receptor mapping. J. Med. Chem. 1973, 16, 147–150. [Google Scholar] [CrossRef]
- The Regents of the University of California Monoamine Oxidase B (MAO-B) Inhibitors. Available online: https://pdcenter.ucsf.edu/monoamine-oxidase-b-mao-b-inhibitors (accessed on 27 November 2021).
- Martin, Y.C.; Holland, J.B.; Jarboe, C.H.; Plotnikoff, N. Discriminant analysis of the relation between physical properties and the inhibition of monoamine oxidase by aminotetralins and aminoindans. J. Med. Chem. 1974, 17, 409–413. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Gross, A.; Finberg, J.P.M. Rasagiline [N-propargyl-1R(+)-aminoindan], a selective and potent inhibitor of mitochondrial monoamine oxidase B. Br. J. Pharmacol. 2001, 132, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Knudsen Gerber, D.S. Selegiline and rasagiline: Twins or distant cousins? Consult Pharm 2011, 26, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Oberlender, R. Structure-activity-relationships of MDMA and related-compounds—A new class of psychoactive-drugs. Ann. N.Y. Acad. Sci. 1990, 600, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Hoffman, A.J.; Oberlender, R.A.; Jacob, P., III; Shulgin, A.T. Derivatives of 1-(1,3-benzodioxol-5-yl)-2-butanamine: Representatives of a novel therapeutic class. J. Med. Chem. 1986, 29, 2009–2015. [Google Scholar] [CrossRef]
- Oberlender, R.; Nichols, D.E. (+)-N-methyl-1-(1,3-benzodioxol-5-yl)-2-butanamine as a discriminative stimulus in studies of 3,4-methylenedioxy-methamphetamine-like behavioral activity. J. Pharmacol. Exp. Ther. 1990, 255, 1098–1106. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. Action on New Drugs. Available online: https://www.emcdda.europa.eu/html.cfm/index96437EN.html (accessed on 27 November 2021).
- United Nations Office on Drugs and Crime. Early Warning Advisory on New Psychoactive Substances: Aminoindanes. Available online: https://www.unodc.org/LSS/SubstanceGroup/Details/8fd64573-c567-4734-a258-76d1d95dca25 (accessed on 27 November 2021).
- Gill, H.; Gill, B.; Chen-Li, D.; El-Halabi, S.; Rodrigues, N.B.; Cha, D.S.; Lipsitz, O.; Lee, Y.; Rosenblat, J.D.; Majeed, A.; et al. The emerging role of psilocybin and MDMA in the treatment of mental illness. Expert Rev. Neurother. 2020, 20, 1263–1273. [Google Scholar] [CrossRef]
- Leach, B. New Drug to Replace Mephedrone as ‘Legal High’. The Telegraph, 18 April 2010. [Google Scholar]
- Corkery, J.M.; Elliott, S.; Schifano, F.; Corazza, O.; Ghodse, A.H. MDAI (5,6-methylenedioxy-2-aminoindane; 6,7-dihydro-5H-cyclopenta[f][1,3]benzodioxol-6-amine; “sparkle”; ’mindy’) toxicity: A brief overview and update. Hum. Psychopharmacol. Clin. Exp. 2013, 28, 345–355. [Google Scholar] [CrossRef]
- Coppola, M. Is the 5-iodo-2-aminoindan (5-IAI) the new MDMA? J. Addict. Res. Ther. 2012, 3, 1–3. [Google Scholar] [CrossRef]
- Coppola, M.; Mondola, R. 5-Iodo-2-aminoindan (5-IAI): Chemistry, pharmacology, and toxicology of a research chemical producing MDMA-like effects. Toxicol. Lett. 2013, 218, 24–29. [Google Scholar] [CrossRef]
- Kavanagh, P.; Sharma, J.; McNamara, S.; Angelov, D.; McDermott, S.; Mullan, D.; Ryder, S. Head Shop ‘Legal Highs’ Active Constituents Identification Chart (June 2010, Post-Ban). Available online: https://www.drugsandalcohol.ie/13204/ (accessed on 8 December 2021).
- PsychonautWiki MDAI/Summary. Available online: https://psychonautwiki.org/wiki/MDAI/Summary (accessed on 8 December 2021).
- Gross, P.; Smith, R.P. Biologic Activity of Hydroxylamine: A Review. Crit. Rev. Toxicol. 1985, 14, 87–99. [Google Scholar] [CrossRef]
- Páleníček, T.; Lhotková, E.; Žídková, M.; Balíková, M.; Kuchař, M.; Himl, M.; Mikšátková, P.; Čegan, M.; Valeš, K.; Tylš, F.; et al. Emerging toxicity of 5,6-methylenedioxy-2-aminoindane (MDAI): Pharmacokinetics, behaviour, thermoregulation and LD50 in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 69, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Monte, A.P.; Maronalewicka, D.; Cozzi, N.V.; Nichols, D.E. Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogs of 3,4-(methylenedioxy)amphetamine. J. Med. Chem. 1993, 36, 3700–3706. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.T.; Assi, S.; Stair, J.L.; Fergus, S.; Corazza, O.; Corkery, J.M.; Schifano, F. 5,6-methylenedioxy-2-aminoindane: From laboratory curiosity to ‘legal high’. Hum. Psychopharmacol. Clin. Exp. 2012, 27, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.; Evans, J. A 3-year review of new psychoactive substances in casework. Forensic Sci. Int. 2014, 243, 55–60. [Google Scholar] [CrossRef]
- Soares, J.; Costa, V.M.; Bastos, M.D.L.; Carvalho, F.; Capela, J.P. An updated review on synthetic cathinones. Arch. Toxicol. 2021, 95, 2895–2940. [Google Scholar] [CrossRef]
- Al-Hebshi, N.; Skaug, N. Khat (Catha edulis)—An updated review. Addict. Biol. 2005, 10, 299–307. [Google Scholar] [CrossRef]
- Alles, G.A.; Fairchild, M.D.; Jensen, M.; Alles, A. Chemical Pharmacology of Catha Edulis. J. Med. Chem. 1960, 3, 323–352. [Google Scholar] [CrossRef]
- Hyde, J.F.; Browning, E.; Adams, R. Synthetic homologues of d,l- ephedrine. J. Am. Chem. Soc. 1928, 50, 2287–2292. [Google Scholar] [CrossRef]
- Saem de Burnaga Sanchez, J. Sur un homologue de l’éphédrine [On a homologue of ephedrine]. Bull. Soc. Chim. Fr. 1929, 45, 284–286. [Google Scholar]
- Mehta, N. Meta chloro substituted-alpha-butylamino-propiophenones. U.S. Patent 3,819,706, 6 June 1974. [Google Scholar]
- Schütte, J. Anorexigenic Propiophenones. U.S. Patent US3001910A, 26 September 1961. [Google Scholar]
- Gardos, G.; Cole, J.O. Evaluation of pyrovalerone in chronically fatigued volunteers. Curr. Ther. Res. Clin. Exp. 1971, 13, 631–635. [Google Scholar]
- Deramos, E.C. The use of diethylpropion in the treatment of obesity. Br. J. Clin. Pract. 1964, 18, 210–211. [Google Scholar] [PubMed]
- Soroko, F.E.; Mehta, N.B.; Maxwell, R.A.; Ferris, R.M.; Schroeder, D. Bupropion hydrochloride ((+/−) alpha-t-butylamino-3-chloro-propiophenone HCl): A novel antidepressant agent. J. Pharm. Pharmacol. 1977, 29, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Stead, L.F.; Hartmann-Boyce, J.; Cahill, K.; Lancaster, T. Antidepressants for smoking cessation. Cochrane Database Syst. Rev. 2014, 1, CD000031. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.H.; Umashanker, D.; Igel, L.I.; Kumar, R.B.; Aronne, L.J. Obesity Pharmacotherapy. Med. Clin. North Am. 2018, 102, 135–148. [Google Scholar] [CrossRef]
- Dal Cason, T.A.; Young, R.; Glennon, R.A. Cathinone: An investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol. Biochem. Behav. 1997, 58, 1109–1116. [Google Scholar] [CrossRef]
- Simão, A.Y.; Antunes, M.; Marques, H.; Rosado, T.; Soares, S.; Gonçalves, J.; Barroso, M.; Andraus, M.; Gallardo, E. Recent bionalytical methods for the determination of new psychoactive substances in biological specimens. Bioanalysis 2020, 12, 1557–1595. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Addiction. European Drug Report Trends and Developments. Available online: https://www.emcdda.europa.eu/publications/edr/trends-developments/2021_en (accessed on 1 October 2021).
