The Association between Tannerella forsythia and the Onset of Fever in Older Nursing Home Residents: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
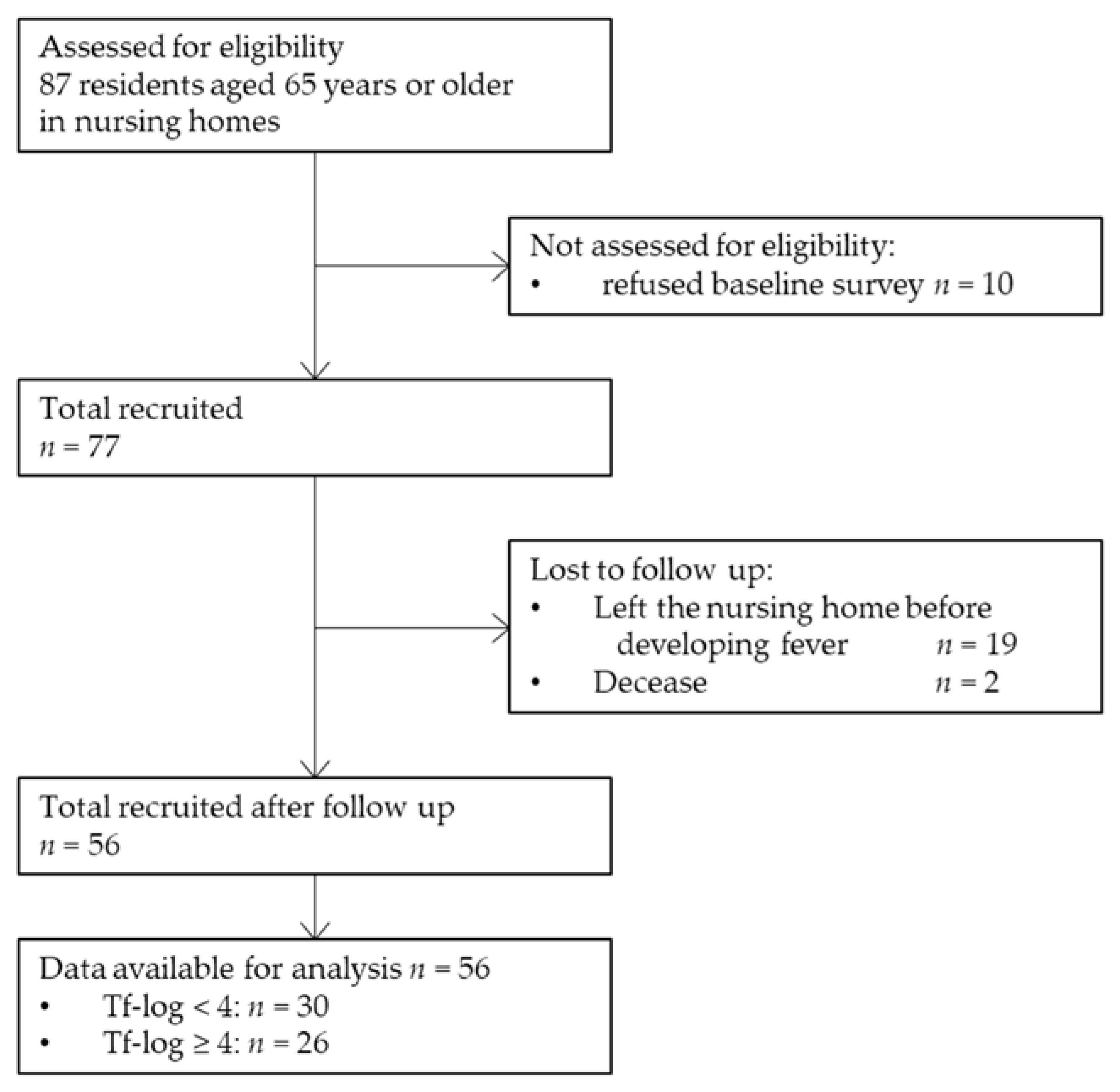
2.1. Study Setting and Population
2.2. Data Collection
2.3. Real-Time Polymerase Chain Reaction
2.4. Outcome
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sund-Levander, M.; Grodzinsky, E. Assessment of body temperature measurement options. Br. J. Nurs. 2013, 22, 942, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.S.; Petkun, W.M.; Freedman, M.L.; Antopol, S.C. Pneumococcal bacteremia in adults: Age-dependent differences in presentation and in outcome. J. Am. Geriatr. Soc. 1983, 31, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ikematsu, H.; Yamaga, S.; Nabeshima, A.; Yamaji, K.; Kakuda, K.; Ueno, K.; Hayashi, J.; Hara, H.; Shirai, T.; Kashiwagi, S. Incidence and duration of febrile episodes in a hospitalized geriatric cohort. Kansenshogaku Zasshi 1996, 70, 1079–1085. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marik, P.E. Aspiration pneumonitis and aspiration pneumonia. N. Engl. J. Med. 2001, 344, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Doggett, D.L.; Tappe, K.A.; Mitchell, M.D.; Chapell, R.; Coates, V.; Turkelson, C.M. Prevention of pneumonia in elderly stroke patients by systematic diagnosis and treatment of dysphagia: An evidence-based comprehensive analysis of the literature. Dysphagia 2001, 16, 279–295. [Google Scholar] [CrossRef]
- Ueno, K. A Relationship between febrile illness, serum albumin level and mortality in elderly hospitalized patients. Fukuoka Igaku Zasshi 2003, 94, 9–19. [Google Scholar]
- Castle, S.C.; Norman, D.C.; Yeh, M.; Miller, D.; Yoshikawa, T.T. Fever response in elderly nursing home residents: Are the older truly colder? J. Am. Geriatr. Soc. 1991, 39, 853–857. [Google Scholar] [CrossRef]
- Norman, D.C.; Grahn, D.; Yoshikawa, T.T. Fever and aging. J. Am. Geriatr. Soc. 1985, 33, 859–863. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef]
- Nishimura, F.; Iwamoto, Y.; Mineshiba, J.; Shimizu, A.; Soga, Y.; Murayama, Y. Periodontal disease and diabetes mellitus: The role of tumor necrosis factor-alpha in a 2-way relationship. J. Periodontol. 2003, 74, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Merchant, A.T.; Vidanapathirana, N.; Yi, F.; Celuch, O.; Zhong, Z.; Jin, Q.; Zhang, J. Association between groups of immunoglobulin G antibodies against periodontal microorganisms and diabetes related mortality. J. Periodontol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: A meta-analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Ferrillo, M.; Migliario, M.; Roccuzzo, A.; Molinero-Mourelle, P.; Falcicchio, G.; Umano, G.R.; Pezzotti, F.; Foglio Bonda, P.L.; Calafiore, D.; de Sire, A. Periodontal Disease and Vitamin D Deficiency in Pregnant Women: Which Correlation with Preterm and Low-Weight Birth? J. Clin. Med. 2021, 10, 4578. [Google Scholar] [CrossRef]
