Determinants of Late HIV Presentation at Ndlavela Health Center in Mozambique
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Data Analysis
2.4. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacCarthy, S.; Bangsberg, D.R.; Fink, G.; Reich, M.; Gruskin, S. Late presentation to HIV/AIDS testing, treatment or continued care: Clarifying the use of CD4 evaluation in the consensus definition. HIV Med. 2014, 15, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Mao, Y.; Tang, W.; Han, J.; Xu, J.; Li, J. “Late for testing, early for antiretroviral therapy, less likely to die”: Results from a large HIV cohort study in China, 2006–2014. BMC Infect. Dis. 2018, 18, 272. [Google Scholar] [CrossRef] [PubMed]
- Monforte, A.D.; the Icona Foundation Study Group; Cozzi-Lepri, A.; Girardi, E.; Castagna, A.; Mussini, C.; Di Giambenedetto, S.; Galli, M.; Cassola, G.; Vullo, V.; et al. Late presenters in new HIV diagnoses from an Italian cohort of HIV-infected patients: Prevalence and clinical outcome. Antivir. Ther. 2011, 16, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- MISAU/INE. Inquérito de Indicadores de Imunização, Malária e HIV/SIDA em Mocambique (IMASIDA) 2015; MISAU/INE: Maputo, Mozambique, 2019.
- Antinori, A.; Coenen, T.; Costagiola, D.; Dedes, N.; Ellefson, M.; Gatell, J.; Girardi, E.; Johnson, M.; Kirk, O.; Lundgren, J.; et al. Late presentation of HIV infection: A consensus definition. HIV Med. 2011, 12, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Mukolo, A.; Villegas, R.; Aliyu, M.; Wallston, K.A. Predictors of Late Presentation for HIV Diagnosis: A Literature Review and Suggested Way Forward. AIDS Behav. 2012, 17, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Girardi, E.; Sabin, C.; Monforte, A.D. Late Diagnosis of HIV Infection: Epidemiological Features, Consequences and Strategies to Encourage Earlier Testing. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 46 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef]
- Moreira, A.L.; Fronteira, I.; Augusto, G.F.; Martins, M.R.O. Unmatched Case-Control Study on Late Presentation of HIV Infection in Santiago, Cape Verde (2004–2011). Int. J. Environ. Res. Public Health 2016, 13, 320. [Google Scholar] [CrossRef]
- Gelaw, Y.A.; Senbete, G.H.; Adane, A.A.; Alene, K.A. Determinants of late presentation to HIV/AIDS care in Southern Tigray Zone, Northern Ethiopia: An institution based case—control study. AIDS Res. Ther. 2015, 12, 40. [Google Scholar] [CrossRef]
- Honge, B.L.; Jespersen, S.; Aunsborg, J.; Mendes, D.V.; Medina, C.; Da Silva, D.; Laursen, A.L.; Erikstrup, C.; Wejse, C.; Hiv, T.B. High prevalence and excess mortality of late presenters among HIV-1, HIV-2 and HIV-1/2 dually infected patients in Guinea-Bissau—A cohort study from West Africa. Pan Afr. Med. J. 2016, 25, 40. [Google Scholar] [CrossRef]
- Agaba, P.A.; Meloni, S.T.; Sule, H.M.; Agbaji, O.O.; Ekeh, P.N.; Job, G.C.; Nyango, N.; Ugoagwu, P.O.; Imade, G.E.; Idoko, J.A.; et al. Patients who present late to HIV care and associated risk factors in Nigeria. HIV Med. 2014, 15, 396–405. [Google Scholar] [CrossRef]
- Luma, H.N.; Jua, P.; Donfack, O.-T.; Kamdem, F.; Ngouadjeu, E.; Mbatchou, H.B.; Doualla, M.-S.; Mapoure, Y.N. Late presentation to HIV/AIDS care at the Douala general hospital, Cameroon: Its associated factors, and consequences. BMC Infect. Dis. 2018, 18, 298. [Google Scholar] [CrossRef] [PubMed]
- Fomundam, H.N.; Tesfay, A.R.; Mushipe, S.A.; Mosina, M.B.; Boshielo, C.T.; Nyambi, H.T.; Larsen, A.; Cheyip, M.; Getahun, A.; Pillay, Y. Prevalence and predictors of late presentation for HIV care in South Africa. S. Afr. Med. J. 2017, 107, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Sogbanmu, O.O.; Goon, D.T.; Obi, L.C.; Iweriebor, B.C.; Nwodo, U.N.; Ajayi, A.I.; Okoh, A.I. Socio-demographic and clinical determinants of late presentation among patients newly diagnosed with HIV in the Eastern Cape, South Africa. Medicine 2019, 98, e14664. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Italiano, C.; Chaiwarith, R.; Ng, O.T.; Vanar, S.; Jiamsakul, A.; Saphonn, V.; Van Nguyen, K.; Kiertiburanakul, S.; Lee, M.P.; et al. Late Presentation into Care of HIV Disease and Its Associated Factors in Asia: Results of TAHOD. AIDS Res. Hum. Retrovir. 2016, 32, 255–261. [Google Scholar] [CrossRef]
- Ministério da Saúde (MISAU) Direcção Nacional de Saúde Pública (DNSP). Tratamento Antiretroviral e Infeccões Oportunistas do Adulto, Adolescente, Grávida e Criança; MISAU: Maputo, Mozambique, 2016.