- European Monitoring Centre for Drugs and Addiction. Injection of Synthetic Cathinones. Available online: http://www.emcdda.europa.eu/system/files/publications/2754/Synthetic cathinones_updated2015.pdf (accessed on 3 December 2021).
- Grifell, M.; Ventura, M.; Carbón, X.; Quintana, P.; Galindo, L.; Palma, A.; Fornis, I.; Gi, C.; Farre, M.; Torrens, M. Patterns of use and toxicity of new para-halogenated substituted cathinones: 4-CMC (clephedrone), 4-CEC (4-chloroethcatinone) and 4-BMC (brephedrone). Hum. Psychopharmacol. Clin. Exp. 2017, 32, e2621. [Google Scholar] [CrossRef]
- Valente, M.J.; Guedes de Pinho, P.; Bastos, M.L.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Wojcieszak, J. Designer cathinones—An emerging class of novel recreational drugs. Forensic Sci. Int. 2013, 231, 42–53. [Google Scholar] [CrossRef]
- Karila, L.; Benyamina, A. The effects and risks associated with synthetic cathinones use in humans. In Synthetic Cathinones; Zawilska, J.B., Ed.; Springer: Cham, Switzerland, 2018; pp. 191–202. [Google Scholar]
- Riley, A.L.; Nelson, K.H.; To, P.; López-Arnau, R.; Xu, P.; Wang, D.; Wang, Y.; Shen, H.; Kuhn, D.M.; Angoa-Perez, M.; et al. Abuse potential and toxicity of the synthetic cathinones (i.e., “Bath salts”). Neurosci. Biobehav. Rev. 2020, 110, 150–173. [Google Scholar] [CrossRef]
- Corkery, J.M.; Guirguis, A.; Papanti, D.G.; Orsolini, L.; Schifano, F. Synthetic cathinones—Prevalence and motivations for use. In Synthetic Cathinones; Zawilska, J.B., Ed.; Springer: Cham, Switzerland, 2018; pp. 153–189. [Google Scholar]
- European Monitoring Centre for Drugs and Addiction. Synthetic cathinones drug profile. Available online: https://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cathinones_en (accessed on 25 November 2021).
- Gołembiowska, K.; Kamińska, K. Effects of Synthetic Cathinones on Brain Neurotransmitters. In Synthetic Cathinones; Zawilska, J.B., Ed.; Springer: Cham, Switzerland, 2018; pp. 117–124. [Google Scholar]
- Baumann, M.H.; Ayestas, M.A., Jr.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The Designer Methcathinone Analogs, Mephedrone and Methylone, are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology 2012, 37, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.N.; Kolanos, R.; Solis, E., Jr.; Glennon, R.A.; de Felice, L.J. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br. J. Pharmacol. 2013, 168, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, N.V.; Sievert, M.K.; Shulgin, A.T.; Jacob, P., III; Ruoho, A.E. Inhibition of plasma membrane monoamine transporters by β-ketoamphetamines. Eur. J. Pharmacol. 1999, 381, 63–69. [Google Scholar] [CrossRef]
- Nagai, F.; Nonaka, R.; Kamimura, K.S.H. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur. J. Pharmacol. 2007, 559, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P. Cathinone derivatives: A review of their chemistry, pharmacology and toxicology. Drug Test. Anal. 2011, 3, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Toennes, S.W.; Harder, S.; Schramm, M.; Niess, C.; Kauert, G.F. Pharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leaves. Br. J. Clin. Pharmacol. 2003, 56, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Widler, P.; Mathys, K.; Brenneisen, R.; Kalix, P.; Fisch, H.U. Pharmacodynamics and pharmacokinetics of khat: A controlled study. Clin. Pharmacol. Ther. 1994, 55, 556–562. [Google Scholar] [CrossRef]
- Novellas, J.; López-Arnau, R.; Carbó, M.L.; Pubill, D.; Camarasa, J.; Escubedo, E. Concentrations of MDPV in rat striatum correlate with the psychostimulant efect. J. Psychopharmacol. 2015, 29, 1209–1218. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; Mateus, J.-A.; Pujadas, M.; Fonseca, F.; Torrens, M.; Olesti, E.; de la Torre, R.; Farré, M. Human pharmacology of mephedrone in comparison with MDMA. Neuropsychopharmacology 2016, 41, 2704–2713. [Google Scholar] [CrossRef]
- Zaitsu, K. Metabolism of Synthetic Cathinones. In Synthetic Cathinones; Zawilska, J.B., Ed.; Springer: Cham, Switzerland, 2018; pp. 71–96. [Google Scholar]
- Negreira, N.; Erratico, C.; Kosjek, T.; van Nuijs, A.L.N.; Heath, E.; Neels, H.; Covaci, A. In vitro Phase I and Phase II metabolism of α-pyrrolidinovalerophenone (α-PVP), methylenedioxypyrovalerone (MDPV) and methedrone by human liver microsomes and human liver cytosol. Anal. Bioanal. Chem. 2015, 407, 5803–5816. [Google Scholar] [CrossRef]
- Židková, M.; Linhart, I.; Balíková, M.; Himl, M.; Dvořáčková, V.; Lhotková, E.; Páleníček, T. Identification of three new phase II metabolites of a designer drug methylone formed in rats by N-demethylation followed by conjugation with dicarboxylic acids. Xenobiotica 2017, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Turcant, A.; Deguigne, M.; Ferec, S.; Bruneau, C.; Leborgne, I.; Lelievre, B.; Gegu, C.; Jegou, F.; Abbara, C.; Roux, G.L.; et al. A 6-year review of new psychoactive substances at the Centre Antipoison Grand-Ouest d’Angers: Clinical and biological data. Toxicol. Anal. Clin. 2017, 29, 18–33. [Google Scholar] [CrossRef]
- Wiergowski, M.; Aszyk, J.; Kaliszan, M.; Wilczewska, K.; Anand, J.S.; Kot-Wasik, A.; Jankowski, Z. Identification of novel psychoactive substances 25B-NBOMe and 4-CMC in biologi- cal material using HPLC-Q-TOF-MS and their quantification in blood using UPLC-MS/MS in case of severe intoxications. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1041–1042, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wurita, A.; Hasegawa, K.; Minakata, K.; Gonmori, K.; Nozawa, H.; Yamagishi, I.; Suzuki, O.; Watanabe, K. Postmortem distri- bution of α-pyrrolidinobutiophenone in body fluids and solid tissues of a human cadaver. Leg. Med. 2014, 16, 241–246. [Google Scholar] [CrossRef]
- Margalho, C.; Castanheira, A.; Real, F.C.; Gallardo, E.; López-Rivadulla, M. Determination of “new psychoactive substances” in postmortem matrices using microwave derivatization and gas chromatography–mass spectrometry. J. Chromatogr. B 2016, 1020, 14–23. [Google Scholar] [CrossRef]
- Antunes, M.; Sequeira, M.; de Caires Pereira, M.; Caldeira, M.J.; Santos, S.; Franco, J.; Barroso, M.; Gaspar, H. Determination of Selected Cathinones in Blood by Solid-Phase Extraction and GC-MS. J. Anal. Toxicol. 2021, 45, 233–242. [Google Scholar] [CrossRef]
- Hemby, S.E.; McIntosh, S.; Leon, F.; Cutler, S.J.; McCurdy, C.R. Abuse liability and therapeutic potential of the Mitragyna speciosa (kratom) alkaloids mitragynine and 7-hydroxymitragynine. Addict. Biol. 2019, 24, 874–885. [Google Scholar] [CrossRef]
- Brandt, S.D.; Freeman, S.; Sumnall, H.R.; Measham, F.; Cole, J. Analysis of NRG ‘legal highs’ in the UK: Identification and formation of novel cathinones. Drug Test. Anal. 2011, 3, 569–575. [Google Scholar] [CrossRef]
- Vignali, C.; Moretti, M.; Groppi, A.; Osculati, A.M.M.; Tajana, L.; Morini, L. Distribution of the synthetic cathinone α-pyrrolidinohexiophenone in biological specimens. J. Anal. Toxicol. 2019, 43, 1–6. [Google Scholar] [CrossRef]
- Adamowicz, P.; Jurczyk, A.; Gil, D.; Szustowski, S. A case of intoxication with a new cathinone derivative α-PiHP—A presentation of concentrations in biological specimens. Leg. Med. 2020, 42, 101626. [Google Scholar] [CrossRef]
- Lelievre, B.; Richeval, C.; Coulon, A.; Iwanikow, D.; Brofferio, M.; Deguigne, M.; Boels, D.; Allorge, D.; Ferec, S.; Drevin, G.; et al. Case report on twocathinones abuse: MPHP and N-ethyl-4′methylnorpentedrone, with a fatal outcome. Forensic Toxicol. 2020, 38, 243–254. [Google Scholar] [CrossRef]