- Czerniuk, M.R.; Surma, S.; Romańczyk, M.; Nowak, J.M.; Wojtowicz, A.; Filipiak, K.J. Unexpected Relationships: Periodontal Diseases: Atherosclerosis–Plaque Destabilization? From the Teeth to a Coronary Event. Biology 2022, 11, 272. [Google Scholar] [CrossRef]
- De Sire, A.; Invernizzi, M.; Ferrillo, M.; Gimigliano, F.; Baricich, A.; Cisari, C.; De Marchi, F.; Foglio Bonda, P.L.; Mazzini, L.; Migliario, M. Functional status and oral health in patients with amyotrophic lateral sclerosis: A cross-sectional study. NeuroRehabilitation 2021, 48, 49–57. [Google Scholar] [CrossRef]
- Heo, S.M.; Sung, R.S.; Scannapieco, F.A.; Haase, E.M. Genetic relationships between Candida albicans strains isolated from dental plaque, trachea, and bronchoalveolar lavage fluid from mechanically ventilated intensive care unit patients. J. Oral Microbiol. 2011, 3, 6362. [Google Scholar] [CrossRef]
- Gomes-Filho, I.S.; de Oliveira, T.F.; da Cruz, S.S.; Passos-Soares Jde, S.; Trindade, S.C.; Oliveira, M.T.; Souza-Machado, A.; Cruz, Á.A.; Barreto, M.L.; Seymour, G.J. Influence of periodontitis in the development of nosocomial pneumonia: A case control study. J. Periodontol. 2014, 85, e82–e90. [Google Scholar] [CrossRef]
- Izumi, M.; Isobe, A.; Akifusa, S. Trypsin-Like Activity in Oral Cavity Is Associated with Risk of Fever Onset in Older Residents of Nursing Homes: An 8-Month Longitudinal Prospective Cohort Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 2255. [Google Scholar] [CrossRef]
- Iwasaki, M.; Usui, M.; Ariyoshi, W.; Nakashima, K.; Nagai-Yoshioka, Y.; Inoue, M.; Nishihara, T. A Preliminary Study on the Ability of the Trypsin-Like Peptidase Activity Assay Kit to Detect Periodontitis. Dent. J. 2020, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000 2005, 38, 72–122. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Christensen, S.; Johansen, M.B.; Christiansen, C.F.; Jensen, R.; Lemeshow, S. Comparison of Charlson comorbidity index with SAPS and APACHE scores for prediction of mortality following intensive care. Clin. Epidemiol. 2011, 3, 203–211. [Google Scholar] [CrossRef]
- Züger, J.; Lüthi-Schaller, H.; Gmür, R. Uncultivated Tannerella BU045 and BU063 are slim segmented filamentous rods of high prevalence but low abundance in inflammatory disease-associated dental plaques. Microbiol. 2007, 153 Pt 11, 3809–3816. [Google Scholar] [CrossRef]
