Mini Review: Co-Existing Diseases and COVID-19—A One Way Ticket?
Abstract
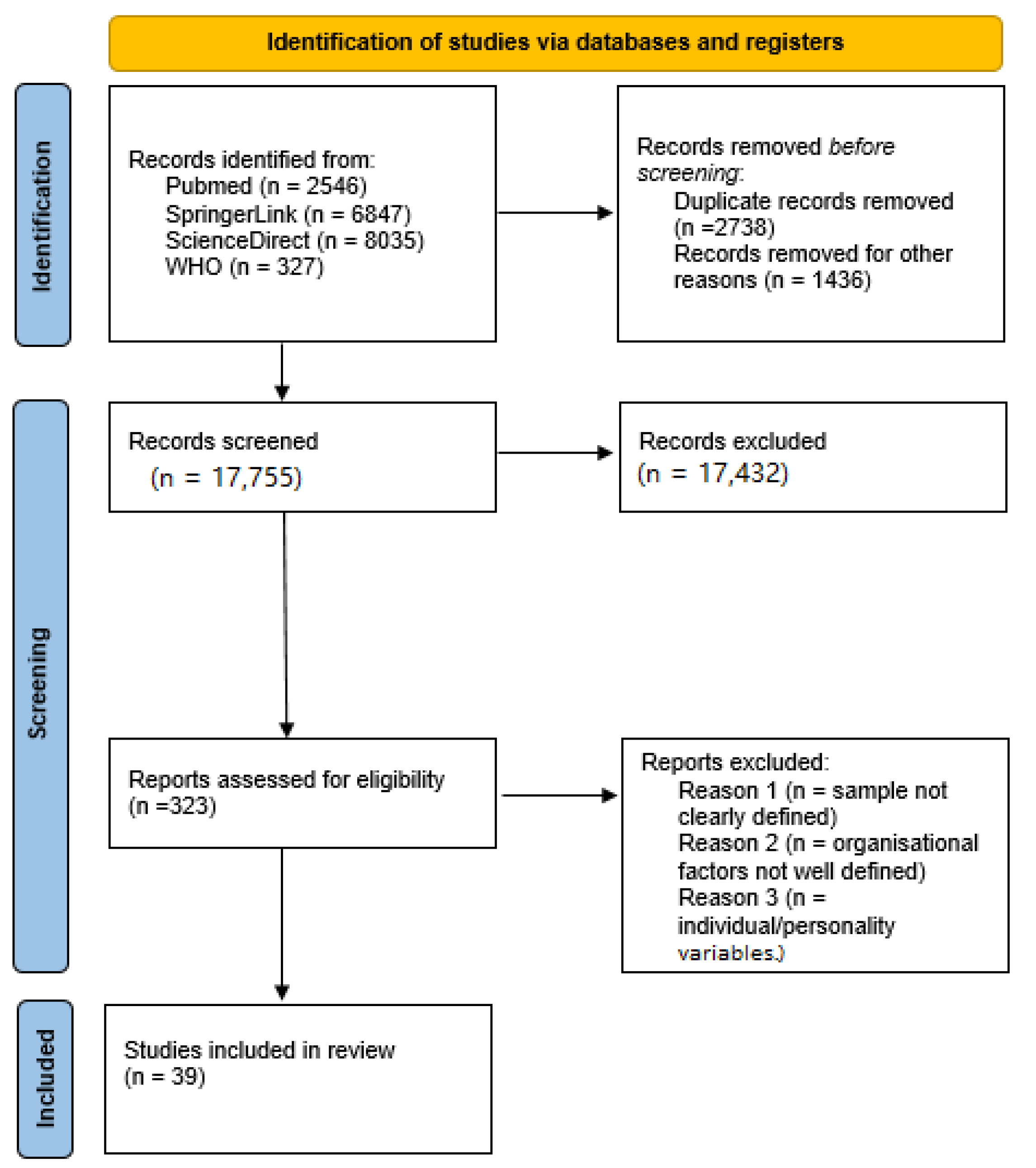
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Immunosuppression and COVID-19
3.2. Cardiovascular Diseases and COVID-19
3.3. Metabolic Syndrome and COVID-19
3.4. Hematologic Diseases and COVID-19
3.5. Kidney Diseases and COVID-19
3.6. Unmodified Factors and COVID-19
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 30 December 2020).