- Pei, Y.; Asif-Malik, A.; Canales, J.J. Trace Amines and the Trace Amine-Associated Receptor 1: Pharmacology, Neurochemistry, and Clinical Implications. Front. Neurosci. 2016, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.D. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J. Neurochem. 2004, 90, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Glennon, R.A.; Ismaiel, A.E.M.; Martin, B.; Poff, D.; Sutton, M. A preliminary behavioral investigation of PMMA, the 4-methoxy analog of methamphetamine. Pharmacol. Biochem. Behav. 1988, 31, 9–13. [Google Scholar] [CrossRef]
- Shulgin, A.T.; Shulgin, A. Pihkal: A Chemical Love Story; Transform Press: Berkeley, CA, USA, 1990; ISBN 0963009605. [Google Scholar]
- Monte, A.P.; Waldman, S.R.; Marona-Lewicka, D.; Wainscott, D.B.; Nelson, D.L.; Sanders-Bush, E.; Nichols, D.E. Dihydrobenzofuran analogues of hallucinogens. 4. Mescaline derivatives. J. Med. Chem. 1997, 40, 2997–3008. [Google Scholar] [CrossRef]
- Collins, M. Some new psychoactive substances: Precursor chemicals and synthesis-driven end-products. Drug Test. Anal. 2011, 3, 404–416. [Google Scholar] [CrossRef]
- Sutherland, R.; Barratt, M. New (and Emerging) Psychoactive Substances (NPS). Available online: https://ndarc.med.unsw.edu.au/sites/default/files/ndarc/resources/NDA073 New Psychoactive Substances %28NPS%29.pdf (accessed on 7 December 2021).
- Davies, S.; Wood, D.M.; Smith, G.; Button, J.; Ramsey, J.; Archer, R.; Holt, D.W.; Dargan, P.I. Purchasing “legal highs” on the Internet-is there consistency in what you get? QJM An Int. J. Med. 2010, 103, 489–493. [Google Scholar] [CrossRef]
- Marek, G.J.; Aghajanian, G.K. LSD and the phenethylamine hallucinogen DOI are potent partial agonists at 5-HT2A receptors on interneurons in rat piriform cortex. J. Pharmacol. Exp. Ther. 1996, 278, 1373–1382. [Google Scholar]
- Marchi, N.C.; Scherer, J.N.; Fara, L.S.; Remy, L.; Ornel, R.; Reis, M.; Zamboni, A.; Paim, M.; Fiorentin, T.R.; Wayhs, C.A.Y.; et al. Clinical and Toxicological Profile of NBOMes: A Systematic Review. Psychosomatics 2019, 60, 129–138. [Google Scholar] [CrossRef]
- Pawar, R.S.; Grundel, E. Overview of regulation of dietary supplements in the USA and issues of adulteration with phenethylamines (PEAs). Drug Test. Anal. 2017, 9, 500–517. [Google Scholar] [CrossRef]
- Lee, J.; Choe, S.; Choi, H.; Heo, S.; Kim, E.; Kim, H.; Bang, E.; Chung, H. Identification of N-ethyl-α-ethylphenethylamine in crystalline powder seized for suspected drug trafficking: A research chemical or a new designer drug? Forensic Toxicol. 2013, 31, 54–58. [Google Scholar] [CrossRef]
- Smith, T.A. Phenethylamine and related compounds in plants. Phytochemistry 1977, 16, 9–18. [Google Scholar] [CrossRef]
- Hill, S.L.; Thomas, S.H.L. Clinical toxicology of newer recreational drugs. Clin. Toxicol. 2011, 49, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.V.; Stellpflug, S.J.; Burnett, A.M.; Engebretsen, K.M. 2C or Not 2C: Phenethylamine Designer Drug Review. J. Med. Toxicol. 2013, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Haroz, R.; Greenberg, M.I. New drugs of abuse in North America. Clin. Lab. Med. 2006, 26, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Carmo, H.; Hengstler, J.G.; de Boer, D.; Ringel, M.; Remião, F.; Carvalho, F.; Fernandes, E.; dos Reys, L.A.; Oesch, F.; de Lourdes Bastos, M. Metabolic pathways of 4-bromo-2,5-dimethoxyphenethylamine (2C-B): Analysis of phase I metabolism with hepatocytes of six species including human. Toxicology 2005, 206, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Theobald, D.S.; Maurer, H.H. No Title Studies on the metabolism and toxicological detection of the designer drug 4-ethyl-2,5-dimethoxy-beta-phenethylamine (2C-E) in rat urine using gas chromatographic-mass spectrometric techniques. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 842, 76–90. [Google Scholar] [CrossRef]
- Šuláková, A.; Nykodemová, J.; Palivec, P.; Jurok, R.; Rimpelová, S.; Leonhardt, T.; Šíchová, K.; Páleníček, T.; Kuchař, M. 25CN-NBOMe Metabolites in Rat Urine, Human Liver Microsomes and C.elegans- Structure Determination and Synthesis of the Most Abundant Metabolites. Metabolites 2021, 11, 212. [Google Scholar] [CrossRef]
- Caspar, A.T.; Brandt, S.D.; Stoever, A.E.; Meyer, M.R.; Maurer, H.H. Metabolic fate and detectability of the new psychoactive substances 2-(4-bromo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25B-NBOMe) and 2-(4-chloro-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25C-NBOMe) in human and rat u. J. Pharm. Biomed. Anal. 2017, 134, 158–169. [Google Scholar] [CrossRef]
- Nielsen, L.M.; Holm, N.B.; Leth-Petersen, S.; Kristensen, J.L.; Olsen, L.; Linnet, K. Characterization of the hepatic cytochrome P450 enzymes involved in the metabolism of 25I-NBOMe and 25I-NBOH. Drug Test. Anal. 2016, 9, 671–679. [Google Scholar] [CrossRef]
- Schifano, F.; Chiappini, S.; Miuli, A.; Corkery, J.M.; Scherbaum, N.; Napoletano, F.; Arillotta, D.; Zangani, C.; Catalani, V.; Vento, A.; et al. New psychoactive substances (NPS) and serotonin syndrome onset: A systematic review. Exp. Neurol. 2021, 339, 113638. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.; Kemp, P.; Harty, L.; Choi, C.; Christensen, D. Postmortem identification and quantitation of 2,5-dimethoxy-4-n-propylthiophenethylamine using GC-MSD and GC-NPD. J. Anal. Toxicol. 2003, 27, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Stellpflug, S.J.; Kealey, S.E.; Hegarty, C.B.; Janis, G.C. 2-(4-Iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe): Clinical case with unique confirmatory testing. J. Med. Toxicol. 2014, 10, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Poklis, J.L.; Devers, K.G.; Arbefeville, E.F.; Pearson, J.M.; Houston, E.; Poklis, A. Postmortem detection of 25I-NBOMe [2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine] in fluids and tissues determined by high performance liquid chromatography with tandem mass spectrometry from a traumatic death. Forensic Sci. Int. 2014, 234, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Imam, A. 25b-NBOMe: A Case Report of Sudden Death and Insightful View of Google Trends Data. Iran. J. Psychiatry Behav. Sci. 2018, 12, e9870. [Google Scholar] [CrossRef]
- Malaca, S.; Lo Faro, A.F.; Tamborra, A.; Pichini, S.; Busardò, F.P.; Huestis, M.A. Toxicology and Analysis of Psychoactive Tryptamines. Int. J. Mol. Sci. 2020, 21, 9279. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.M.; Carvalho, F.; de Lourdes Bastos, M.; Guedes de Pinho, P.; Carvalho, M. The hallucinogenic world of tryptamines: An updated review. Arch. Toxicol. 2015, 89, 1151–1173. [Google Scholar] [CrossRef]
- Liechti, M.E. Novel psychoactive substances (designer drugs): Overview and pharmacology of modulators of monoamine signalling. Swiss Med. Wkly. 2015, 145, w14043. [Google Scholar] [CrossRef]
- Tittarelli, R.; Mannocchi, G.; Pantano, F.; Romolo, F. Recreational Use, Analysis and Toxicity of Tryptamines. Curr. Neuropharmacol. 2014, 13, 26–46. [Google Scholar] [CrossRef]
- Simão, A.Y.; Gonçalves, J.; Duarte, A.P.; Barroso, M.; Cristóvão, A.C.; Gallardo, E. Toxicological Aspects and Determination of the Main Components of Ayahuasca: A Critical Review. Medicines 2019, 6, 106. [Google Scholar] [CrossRef]
- Vargas, A.S.; Luís, Â.; Barroso, M.; Gallardo, E.; Pereira, L. Psilocybin as a New Approach to Treat Depression and Anxiety in the Context of Life-Threatening Diseases-A Systematic Review and Meta-Analysis of Clinical Trials. Biomedicines 2020, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Kargbo, R.B. Psilocybin Therapeutic Research: The Present and Future Paradigm. ACS Med. Chem. Lett. 2020, 11, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Griffiths, R.R. Potential Therapeutic Effects of Psilocybin. Neurotherapeutics 2017, 14, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Global Drug Survey GDS 2020 Psychedelics Report. Available online: https://www.globaldrugsurvey.com/gds-2020-psychedelics-report/ (accessed on 1 December 2021).