- Tanner, A.C.; Izard, J. Tannerella forsythia, a periodontal pathogen entering the genomic era. Periodontology 2000 2006, 42, 88–113. [Google Scholar] [CrossRef]
- Ardila, C.M.; Olarte-Sossa, M.; Guzmán, I.C. Association between immunoglobulin G1 against Tannerella forsythia and reduction in the loss of attachment tissue. J. Periodontal Implant Sci. 2014, 44, 274–279. [Google Scholar] [CrossRef]
- Hamlet, S.M.; Ganashan, N.; Cullinan, M.P.; Westerman, B.; Palmer, J.E.; Seymour, G.J. A 5-year longitudinal study of Tannerella forsythia prtH genotype: Association with loss of attachment. J. Periodontol. 2008, 79, 144–149. [Google Scholar] [CrossRef]
- Honma, K.; Mishima, E.; Sharma, A. Role of Tannerella forsythia NanH sialidase in epithelial cell attachment. Infect. Immun. 2011, 79, 393–401. [Google Scholar] [CrossRef]
- Grenier, D. Nutritional interactions between two suspected periodontopathogens, Treponema denticola and Porphyromonas gingivalis. Infect. Immun. 1992, 60, 5298–5301. [Google Scholar] [CrossRef]
- Ishikura, H.; Arakawa, S.; Nakajima, T.; Tsuchida, N.; Ishikawa, I. Cloning of the Tannerella forsythensis (Bacteroides forsythus) siaHI gene and purification of the sialidase enzyme. J. Med. Microbiol. 2003, 52 Pt 12, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.; Homer, K.A.; Rao, S.; Booth, V.; Hosie, A.H. An orthologue of Bacteroides fragilis NanH is the principal sialidase in Tannerella forsythia. J. Bacteriol. 2009, 191, 3623–3628. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sojar, H.T.; Glurich, I.; Honma, K.; Kuramitsu, H.K.; Genco, R.J. Cloning, expression, and sequencing of a cell surface antigen containing a leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect. Immun. 1998, 66, 5703–5710. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.V.; Malki, G.; Loo, C.Y.; Tanner, A.C.; Ganeshkumar, N. Cloning and expression of alpha-D-glucosidase and N-acetyl-beta-glucosaminidase from the periodontal pathogen, Tannerella forsythensis (Bacteroides forsythus). Oral Microbiol. Immunol. 2003, 18, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Higuchi, N.; Nakamura, H.; Yoshimura, F.; Oppenheim, F.G. Bacteroides forsythus hemagglutinin is inhibited by N-acetylneuraminyllactose. Oral Microbiol. Immunol. 2002, 17, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Sabet, M.; Lee, S.W.; Nauman, R.K.; Sims, T.; Um, H.S. The surface (S-) layer is a virulence factor of Bacteroides forsythus. Microbiol. (Read.) 2003, 149 Pt 12, 3617–3627. [Google Scholar] [CrossRef]