- Lietuvos Statistikos Departamento Informacija ir Interaktyvus Protrūkių Žemėlapis. Available online: https://osp.stat.gov.lt/ (accessed on 1 March 2022).
- Liu, C.; Zhao, Y.; Okwan-Duodu, E.A.D.; Basho, R.; Cui, X. COVID-19 in cancer patients: Risk, clinical features, and management. Cancer Biol. Med. 2020, 17, 519–527. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Gosain, R.; Abdou, Y.; Singh, A.; Rana, N.; Puzanov, I.; Ernstoff, M.S. COVID-19 and Cancer: A Comprehensive Review. Curr. Oncol. Rep. 2020, 22, 53. [Google Scholar] [CrossRef]
- Fernández-Ruiz, M.; Andrés, A.; Loinaz, C.; Delgado, J.F.; López-Medrano, F.; Juan, R.S.; González, E.; Polanco, N.; Folgueira, M.D.; Lalueza, A.; et al. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am. J. Transplant. 2020, 20, 1849–1858. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Pranata, R.; Huang, I.; Lim, M.A.; Wahjoepramono, E.J.; July, J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J. Stroke Cerebrovasc. Dis. 2020, 29, 104949. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.R.; Kim, M.-N.; Shim, W.J.; Park, S.-M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: A systematic review and meta-analysis. Heart 2020, 107, 373–380. [Google Scholar] [CrossRef]
- Bansal, R.; Gubbi, S.; Muniyappa, R. Metabolic Syndrome and COVID 19: Endocrine-Immune-Vascular Interactions Shapes Clinical Course. Endocrinology 2020, 161, bqaa112. [Google Scholar] [CrossRef]
- Marhl, M.; Grubelnik, V.; Magdič, M.; Markovič, R. Diabetes and metabolic syndrome as risk factors for COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 671–677. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Petrakis, D.; Margină, D.; Tsarouhas, K.; Tekos, F.; Stan, M.; Nikitovic, D.; Kouretas, D.; Spandidos, D.A.; Tsatsakis, A. Obesity—A risk factor for increased COVID-19 prevalence, severity and lethality (Review). Mol. Med. Rep. 2020, 22, 9–19. [Google Scholar] [CrossRef]
- Costa, F.F.; Rosário, W.R.; Farias, A.C.R.; de Souza, R.G.; Gondim, R.S.D.; Barroso, W.A. Metabolic syndrome and COVID-19: An update on the associated comorbidities and proposed therapies. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 809–814. [Google Scholar] [CrossRef]
- Von Lilienfeld-Toal, M.; EHA Infectious Disease Scientific Working Group; Vehreschild, J.J.; Cornely, O.; Pagano, L.; Compagno, F.; Hirsch, H.H. Frequently asked questions regarding SARS-CoV-2 in cancer patients—Recommendations for clinicians caring for patients with malignant diseases. Leukemia 2020, 34, 1487–1494. [Google Scholar] [CrossRef]
- Girmenia, C.; Gentile, G.; Micozzi, A.; Petrucci, L.; Malaspina, F.; Di Prima, A.; Baldacci, E.; Bianchi, S.; Pugliese, P.; Turriziani, O.; et al. COVID-19 in Patients with Hematologic Disorders Undergoing Therapy: Perspective of a Large Referral Hematology Center in Rome. Acta Haematol. 2020, 143, 574–582. [Google Scholar] [CrossRef]
- He, W.; Chen, L.; Yuan, G.; Fang, Y.; Chen, W.; Wu, D.; Liang, B.; Lu, X.; Ma, Y.; Li, L.; et al. COVID-19 in persons with haematological cancers. Leukemia 2020, 34, 1637–1645. [Google Scholar] [CrossRef]
- Malard, F.; Genthon, A.; Brissot, E.; Van De Wyngaert, Z.; Marjanovic, Z.; Ikhlef, S.; Banet, A.; Lapusan, S.; Sestilli, S.; Corre, E.; et al. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020, 55, 2180–2184. [Google Scholar] [CrossRef]