- Bilhimer, M.H.; Schult, R.F.; Higgs, K.V.; Wiegand, T.J.; Gorodetsky, R.M.; Acquisto, N.M. Acute Intoxication following Dimethyltryptamine Ingestion. Case Rep. Emerg. Med. 2018, 2018, 1–3. [Google Scholar] [CrossRef]
- Honyiglo, E.; Franchi, A.; Cartiser, N.; Bottinelli, C.; Advenier, A.S.; Bévalot, F.; Fanton, L. Unpredictable Behavior Under the Influence of “Magic Mushrooms”: A Case Report and Review of the Literature. J. Forensic Sci. 2019, 64, 1266–1270. [Google Scholar] [CrossRef]
- Attema-de Jonge, M.E.; Portier, C.B.; Franssen, E.J.F. Automutilation after consumption of hallucinogenic mushrooms. Ned Tijdschr Geneeskd. 2007, 151, 2869–2872. [Google Scholar]
- Sklerov, J.; Levine, B.; Moore, K.A.; King, T.; Fowler, D. A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation. J. Anal. Toxicol. 2005, 29, 838–841. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; Pérez-Acevedo, A.P.; Hladun, O.; Torres-Moreno, M.C.; Muga, R.; Torrens, M.; Farré, M. Cannabinoids: From pot to lab. Int. J. Med. Sci. 2018, 15, 1286. [Google Scholar] [CrossRef]
- Wiley, J.L.; Marusich, J.A.; Huffman, J.W. Moving around the molecule: Relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci. 2014, 97, 55–63. [Google Scholar] [CrossRef]
- Auwärter, V.; Dresen, S.; Weinmann, W.; Müller, M.; Pütz, M.; Ferreirós, N. ‘Spice’ and other herbal blends: Harmless incense or cannabinoid designer drugs? J. Mass Spectrom. 2009, 44, 832–837. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Synthetic Cannabinoids and “Spice” Drug Profile. Available online: https://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids_en (accessed on 1 December 2021).
- United Nations Office on Drugs and Crime. Recommended Methods for the Identification and Analysis of Synthetic Cannabinoid Receptor Agonists in Seized Materials (Revised and Updated) Manual for Use by National Drug Analysis Laboratories; United Nations Office at Vienna: Vienna, Austria, 2020. [Google Scholar]
- Lefever, T.W.; Marusich, J.A.; Thomas, B.F.; Barrus, D.G.; Peiper, N.C.; Kevin, R.C.; Wiley, J.L. Vaping Synthetic Cannabinoids: A Novel Preclinical Model of E-Cigarette Use in Mice. Subst. Abus. Res. Treat. 2017, 11, 1178221817701739. [Google Scholar] [CrossRef] [PubMed]
- Blundell, M.S.; Dargan, P.I.; Wood, D.M. The dark cloud of recreational drugs and vaping. QJM 2018, 111, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Breitbarth, A.K.; Morgan, J.; Jones, A.L. E-cigarettes—An unintended illicit drug delivery system. Drug Alcohol Depend. 2018, 192, 98–111. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. Synthetic Cannabinoids in Europe—A Review. Available online: https://www.emcdda.europa.eu/system/files/publications/14035/Synthetic-cannabinoids-in-Europe-EMCDDA-technical-report.pdf (accessed on 1 December 2021).
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic cannabinoids: Epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Synthetic Cannabinoids in Europe. Available online: https://www.emcdda.europa.eu/topics/pods/synthetic-cannabinoids_en (accessed on 1 December 2021).
- United Nations Office on Drugs and Crime. Current NPS Threats Volume IV; UNODC: Vienna, Austria, 2021. [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. New Report Highlights Public Health and Social Risks Posed by Synthetic Cannabinoids in Europe. Available online: https://www.emcdda.europa.eu/news/2021/new-report-highlights-public-health-and-social-risks-posed-synthetic-cannabinoids-europe_en (accessed on 9 December 2021).
- Zuba, D.; Byrska, B.; MacIow, M. Comparison of “herbal highs” composition. Anal. Bioanal. Chem. 2011, 400, 119–126. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network The DAWN Report; Drug-Related Emergency Department Visits Involving Synthetic Cannabinoids; SAMHSA: Rockville, MD, USA, 2014.