- Maiden, M.F.; Pham, C.; Kashket, S. Glucose toxicity effect and accumulation of methylglyoxal by the periodontal anaerobe Bacteroides forsythus. Anaerobe 2004, 10, 27–32. [Google Scholar] [CrossRef]
- Karim, A.Y.; Kulczycka, M.; Kantyka, T.; Dubin, G.; Jabaiah, A.; Daugherty, P.S.; Thogersen, I.B.; Enghild, J.J.; Nguyen, K.A.; Potempa, J. A novel matrix metalloprotease-like enzyme (karilysin) of the periodontal pathogen Tannerella forsythia ATCC 43037. Biol. Chem. 2010, 391, 105–117. [Google Scholar] [CrossRef]
- Nakajima, T.; Tomi, N.; Fukuyo, Y.; Ishikura, H.; Ohno, Y.; Arvind, R.; Arai, T.; Ishikawa, I.; Arakawa, S. Isolation and identification of a cytopathic activity in Tannerella forsythia. Biochem. Biophys. Res. Commun. 2006, 351, 133–139. [Google Scholar] [CrossRef]
- Sharma, A. Virulence mechanisms of Tannerella forsythia. Periodontology 2000 2010, 54, 106–116. [Google Scholar] [CrossRef]
- Sharma, A.; Inagaki, S.; Honma, K.; Sfintescu, C.; Baker, P.J.; Evans, R.T. Tannerella forsythia-induced alveolar bone loss in mice involves leucine-rich-repeat BspA protein. J. Dent. Res. 2005, 84, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, T.; Kurihara, H.; Dahlen, G. Characterization of Bacteroides forsythus isolates. J. Clin. Microbiol. 1997, 35, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.M.R.; Saraiva, L.; Romito, G.A.; Pannuti, C.M.; Oliveira, H.P.; Peres, M.; Carmona, M.J.C.; Villar, C.C. Periodontopathogenic bacteria in subglottic samples from patients undergoing elective intubation for general anesthesia: A pilot study. J. Periodontol. 2021, 92, e94–e102. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, G.; Flöel, A.; Stahl, B. Cross-Reactivity as a Mechanism Linking Infections to Stroke. Front. Neurol. 2019, 10, 469. [Google Scholar] [CrossRef]
- Porto, A.N.; Borges, A.H.; Rocatto, G.; Matos, F.Z.; Borba, A.M.; Pedro, F.L.; Lima, S.L.; Tonetto, M.R.; Bandéca, M.C.; Aranha, A.M. Periodontal and Microbiological Profile of Intensive Care Unit Inpatients. J. Contemp. Dent. Pract. 2016, 17, 807–814. [Google Scholar]
- Benedyk, M.; Mydel, P.M.; Delaleu, N.; Płaza, K.; Gawron, K.; Milewska, A.; Maresz, K.; Koziel, J.; Pyrc, K.; Potempa, J. Gingipains: Critical Factors in the Development of Aspiration Pneumonia Caused by Porphyromonas gingivalis. J. Innate Immun. 2016, 8, 185–198. [Google Scholar] [CrossRef]
- Kamio, N.; Hayata, M.; Tamura, M.; Tanaka, H.; Imai, K. Porphyromonas gingivalis enhances pneumococcal adhesion to human alveolar epithelial cells by increasing expression of host platelet-activating factor receptor. FEBS Lett. 2021, 595, 1604–1612. [Google Scholar] [CrossRef]