- Hassanein, M.; Radhakrishnan, Y.; Sedor, J.; Vachharajani, T.; Vachharajani, V.T.; Augustine, J.; Demirjian, S.; Thomas, G. COVID-19 and the kidney. Clevel. Clin. J. Med. 2020, 87, 619–631. [Google Scholar] [CrossRef]
- Farouk, S.S.; Fiaccadori, E.; Cravedi, P.; Campbell, K.N. COVID-19 and the kidney: What we think we know so far and what we don’t. J. Nephrol. 2020, 33, 1213–1218. [Google Scholar] [CrossRef]
- Shao, M.; Li, X.; Liu, F.; Tian, T.; Luo, J.; Yang, Y. Acute kidney injury is associated with severe infection and fatality in patients with COVID-19: A systematic review and meta-analysis of 40 studies and 24,527 patients. Pharmacol. Res. 2020, 161, 105107. [Google Scholar] [CrossRef]
- Rombolà, G.; Heidempergher, M.; Pedrini, L.; Farina, M.; Aucella, F.; Messa, P.; Brunori, G. Practical indications for the prevention and management of SARS-CoV-2 in ambulatory dialysis patients: Lessons from the first phase of the epidemics in Lombardy. J. Nephrol. 2020, 33, 193–196. [Google Scholar] [CrossRef] [Green Version]
- Jutzeler, C.R.; Bourguignon, L.; Weis, C.V.; Tong, B.; Wong, C.; Rieck, B.; Pargger, H.; Tschudin-Sutter, S.; Egli, A.; Borgwardt, K.; et al. Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID-19: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 37, 101825. [Google Scholar] [CrossRef]
- Bienvenu, L.A.; Noonan, J.; Wang, X.; Peter, K. Higher mortality of COVID-19 in males: Sex differences in immune response and cardiovascular comorbidities. Cardiovasc. Res. 2020, 116, 2197–2206. [Google Scholar] [CrossRef]
- Jin, J.M.; Bai, P.; He, W.; Wu, F.; Liu, X.F.; Han, D.M.; Liu, S.; Yang, J.K. Gender Differences in Patients with COVID-19: Focus on Severity and Mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Acharya, Y.; Pant, S.; Gyanwali, P.; Dangal, G.; Karki, P.; Bista, N.R.; Tandan, M. Gender Disaggregation in COVID-19 and Increased Male Susceptibility. J. Nepal Heal. Res. Counc. 2020, 18, 345–350. [Google Scholar] [CrossRef]
- Guzek, D.; Skolmowska, D.; Głąbska, D. Analysis of Gender-Dependent Personal Protective Behaviors in a National Sample: Polish Adolescents’ COVID-19 Experience (PLACE-19) Study. Int. J. Environ. Res. Public Health 2020, 17, 5770. [Google Scholar] [CrossRef]
- O’Brien, J.; Du, K.Y.; Peng, C. Incidence, clinical features, and outcomes of COVID-19 in Canada: Impact of sex and age. J. Ovarian Res. 2020, 13, 137. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, B.; Liang, S.; Yang, J.-W.; Lu, H.-W.; Chai, Y.-H.; Wang, L.; Zhang, L.; Li, Q.-H.; Zhao, L.; et al. Association between age and clinical characteristics and outcomes of COVID-19. Eur. Respir. J. 2020, 55, 2001112. [Google Scholar] [CrossRef] [Green Version]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; de Virgilio, A.; de Marco, F.; di Domenico, E.G.; et al. Obesity May Hamper SARS-CoV-2 Vaccine Immunogenicity; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2021. [Google Scholar] [CrossRef]
- Ealey, K.N.; Phillips, J.; Sung, H.-K. COVID-19 and obesity: Fighting two pandemics with intermittent fasting. Trends Endocrinol. Metab. 2021, 32, 706–720. [Google Scholar] [CrossRef]
- WHO. Corona Virus (COVID-19) Disease Severity Classification; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Corona Virus Disease (COVID-19) Tobacco. Available online: https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-tobacco (accessed on 28 December 2020).