- Barratt, M.J.; Cakic, V.; Lenton, S. Patterns of synthetic cannabinoid use in Australia. Drug Alcohol Rev. 2013, 32, 141–146. [Google Scholar] [CrossRef]
- Hu, X.; Primack, B.A.; Barnett, T.E.; Cook, R.L. College students and use of K2: An emerging drug of abuse in young persons. Subst. Abuse Treat. Prev. Policy 2011, 6, 16. [Google Scholar] [CrossRef]
- Schaefer, N.; Kettner, M.; Laschke, M.W.; Schlote, J.; Ewald, A.H.; Menger, M.D.; Maurer, H.H.; Schmidt, P.H. Distribution of Synthetic Cannabinoids JWH-210, RCS-4 and ∆ 9-Tetrahydrocannabinol After Intravenous Administration to Pigs. Curr. Neuropharmacol. 2017, 15, 713. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, M.; Iszard, M.; Cole, R.B.; Wang, W.; Wang, G. In vitro metabolism of R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo [1,2,3-de]1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate, a cannabinoid receptor agonist. Drug Metab. Dispos. 2002, 30, 1077–1086. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, P.; Wang, W.; Cole, R.B.; Wang, G. Characterization of rat liver microsomal metabolites of AM-630, a potent cannabinoid receptor antagonist, by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2004, 39, 672–681. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, P.; Cole, R.B.; Wang, G. Identification of in vitro metabolites of JWH-015, an aminoalkylindole agonist for the peripheral cannabinoid receptor (CB2) by HPLC-MS/MS. Anal. Bioanal. Chem. 2006, 386, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Sobolevsky, T.; Prasolov, I.; Rodchenkov, G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci. Int. 2010, 200, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Möller, I.; Wintermeyer, A.; Bender, K.; Jübner, M.; Thomas, A.; Krug, O.; Schänzer, W.; Thevis, M. Screening for the synthetic cannabinoid JWH-018 and its major metabolites in human doping controls. Drug Test. Anal. 2011, 3, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Toennes, S.W.; Geraths, A.; Pogoda, W.; Paulke, A.; Wunder, C.; Theunissen, E.L.; Ramaekers, J.G. Pharmacokinetic properties of the synthetic cannabinoid JWH-018 and of its metabolites in serum after inhalation. J. Pharm. Biomed. Anal. 2017, 140, 215–222. [Google Scholar] [CrossRef]
- Moran, C.L.; Le, V.H.; Chimalakonda, K.C.; Smedley, A.L.; Lackey, F.D.; Owen, S.N.; Kennedy, P.D.; Endres, G.W.; Ciske, F.L.; Kramer, J.B.; et al. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal. Chem. 2011, 83, 4228–4236. [Google Scholar] [CrossRef]
- Chimalakonda, K.C.; Bratton, S.M.; Le, V.H.; Yiew, K.H.; Dineva, A.; Moran, C.L.; James, L.P.; Moran, J.H.; Radominska-Pandya, A. Conjugation of Synthetic Cannabinoids JWH-018 and JWH-073, Metabolites by Human UDP-Glucuronosyltransferases. Drug Metab. Dispos. 2011, 39, 1967. [Google Scholar] [CrossRef]
- Wintermeyer, A.; Möller, I.; Thevis, M.; Jübner, M.; Beike, J.; Rothschild, M.A.; Bender, K. In vitro phase I metabolism of the synthetic cannabimimetic JWH-018. Anal. Bioanal. Chem. 2010, 398, 2141–2153. [Google Scholar] [CrossRef]
- Kong, T.Y.; Kim, J.-H.; Kim, D.K.; Lee, H.S. Synthetic cannabinoids are substrates and inhibitors of multiple drug-metabolizing enzymes. Arch. Pharm. Res. 2018, 41, 691–710. [Google Scholar] [CrossRef]
- Abbate, V.; Schwenk, M.; Presley, B.C.; Uchiyama, N. The ongoing challenge of novel psychoactive drugs of abuse. Part I. Synthetic cannabinoids (IUPAC Technical Report). Pure Appl. Chem. 2018, 90, 1255–1282. [Google Scholar] [CrossRef]
- Chimalakonda, K.C.; Seely, K.A.; Bratton, S.M.; Brents, L.K.; Moran, C.L.; Endres, G.W.; James, L.P.; Hollenberg, P.F.; Prather, P.L.; Radominska-Pandya, A.; et al. Cytochrome P450-Mediated Oxidative Metabolism of Abused Synthetic Cannabinoids Found in K2/Spice: Identification of Novel Cannabinoid Receptor Ligands. Drug Metab. Dispos. 2012, 40, 2174–2184. [Google Scholar] [CrossRef]
- Giorgetti, A.; Mogler, L.; Haschimi, B.; Halter, S.; Franz, F.; Westphal, F.; Fischmann, S.; Riedel, J.; Pütz, M.; Auwärter, V. Detection and phase I metabolism of the 7-azaindole-derived synthetic cannabinoid 5F-AB-P7AICA including a preliminary pharmacokinetic evaluation. Drug Test. Anal. 2020, 12, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.; Fantegrossi, W.E. Pharmacological and Toxicological Effects of Synthetic Cannabinoids and Their Metabolites. In Neuropharmacology of New Psychoactive Substances (NPS). Current Topics in Behavioral Neurosciences; Baumann, M., Glennon, R., Wiley, J., Eds.; Springer: Cham, Switzerland, 2016; Volume 32, pp. 249–262. [Google Scholar]
- Lapoint, J.; James, L.P.; Moran, C.L.; Nelson, L.S.; Hoffman, R.S.; Moran, J.H. Severe Toxicity Following Synthetic Cannabinoid Ingestion. Clin. Toxicol. 2011, 49, 760. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.A.; Lonati, D.; Giampreti, A.; Petrolini, V.; Vecchio, S.; Rognoni, C.; Bigi, S.; Buscaglia, E.; Mazzoleni, M.; Manzo, L.; et al. New Synthetic Cannabinoids Intoxications in Italy: Clinical Identification and Analytical Confirmation of Cases. J. Emerg. Med. 2011, 41, 220. [Google Scholar] [CrossRef]
- Cohen, J.; Morrison, S.; Greenberg, J.; Saidinejad, M. Clinical Presentation of Intoxication Due to Synthetic Cannabinoids. Pediatrics 2012, 129, e1064–e1067. [Google Scholar] [CrossRef]
- Hoyte, C.O.; Jacob, J.; Monte, A.A.; Al-Jumaan, M.; Bronstein, A.C.; Heard, K.J. A Characterization of Synthetic Cannabinoid Exposures Reported to the National Poison Data System in 2010. Ann. Emerg. Med. 2012, 60, 435–438. [Google Scholar] [CrossRef]
- Tait, R.J.; Caldicott, D.; Mountain, D.; Hill, S.L.; Lenton, S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin. Toxicol. 2016, 54, 1–13. [Google Scholar] [CrossRef]
- Yeruva, R.R.; Mekala, H.M.; Sidhu, M.; Lippmann, S. Synthetic Cannabinoids—”Spice” Can Induce a Psychosis: A Brief Review. Innov. Clin. Neurosci. 2019, 16, 31. [Google Scholar]
- Pacher, P.; Steffens, S.; Haskó, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2017, 15, 151–166. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wurita, A.; Minakata, K.; Gonmori, K.; Yamagishi, I.; Nozawa, H.; Watanabe, K.; Suzuki, O. Identification and quantitation of 5-fluoro-ADB, one of the most dangerous synthetic cannabinoids, in the stomach contents and solid tissues of a human cadaver and in some herbal products. Forensic Toxicol. 2015, 33, 112–121. [Google Scholar] [CrossRef]
- Hasegawa, K.; Wurita, A.; Minakata, K.; Gonmori, K.; Nozawa, H.; Yamagishi, I.; Watanabe, K.; Suzuki, O. Postmortem distribution of MAB-CHMINACA in body fluids and solid tissues of a human cadaver. Forensic Toxicol. 2015, 33, 380–387. [Google Scholar] [CrossRef]
- Minakata, K.; Yamagishi, I.; Nozawa, H.; Hasegawa, K.; Suzuki, M.; Gonmori, K.; Suzuki, O.; Watanabe, K. Sensitive identification and quantitation of parent forms of six synthetic cannabinoids in urine samples of human cadavers by liquid chromatography–tandem mass spectrometry. Forensic Toxicol. 2017, 35, 275–283. [Google Scholar] [CrossRef]
- Angerer, V.; Jacobi, S.; Franz, F.; Auwärter, V.; Pietsch, J. Three fatalities associated with the synthetic cannabinoids 5F-ADB, 5F-PB-22, and AB-CHMINACA. Forensic Sci. Int. 2017, 281, e9–e15. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.; Stockhausen, S.; Kernbach-Wighton, G.; Madea, B. Death due to diabetic ketoacidosis: Induction by the consumption of synthetic cannabinoids? Forensic Sci. Int. 2015, 257, e6–e11. [Google Scholar] [CrossRef] [PubMed]
- Wallach, J.; Brandt, S.D. Phencyclidine-based new psychoactive substances. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2018; Volume 252, pp. 261–303. [Google Scholar]
- Morris, H.; Wallach, J. From PCP to MXE: A comprehensive review of the non-medical use of dissociative drugs. Drug Test. Anal. 2014, 6, 614–632. [Google Scholar] [CrossRef] [PubMed]
- Fujigaki, H.; Mouri, A.; Yamamoto, Y.; Nabeshima, T.; Saito, K. Linking phencyclidine intoxication to the tryptophan-kynurenine pathway: Therapeutic implications for schizophrenia. Neurochem. Int. 2019, 125, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Lindstedt, D.; Roman, M.; Thelander, G.; Nielsen, E.I.; Lennborn, U.; Sandler, H.; Rubertsson, S.; Ahlner, J.; Kronstrand, R.; et al. A non-fatal intoxication and seven deaths involving the dissociative drug 3-MeO-PCP. Forensic Sci. Int. 2017, 275, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Carroll, F.I.; Lewin, A.H.; Mascarella, S.W.; Seltzman, H.H.; Reddy, P.A. Designer drugs: A medicinal chemistry perspective. Ann. N.Y. Acad. Sci. 2012, 1248, 18–38. [Google Scholar] [CrossRef]
- Vargas, A.S.; Caramelo, D.; Simão, A.Y.; Gonçalves, J.; Rosado, T.; Antunes, M.; Barroso, M.; Gallardo, E. Ketamine and Other Phencyclidine Analogues: A Review of Their Use as Drugs of Abuse, Toxicological Aspects and Bioanalytical Approaches. In Ketamine: History, Uses and Health Effects; McBride, L.A., Ed.; Nova Publisher: New York, NY, USA, 2020; pp. 73–183. ISBN 978-1-53616-731-3. [Google Scholar]
- Ho, J.H.; Dargan, P.I. Arylcyclohexamines (Ketamine, Phencyclidine, and Analogues). In Critical Care Toxicology; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–46. [Google Scholar]
- Bertron, J.L.; Seto, M.; Lindsley, C.W. DARK Classics in Chemical Neuroscience: Phencyclidine (PCP). ACS Chem. Neurosci. 2018, 9, 2459–2474. [Google Scholar] [CrossRef]
- Journey, J.D.; Bentley, T.P. Phencyclidine Toxicity. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507865/ (accessed on 10 December 2021).