- Ishihara, K.; Nabuchi, A.; Ito, R.; Miyachi, K.; Kuramitsu, H.K.; Okuda, K. Correlation between detection rates of periodontopathic bacterial DNA in coronary stenotic artery plaque and in dental plaque samples. J. Clin. Microbiol. 2004, 42, 1313–1315. [Google Scholar] [CrossRef]
- Iwai, T.; Inoue, Y.; Umeda, M.; Huang, Y.; Kurihara, N.; Koike, M.; Ishikawa, I. Oral bacteria in the occluded arteries of patients with Buerger disease. J. Vasc. Surg. 2005, 42, 107–115. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Wang, M.; Bagby, G.J.; Nelson, S. Importance of TLR2 in early innate immune response to acute pulmonary infection with Porphyromonas gingivalis in mice. J. Immunol. 2008, 181, 4141–4149. [Google Scholar] [CrossRef]
- Leuckfeld, I.; Obregon-Whittle, M.V.; Lund, M.B.; Geiran, O.; Bjørtuft, Ø.; Olsen, I. Severe chronic obstructive pulmonary disease: Association with marginal bone loss in periodontitis. Respir. Med. 2008, 102, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Strong, C.A.; McTeague, M.; Marina, M. The importance of black-pigmented gram-negative anaerobes in human infections. FEMS Immunol. Med. Microbiol. 1993, 6, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.C.; Yeh, M.; Toledo, S.; Yoshikawa, T.T.; Norman, D.C. Lowering the temperature criterion improves detection of infections in nursing home residents. Aging Immun. Infect. Dis. 1993, 4, 67–76. [Google Scholar]
- Chapman, S.W.; Dismukes, W.E.; Proia, L.A.; Bradsher, R.W.; Pappas, P.G.; Threlkeld, M.G.; Kauffman, C.A. Clinical Practice Guidelines for the Management of Blastomycosis: 2008 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 1801–1812. [Google Scholar] [CrossRef]
- Ohse, H.; Ito, T.; Saito, K.; Kohno, Y.; Kobayashi, M.; Takeuchi, R.; Shimizu, Y.; Matsuzaki, H.; Wadano, Y. Examination of the exothermic cases in the recovery rehabilitation unit of our hospita1. Med. J. Ibaraki Prefec. Hosp. 2014, 31, 23–27. [Google Scholar]

| Bacteria | Primers |
|---|---|
| Porphyromonas gingivalis | Forward: CCGCATACACTTGTATTATTGCATGATATT |
| Reverse: AAGAAGTTTACAATCCTTAGGACTGTCT | |
| Tannerella forsythia | Forward: ATCCTGGCTCAGGATGAACG |
| Reverse: TACGCATRCCCATCCGCAA | |
| Treponema denticola | Forward: CCTTGAACAAAAACCGGAAA |
| Reverse: GGGAAAAGCAGGAAGCATAA | |
| Universal primer | Forward: TTAAACTCAAAGGAATTGACGG |
| Reverse: CTCACGACACGAGCTGACGAC |
| Bacterial Counts | Onset of Fever | p-Value † | |
|---|---|---|---|
| (−) n = 28 | (+) n = 28 | ||
| Pg-log | 3.6 (0–6.4) | 2.3 (0–5.6) | 0.414 |
| Td-log | 0 (0–4.5) | 0 (0–6.0) | 0.060 |
| Tf-log | 3.5 (0–5.6) | 4.2 (1.9–5.3) | 0.044 |
| Univ-log | 7.1 (6.5–8.2) | 7.2 (6.3–8.2) | 0.610 |
| Variables | Tf-log < 4 (n = 30) | Tf-log ≥ 4 (n = 26) | p-Value |