- WHO. Alcohol Consumption and Alcohol-Related Problems. Available online: https://www.who.int/publications/i/item/alcohol-consumption-and-alcohol-related-problems (accessed on 28 December 2020).
- World Health Organization. Advice for the Public: Coronavirus Disease (COVID-19). 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public (accessed on 9 April 2020).
| Investigator (Year) | Location | Study Design | Number of Patients | Study Population | Findings |
|---|---|---|---|---|---|
| Liu et al., 2020 [3] | China | Review | 2007 | COVID-19 and cancer | Might impact cancer diagnosis |
| Liang et al., 2020 [4] | China | Cohort | 18 | COVID-19 and cancer | Patients with cancer—severe consequences |
| Gosain et al., 2020 [5] | USA | Review | 53 | COVID-19 and immunosuppression | Remdesivir may have clinical benefits |
| Ruiz et al., 2020 [6] | Spain | Observational study | 18 | Coronavirus after solid organ transplant | SARS-CoV-2 infection has a severe course in SOT patients |
| Wu et al., 2020 [7] | China | Viewpoint | 72,314 | Key findings from diagnosed cases | Characteristics of COVID-19 outbreak |
| Grasselli et al., 2020 [8] | Italy | Investigation study | 1591 | Characteristics of COVID-19 in ICU | Mechanical ventilation and mortality |
| Huang et al., 2020 [9] | China | Observational study | 41 | COVID-19 clinical features | COVID-19 was associated with high mortality |
| Pranata et al., 2020 [10] | Indonesia | Meta-analysis | 4448 | Cardiovascular diseases and COVID-19 | Increased risk for poor outcomes |
| Bae et al., 2021 [11] | South Korea | Meta-analysis | 48,317 | COVID-19 and hypertension + diabetes | Increased risk of fatal outcomes |
| Bansal et al., 2020 [12] | USA | Review | 63,636 | Metabolic syndrome and COVID-19 | Poor outcomes and increased mortality |
| Marhl et al., 2020 [13] | Slovenia | Observational study | 82,511 | COVID-19 and diabetes | Higher risk for COVID-19 |
| Popkin et al., 2020 [14] | USA | Review | 399,461 | Obesity and COVID-19 | More at risk for COVID-positive |
| Petrakis et al., 2020 [15] | Romania | Review | - | Obesity and COVID-19 | Negative prognosis for obese people |
| Costa et al., 2020 [16] | Brazil | Review | 108,287 | Metabolic syndrome and COVID-19 | MS is a risk factor |
| Lilienfield-Toal et al., 2020 [17] | Germany | Review | - | SARS-CoV-2 in cancer patients | Increased risk for worse outcome |
| Girmenia et al., 2020 [18] | Italy | Observational study | 2513 | COVID-19 and hematologic disorders | No significant correlations |
| He et al., 2020 [19] | China | Cohort | 354 | COVID-19 and hematological cancers | Severe COVID-19 and more deaths |
| Malard et al., 2020 [20] | France | Review | 25 | COVID-19 and hematologic disorders | Higher incidence of severe events |
| Hassanein et al., 2020 [21] | USA | Review | 2072 | COVID-19 and kidneys | Acute kidney injury (AKI) is common in COVID-19 |
| Farouk et al., 2020 [22] | USA | Review | 8776 | COVID-19 and kidneys | AKI increased mortality |
| Shao et al., 2020 [23] | China | Meta-analysis | 24,527 | AKI and COVID-19 | AKI is associated with higher mortality rates |
| Rombola et al., 2020 [24] | Italy | Review | 60 | COVID-19 and dialysis patients | Accelerated healthcare protocols |
| Jutzeler et al., 2020 [25] | Switzerland | Meta-analysis | 12,149 | Comorbidities and COVID-19 | Comorbidities are associated with severity and mortality |
| Bienvenu et al., 2020 [26] | Australia | Review | 17,000,000 | COVID-19 and males | Male sex is a risk factor |
| Jin et al., 2020 [27] | China | Review | 1623 | COVID-19 and sex differences | Males with COVID-19 are at risk for worse outcomes |