- Thornton, S.; Lisbon, D.; Lin, T.; Gerona, R. Beyond Ketamine and Phencyclidine: Analytically Confirmed Use of Multiple Novel Arylcyclohexylamines. J. Psychoactive Drugs 2017, 49, 289–293. [Google Scholar] [CrossRef]
- Kintz, P.; Ameline, A.; Walch, A.; Farrugia, A.; Raul, J.S. Murdered while under the influence of 3-MeO-PCP. Int. J. Legal Med. 2019, 133, 475–478. [Google Scholar] [CrossRef]
- Drug Enforcement Justice. Ketamine. Available online: https://www.dea.gov/sites/default/files/2020-06/Ketamine-2020.pdf (accessed on 29 November 2021).
- FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic. Case Med. Res. 2019. [CrossRef]
- Kjellgren, A.; Jonsson, K. Methoxetamine (MXE)—A Phenomenological Study of Experiences Induced by a “Legal High” from the Internet. J. Psychoactive Drugs 2013, 45, 276–286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pourmand, A.; Armstrong, P.; Mazer-Amirshahi, M.; Shokoohi, H. The evolving high: New designer drugs of abuse. Hum. Exp. Toxicol. 2014, 33, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Botanas, C.J.; Perez Custodio, R.J.; Kim, H.J.; de la Pena, J.B.; Sayson, L.V.; Ortiz, D.M.; Kim, M.; Lee, H.J.; Acharya, S.; Kim, K.-M.; et al. R (−)-methoxetamine exerts rapid and sustained antidepressant effects and fewer behavioral side effects relative to S (+)-methoxetamine. Neuropharmacology 2021, 193, 108619. [Google Scholar] [CrossRef] [PubMed]
- Berar, A.; Allain, J.-S.; Allard, S.; Lefevre, C.; Baert, A.; Morel, I.; Bouvet, R.; Gicquel, T. Intoxication with 3-MeO-PCP alone. Medicine 2019, 98, e18295. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime. Depressants 2019. Available online: https://wdr.unodc.org/wdr2019/en/depressants.html (accessed on 9 December 2021).
- Luginbühl, M.; Angelova, S.; Gaugler, S.; Längin, A.; Weinmann, W. Automated high-throughput analysis of tramadol and O-desmethyltramadol in dried blood spots. Drug Test. Anal. 2020, 12, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Pichini, S.; Solimini, R.; Berretta, P.; Pacifici, R.; Busardò, F.P. Acute Intoxications and Fatalities from Illicit Fentanyl and Analogues: An Update. Ther. Drug Monit. 2018, 40, 38–51. [Google Scholar] [CrossRef]
- Vicknasingam, B.; Narayanan, S.; Singh, D.; Corazza, O. Global strategy for New Psychoactive Substances: An update. Curr. Opin. Psychiatry 2020, 33, 295–300. [Google Scholar] [CrossRef]
- Armenian, P.; Vo, K.T.; Barr-Walker, J.; Lynch, K.L. Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology 2018, 134, 121–132. [Google Scholar] [CrossRef]
- Kuczyńska, K.; Grzonkowski, P.; Kacprzak, Ł.; Zawilska, J.B. Abuse of fentanyl: An emerging problem to face. Forensic Sci. Int. 2018, 289, 207–214. [Google Scholar] [CrossRef]
- Stanley, T.H. The Fentanyl Story. J. Pain 2014, 15, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Natsuka, K.; Nakamura, H.; Nishikawa, Y.; Negoro, T.; Uno, H.; Nishimura, H. Synthesis and structure-activity relationships of 1-substituted 4-(1,2-diphenylethyl)piperazine derivatives having narcotic agonist and antagonist activity. J. Med. Chem. 1987, 30, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Natsuka, K.; Nakamura, H.; Uno, H.; Umemoto, S. 1-Substituted 4-(1,2-diphenylethyl)piperazine derivatives and their analgesic activities. 1. J. Med. Chem. 1975, 18, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. EMCDDA-Europol Joint Report on a New Psychoactive Substance: 1-cyclohexyl-4-(1,2-diphenylethyl)piperazine (‘MT-45’); EMCDDA: Lisbon, Portugal, 2014.
- Lucyk, S.N.; Nelson, L.S. Novel Synthetic Opioids: An Opioid Epidemic Within an Opioid Epidemic. Ann. Emerg. Med. 2017, 69, 91–93. [Google Scholar] [CrossRef]
- Rudd, R.A.; Seth, P.; David, F.; Scholl, L. Increases in Drug and Opioid-Involved Overdose Deaths-United States, 2010–2015. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 1445–1452. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. The Growing Complexity of the Opioid Crisis. Global Smart Update. Available online: https://www.unodc.org/documents/scientific/Global_SMART-2020-Vol_24_web.pdf (accessed on 3 December 2021).
- United Nations Office on Drugs and Crime. Recommended Methods for The Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens. Available online: https://www.unodc.org/unodc/en/scientists/recommended-methods-for-the-identification-and-analysis-of-fentanyl-and-its-analogues-in-biological-specimens.html (accessed on 19 December 2021).
- Karila, L.; Marillier, M.; Chaumette, B.; Billieux, J.; Franchitto, N.; Benyamina, A. New synthetic opioids: Part of a new addiction landscape. Neurosci. Biobehav. Rev. 2019, 106, 133–140. [Google Scholar] [CrossRef]
- Zawilska, J.B. An Expanding World of Novel Psychoactive Substances: Opioids. Front. Psychiatry 2017, 8, 110. [Google Scholar] [CrossRef]
- Beardsley, P.M.; Zhang, Y. Synthetic opioids. Handb. Exp. Pharmacol. 2018, 252, 353–381. [Google Scholar] [CrossRef]
- Lovrecic, B.; Lovrecic, M.; Gabrovec, B.; Carli, M.; Pacini, M.; Maremmani, A.G.I.; Maremmani, I. Non-Medical Use of Novel Synthetic Opioids: A New Challenge to Public Health. Int. J. Environ. Res. Public Health 2019, 16, 177. [Google Scholar] [CrossRef]
- United States Drug Enforcement Administration. Oxycodone. Available online: https://www.dea.gov/factsheets/oxycodone (accessed on 19 December 2021).