|---|---|---|---|
| Age; m | 90 (69–98) | 86 (62–98) | 0.175 |
| Sex; n (%) | |||
| Man | 9 (30.0) | 11 (42.3) | 0.408 |
| Woman | 21 (70.0) | 15 (57.7) | |
| Number of onsets of fever (days; m) | 0 (0–17) | 1 (0–16) | 0.120 |
| During initial onset of fever (days; m) | 12 (1–12) | 2 (1–12) | 0.027 |
| Charlson comorbidity index; m | 1 (0–3) | 1 (0–4) | 0.773 |
| Body mass index (kg/m2; m) | 20.8 (16–24.9) | 20.7 (14.5–27.3) | 0.889 |
| Number of teeth; m | 5.5 (0–23) | 11.5 (0-30) | 0.411 |
| BOP; m | 0 (0–10) [0 (0–100)] | 1 (0–10) [1 (0–10)] | 0.014 [0.010] |
| %BOP; m | 0 (0–100) [0 (0–10)] | 11.1 (0–63.0) [11.1 (0–63.0)] | 0.010 [0.007] |
| PPD ≥ 4 mm; m | 0.5 (0–16) [2 (0–16)] | 3.5 (0–20) [5 (0–20)] | 0.160 [0.065] |
| PPD ≥ 6 mm; m | 0 (0–1) [0 (0–1)] | 0 (0–12) [1 (0–12)] | 0.044 [0.026] |
| Pg-log; m | 2.7 (0–5.8) | 3.4 (0–6.4) | 0.425 |
| Td-log; m | 0 (0–4.5) | 0 (0–6) | 0.642 |
| Univ-log; m | 7.1 (6.5–8.2) | 7.2 (6.3–8.2) | 0.402 |
| Crude Model | Adjusted Model † | |||||
|---|---|---|---|---|---|---|
| Variable | B ± SE | HR (95% CI) | p-Value | B ± SE | HR (95% CI) | p-Value |
| Tf-log | ||||||
| <4 | 1 (reference) | 1 (reference) | ||||
| ≥4 | 0.9 ± 0.4 | 2.4 (1.1–5.3) | 0.030 | 1.3 ± 0.5 | 3.7 (1.3–10.2) | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koga, A.; Ariyoshi, W.; Kobayashi, K.; Izumi, M.; Isobe, A.; Akifusa, S.; Nishihara, T. The Association between Tannerella forsythia and the Onset of Fever in Older Nursing Home Residents: A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 4734. https://doi.org/10.3390/ijerph19084734
Koga A, Ariyoshi W, Kobayashi K, Izumi M, Isobe A, Akifusa S, Nishihara T. The Association between Tannerella forsythia and the Onset of Fever in Older Nursing Home Residents: A Prospective Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(8):4734. https://doi.org/10.3390/ijerph19084734
Chicago/Turabian StyleKoga, Ayaka, Wataru Ariyoshi, Kaoru Kobayashi, Maya Izumi, Ayaka Isobe, Sumio Akifusa, and Tatsuji Nishihara. 2022. "The Association between Tannerella forsythia and the Onset of Fever in Older Nursing Home Residents: A Prospective Cohort Study" International Journal of Environmental Research and Public Health 19, no. 8: 4734. https://doi.org/10.3390/ijerph19084734
APA StyleKoga, A., Ariyoshi, W., Kobayashi, K., Izumi, M., Isobe, A., Akifusa, S., & Nishihara, T. (2022). The Association between Tannerella forsythia and the Onset of Fever in Older Nursing Home Residents: A Prospective Cohort Study. International Journal of Environmental Research and Public Health, 19(8), 4734. https://doi.org/10.3390/ijerph19084734