| Acharya et al., 2020 [28] | Ireland | Review | 44,672 | Gender disaggregation in COVID-19 | Higher COVID-19 susceptibility in males |
| Guzek et al., 2020 [29] | Poland | Observational study | 2,170,464 | COVID-19 and personal behaviors | Differences between gender |
| O’Brien et al., 2020 [30] | Canada | Review | 101,121 | Impact of age and sex on COVID-19 | Male sex and older age—worse outcomes |
| Liu et al., 2020 [31] | China | Comment | 221 | Age and COVID-19 | Prognosis was worse in patients older than 60 years |
| Pellini et al., 2021 [32] | Italy | Observational study | 248 | COVID-19 and vaccination | Correlation of BMI classes with antibody titres |
| Ealey et al., 2021 [33] | Canada | Review | 900,000 | COVID-19 and obesity | Vaccines are less effective in obese individuals |
| Comorbidities | Results | References |
|---|---|---|
| Immunosuppression | Cancer patients: higher risk for unfavorable and lethal outcomes, more likely to be admitted to ICU and receive invasive ventilation; Organ recipients: high percentage develop respiratory deficiency; hard to manage with additional comorbidities. | Liu et al., 2020 [3] Liang et al., 2020 [4] Gosain et al., 2020 [5] Fernandez-Ruiz et al., 2020 [6] Wu et al., 2020 [7] |
| Cardiovascular | Suggestion that hypertension is the most common comorbidity among COVID-19 patients; severe forms of COVID-19; high fatality rate; requiring aggressive ventilation at the ICU. | Wu et al., 2020 [7] Grasselli et al., 2020 [8] Huang et al., 2020 [9] |
| Metabolic syndrome | Increased hospitalization rates among diabetic and obese patients; acquire complications such as respiratory distress, AKI, septic shock; poorly controlled diabetes worsens course of the disease, higher fatality rate. | Bansal et al., 2020 [12] Marhl et al., 2020 [13] Costa et al., 2021 [16] |
| Hematologic | Higher risk of severe events, need of ventilation, higher fatality rate; severe COVID-19 development. | Liang et al., 20202 [4] von Lilienfeld-Toal et al., 2020 [17] Girmenia et al., 2020 [18] |
| Renal | High AKI incident rate; dialysis patients hard to manage and protect from COVID-19. | Shao et al., 2020 [23] Rombola et al., 2020 [24] |
| Unmodified factors | Higher percentage of COVID-19 related to men; men develop severe forms of disease; no correlation between smoking and COVID-19, patients >60 years need prolonged treatment, higher rate of respiratory failure. | Bienvenu et al., 2020 [26] Jin et al., 2020 [27] O’Brien et al., 2020 [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eidininkienė, M.; Cesarskaja, J.; Talačkaitė, S.; Traškaitė-Juškevičienė, V.; Macas, A. Mini Review: Co-Existing Diseases and COVID-19—A One Way Ticket? Int. J. Environ. Res. Public Health 2022, 19, 4738. https://doi.org/10.3390/ijerph19084738
Eidininkienė M, Cesarskaja J, Talačkaitė S, Traškaitė-Juškevičienė V, Macas A. Mini Review: Co-Existing Diseases and COVID-19—A One Way Ticket? International Journal of Environmental Research and Public Health. 2022; 19(8):4738. https://doi.org/10.3390/ijerph19084738
Chicago/Turabian StyleEidininkienė, Mantė, Jelena Cesarskaja, Simona Talačkaitė, Vilma Traškaitė-Juškevičienė, and Andrius Macas. 2022. "Mini Review: Co-Existing Diseases and COVID-19—A One Way Ticket?" International Journal of Environmental Research and Public Health 19, no. 8: 4738. https://doi.org/10.3390/ijerph19084738
APA StyleEidininkienė, M., Cesarskaja, J., Talačkaitė, S., Traškaitė-Juškevičienė, V., & Macas, A. (2022). Mini Review: Co-Existing Diseases and COVID-19—A One Way Ticket? International Journal of Environmental Research and Public Health, 19(8), 4738. https://doi.org/10.3390/ijerph19084738