- European Monitoring Centre for Drugs and Drug Addiction and Europol EMCDDA-Europol Joint Report on a New Psychoactive Substance: AH-7921 3,4-dichloro-N-((1-(dimethylamiono)cyclohexyl))methyl)menzamide. Available online: https://www.emcdda.europa.eu/publications/joint-report/AH-7921_en (accessed on 12 December 2021).
- McCabe, S.E.; Cranford, J.A.; Boyd, C.J.; Teter, C.J. Motives, diversion and routes of administration associated with nonmedical use of prescription opioids. Addict. Behav. 2007, 32, 562–575. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Fentanyl Drug Profile. Available online: https://www.emcdda.europa.eu/publications/drug-profiles/fentanyl#use. (accessed on 19 December 2021).
- European Monitoring Centre for Drugs and Addiction. New Psychoactive Substances: Global Markets, Global Threats and the COVID-19 Pandemic. An Update from the EU Early Warning System. Available online: https://www.emcdda.europa.eu/publications/rapid-communication/new-psychoactive-substances-global-markets-glocal-threats-and-covid-19-pandemic_en (accessed on 7 December 2021).
- Coon, T.P.; Miller, M.; Kaylor, D.; Jones-Spangle, K. Rectal Insertion of Fentanyl Patches: A New Route of Toxicity. Ann. Emerg. Med. 2005, 46, 473. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Drug and Drug Addiction (EMCDDA). Spotlight on… Fentanils and Other New Opioids. Available online: https://www.emcdda.europa.eu/spotlights/fentanils-and-other-new-opioids_en (accessed on 19 December 2021).
- European Monitoring Centre for Drugs and Drug Addiction. Europol EU Drug Markets Report 2019. Available online: www.emcdda.europa.eu (accessed on 5 December 2021).
- United Nations Office on Drugs and Crime. Data from the UNODC Early Warning Advisory on New Psychoactive Substances. Available online: https://www.unodc.org/unodc/en/scientists/ewa/data.html (accessed on 7 December 2021).
- Lutfy, K. Opioid Crisis-An Emphasis on Fentanyl Analogs. Brain Sci. 2020, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- McClain, D.A.; Hug, C.C., Jr. Intravenous fentanyl kinetics. Clin. Pharmacol. Ther. 1980, 28, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, I.; Mystakidou, K. Intranasal fentanyl: From pharmacokinetics and bioavailability to current treatment applications. Expert Rev. Anticancer Ther. 2010, 10, 1009–1021. [Google Scholar] [CrossRef]
- Foster, D.; Upton, R.; Christrup, L.; Popper, L. Pharmacokinetics and Pharmacodynamics of Intranasal Versus Intravenous Fentanyl in Patients with Pain after Oral Surgery. Ann. Pharmacother. 2008, 42, 1380–1387. [Google Scholar] [CrossRef]
- Labroo, R.B.; Paine, M.F.; Thummel, K.E.; Kharasch, E.D. Fentanyl Metabolism by Human Hepatic and Intestinal Cytochrome P450 3A4: Implications for Interindividual Variability in Disposition, Efficacy, and Drug Interactions. Drug Metab. Dispos. 1997, 25, 1072–1080. [Google Scholar]
- Tateishi, T.; Krivoruk, Y.; Ueng, Y.-F.; Wood, A.J.J.; Guengerich, F.P.; Wood, M. Identification of Human Liver Cytochrome P-450 3A4 as the Enzyme Responsible for Fentanyl and Sufentanil N-Dealkylation. Anesth. Analg. 1996, 82, 167–172. [Google Scholar]
- Kong, L.; Walz, A.J. Identification of human cytochrome P450 isozymes involved in the oxidative metabolism of carfentanil. Toxicol. Lett. 2021, 343, 28–33. [Google Scholar] [CrossRef]
- Watanabe, S.; Vikingsson, S.; Roman, M.; Green, H.; Kronstrand, R.; Wohlfarth, A. In Vitro and In Vivo Metabolite Identification Studies for the New Synthetic Opioids Acetylfentanyl, Acrylfentanyl, Furanylfentanyl, and 4-Fluoro-Isobutyrylfentanyl. AAPS J. 2017, 19, 1102–1122. [Google Scholar] [CrossRef]
- Allibe, N.; Richeval, C.; Phanithavong, M.; Faure, A.; Allorge, D.; Paysant, F.; Stanke-Labesque, F.; Eysseric-Guerin, H.; Gaulier, J.-M. Fatality involving ocfentanil documented by identification of metabolites. Drug Test. Anal. 2018, 10, 995–1000. [Google Scholar] [CrossRef]
- Coppola, M.; Mondola, R. AH-7921: A new synthetic opioid of abuse. Drug Alcohol Rev. 2015, 34, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Prekupec, M.P.; Mansky, P.A.; Baumann, M.H. Misuse of Novel Synthetic Opioids: A Deadly New Trend. J. Addict. Med. 2017, 11, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Loew, G.; Lawson, J.; Toll, L.; Frenking, G.; Berzetei-Gurske, I.; Polgar, W. Structure activity studies of two classes of beta-amino-amides: The search for kappa-selective opioids. NIDA Res. Monogr. 1988, 90, 144–151. [Google Scholar] [PubMed]
- Mohr, A.L.A.; Friscia, M.; Papsun, D.; Kacinko, S.L.; Buzby, D.; Logan, B.K. Analysis of Novel Synthetic Opioids U-47700, U-50488 and Furanyl Fentanyl by LC-MS/MS in Postmortem Casework. J. Anal. Toxicol. 2016, 40, 709–717. [Google Scholar] [CrossRef]
- Bilel, S.; Azevedo, N.J.; Arfè, R.; Tirri, M.; Gregori, A.; Serpelloni, G.; De-Giorgio, F.; Frisoni, P.; Neri, M.; Calò, G.; et al. In vitro and in vivo pharmacological characterization of the synthetic opioid MT-45. Neuropharmacology 2020, 171, 108110. [Google Scholar] [CrossRef]
- Frisoni, P.; Bacchio, E.; Bilel, S.; Talarico, A.; Gaudio, R.M.; Barbieri, M.; Neri, M.; Marti, M. Novel Synthetic Opioids: The Pathologist’s Point of View. Brain Sci. 2018, 8, 170. [Google Scholar] [CrossRef]
- Sinicina, I.; Sachs, H.; Keil, W. Post-mortem review of fentanyl-related overdose deaths among identified drug users in Southern Bavaria, Germany, 2005–2014. Drug Alcohol Depend. 2017, 180, 286–291. [Google Scholar] [CrossRef]
- Helander, A.; Bäckberg, M.; Signell, P.; Beck, O. Intoxications involving acrylfentanyl and other novel designer fentanyls–results from the Swedish STRIDA project. Clin. Toxicol. 2017, 55, 589–599. [Google Scholar] [CrossRef]
- Bäckberg, M.; Beck, O.; Jönsson, K.-H.; Helander, A. Opioid intoxications involving butyrfentanyl, 4-fluorobutyrfentanyl, and fentanyl from the Swedish STRIDA project. Clin. Toxicol. 2015, 53, 609–617. [Google Scholar] [CrossRef]
- Wilde, M.; Sommer, M.J.; Auwärter, V.; Hermanns-Clausen, M. Acute severe intoxication with cyclopropylfentanyl, a novel synthetic opioid. Toxicol. Lett. 2020, 320, 109–112. [Google Scholar] [CrossRef]
- Cunningham, S.M.; Haikal, N.A.; Kraner, J.C. Fatal Intoxication with Acetyl Fentanyl. J. Forensic Sci. 2016, 61, S276–S280. [Google Scholar] [CrossRef] [PubMed]
- Coopman, V.; Cordonnier, J.; De Leeuw, M.; Cirimele, V. Ocfentanil overdose fatality in the recreational drug scene. Forensic Sci. Int. 2016, 266, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, D.; Rapp, E.; Roman, M.; Thelander, G.; Kronstrand, R. Acrylfentanyl: Another new psychoactive drug with fatal consequences. Forensic Sci. Int. 2017, 277, e21–e29. [Google Scholar] [CrossRef] [PubMed]
- Hikin, L.; Smith, P.R.; Ringland, E.; Hudson, S.; Morley, S.R. Multiple fatalities in the North of England associated with synthetic fentanyl analogue exposure: Detection and quantitation a case series from early 2017. Forensic Sci. Int. 2018, 282, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.P.; Hernandez Lopez, E. A Series of Deaths Involving Carfentanil in the UK and Associated Post-mortem Blood Concentrations. J. Anal. Toxicol. 2018, 42, e41–e45. [Google Scholar] [CrossRef]
- Kronstrand, R.; Thelander, G.; Lindstedt, D.; Roman, M.; Kugelberg, F.C. Fatal Intoxications Associated with the Designer Opioid AH-7921. J. Anal. Toxicol. 2014, 38, 599–604. [Google Scholar] [CrossRef]
- Elliott, S.P.; Brandt, S.D.; Smith, C. The first reported fatality associated with the synthetic opioid 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide (U-47700) and implications for forensic analysis. Drug Test. Anal. 2016, 8, 875–879. [Google Scholar] [CrossRef]
- Helander, A.; Bradley, M.; Hasselblad, A.; Norlén, L.; Vassilaki, I.; Bäckberg, M.; Lapins, J. Acute skin and hair symptoms followed by severe, delayed eye complications in subjects using the synthetic opioid MT-45. Br. J. Dermatol. 2017, 176, 1021–1027. [Google Scholar] [CrossRef]
- Fels, H.; Krueger, J.; Sachs, H.; Musshoff, F.; Graw, M.; Roider, G.; Stoever, A. Two fatalities associated with synthetic opioids: AH-7921 and MT-45. Forensic Sci. Int. 2017, 277, e30–e35. [Google Scholar] [CrossRef]
- Papsun, D.; Krywanczyk, A.; Vose, J.C.; Bundock, E.A.; Logan, B.K. Analysis of MT-45, a Novel Synthetic Opioid, in Human Whole Blood by LC–MS-MS and Its Identification in a Drug-Related Death. J. Anal. Toxicol. 2016, 40, 313–317. [Google Scholar] [CrossRef]
- Feng, L.Y.; Battulga, A.; Han, E.; Chung, H.; Li, J.H. New psychoactive substances of natural origin: A brief review. J. Food Drug Anal. 2017, 25, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Lukić, V.; Micić, R.; Arsić, B.; Nedović, B.; Radosavljević, Ž. Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs. Open Chem. 2021, 19, 60–106. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. Early Warning Advisory on New Psychoactive Substances. Available online: https://www.unodc.org/LSS/SubstanceGroup/Details/4b17fe10-91da-477c-bc3d-593136040668 (accessed on 18 November 2021).
- Wabe, N.T. Chemistry, pharmacology, and toxicology of khat (catha edulis forsk): A review. Addict. Heal. 2011, 3, 137–149. [Google Scholar]
- González, D.; Riba, J.; Bouso, J.C.; Gómez-Jarabo, G.; Barbanoj, M.J. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006, 85, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, C.D.; Carreiro, S.P.; Babu, K.M. Here Today, Gone Tomorrow. and Back Again? A Review of Herbal Marijuana Alternatives (K2, Spice), Synthetic Cathinones (Bath Salts), Kratom, Salvia divinorum, Methoxetamine, and Piperazines. J. Med. Toxicol. 2012, 8, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; Domingos, S.; Gallardo, E.; Martinho, A. A unique natural selective kappa-opioid receptor agonist, salvinorin A, and its roles in human therapeutics. Phytochemistry 2017, 137, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Margalho, C.; Corte-Real, F.; López-Rivadulla, M.; Gallardo, E. Salvia divinorum: Toxicological aspects and analysis in human biological specimens. Bioanalysis 2016, 8, 1415–1425. [Google Scholar] [CrossRef]
- Ramanathan, S.; McCurdy, C.R. Kratom (Mitragyna speciosa): Worldwide issues. Curr. Opin. Psychiatry 2020, 33, 312–318. [Google Scholar] [CrossRef]
- Firmansyah, A.; Sundalian, M.; Taufiq, M. Kratom (Mitragyna speciosa korth) for a new medicinal: A review of pharmacological and compound analysis. Biointerface Res. Appl. Chem. 2021, 11, 9704–9718. [Google Scholar] [CrossRef]
- Ya, K.; Tangamornsuksan, W.; Scholfield, C.N.; Methaneethorn, J.; Lohitnavy, M. Pharmacokinetics of mitragynine, a major analgesic alkaloid in kratom (Mitragyna speciosa): A systematic review. Asian J. Psychiatr. 2019, 43, 73–82. [Google Scholar] [CrossRef]
- Gee, P.; Richardson, S.; Woltersdorf, W.; Moore, G. Toxic effects of BZP-based herbal party pills in humans: A prospective study in Christchurch, New Zealand. N. Z. Med. J. 2005, 118, 1–10. [Google Scholar]
- Alansari, M.; Hamilton, D. Nephrotoxicity of BZP-based herbal party pills: A New Zealand case report. N. Z. Med. J. 2006, 119, 1–3. [Google Scholar]
- Gee, P.; Jerram, T.; Bowie, D. Multiorgan failure from 1-benzylpiperazine ingestion legal high or lethal high. Clin. Toxicol. 2010, 48, 230–233. [Google Scholar] [CrossRef]
- Austin, H.; Monasterio, E. Acute psychosis following ingestion of ‘Rapture’. Australas. Psychiatry 2015, 12, 406–408. [Google Scholar]
- Balmelli, C.; Kupferschmidt, H.; Rentsch, K.; Schneemann, M. Tödliches hirnödem nach einnahme von ecstasy und benzylpiperazin. Dtsch. Medizinische Wochenschrift 2001, 126, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, J.; Ir, E.D.; De Paepe, P.; Verstraete, A. Acute chlorophenylpiperazine overdose: A case report and review of the literature. Ther. Drug Monit. 2008, 30, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Gee, P.; Gilbert, M.; Richardson, S.; Moore, G.; Paterson, S.; Graham, P. Toxicity from the recreational use of 1-benzylpiperazine. Clin. Toxicol. 2008, 46, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.E.; Dargan, P.I.; Wood, D.M.; Puchnarewicz, M.; Davies, S.; Waring, W.S. Methoxetamine associated reversible cerebellar toxicity: Three cases with analytical confirmation. Clin. Toxicol. 2012, 50, 438–440. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simão, A.Y.; Antunes, M.; Cabral, E.; Oliveira, P.; Rosendo, L.M.; Brinca, A.T.; Alves, E.; Marques, H.; Rosado, T.; Passarinha, L.A.; et al. An Update on the Implications of New Psychoactive Substances in Public Health. Int. J. Environ. Res. Public Health 2022, 19, 4869. https://doi.org/10.3390/ijerph19084869
Simão AY, Antunes M, Cabral E, Oliveira P, Rosendo LM, Brinca AT, Alves E, Marques H, Rosado T, Passarinha LA, et al. An Update on the Implications of New Psychoactive Substances in Public Health. International Journal of Environmental Research and Public Health. 2022; 19(8):4869. https://doi.org/10.3390/ijerph19084869
Chicago/Turabian StyleSimão, Ana Y., Mónica Antunes, Emanuel Cabral, Patrik Oliveira, Luana M. Rosendo, Ana Teresa Brinca, Estefânia Alves, Hernâni Marques, Tiago Rosado, Luís A. Passarinha, and et al. 2022. "An Update on the Implications of New Psychoactive Substances in Public Health" International Journal of Environmental Research and Public Health 19, no. 8: 4869. https://doi.org/10.3390/ijerph19084869
APA StyleSimão, A. Y., Antunes, M., Cabral, E., Oliveira, P., Rosendo, L. M., Brinca, A. T., Alves, E., Marques, H., Rosado, T., Passarinha, L. A., Andraus, M., Barroso, M., & Gallardo, E. (2022). An Update on the Implications of New Psychoactive Substances in Public Health. International Journal of Environmental Research and Public Health, 19(8), 4869. https://doi.org/10.3390/ijerph19084869