- Scognamiglio, P.; Chiaradia, G.; De Carli, G.; Giuliani, M.; Mastroianni, C.M.; Barbacci, S.A.; Buonomini, A.R.; Grisetti, S.; Sampaolesi, A.; Corpolongo, A.; et al. The potential impact of routine testing of individuals with HIV indicator diseases in order to prevent late HIV diagnosis. BMC Infect. Dis. 2013, 13, 473. [Google Scholar] [CrossRef]
- Yombi, J.C.; Jonckheere, S.; Vincent, A.; Wilmes, D.; Vandercam, B.; Belkhir, L. Late presentation for human immunodeficiency virus HIV diagnosis results of a Belgian single centre. Acta Clin. Belg. 2014, 69, 33–39. [Google Scholar] [CrossRef]
- Drain, P.; Losina, E.; Parker, G.; Giddy, J.; Ross, D.; Katz, J.N.; Coleman, S.M.; Bogart, L.M.; Freedberg, K.A.; Walensky, R.P.; et al. Risk Factors for Late-Stage HIV Disease Presentation at Initial HIV Diagnosis in Durban, South Africa. PLoS ONE 2013, 8, e55305. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, W.; Han, Z.; Tangthanasup, T.M.; Zhong, F.; Qin, F.; Xu, H. Late Presentation of HIV Infection: Prevalence, Trends, and the Role of HIV Testing Strategies in Guangzhou, China, 2008–2013. BioMed Res. Int. 2016, 2016, 1631878. [Google Scholar] [CrossRef]
- Nyika, H.; Mugurungi, O.; Shambira, G.; Gombe, N.T.; Bangure, D.; Mungati, M.; Tshimanga, M. Factors associated with late presentation for HIV/AIDS care in Harare City, Zimbabwe, 2015. BMC Public Health 2016, 16, 369. [Google Scholar] [CrossRef]
- Heath, K.; Levi, J.; Hill, A. The Joint United Nations Programme on HIV/AIDS 95–95–95 targets: Worldwide clinical and cost benefits of generic manufacture. AIDS 2021, 35, S197–S203. [Google Scholar] [CrossRef]
- Maquera-Afaray, J.; Cvetkovlc-Vega, A.; Cárdenas, M.M.; Kälvläinen, H.; Meja, C.R. Diagnóstico tardío y enfermedad avanzada de vih en pacientes adultos en un hospital de la seguridad social de Perú. Rev. Chil. Infectol. 2016, 33, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Shetty, A.K.; Av, S.; Madi, D.; Unnikrishnan, B. Correlates of Late Presentation to HIV care in a South Indian Cohort. Am. J. Trop. Med. Hyg. 2018, 99, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Kigozi, I.M.; Dobkin, L.M.; Martin, J.N.; Geng, E.H.; Muyindike, W.; Emenyonu, N.I.; Bangsberg, D.R.; Hahn, J.A. Late-Disease Stage at Presentation to an HIV Clinic in the Era of Free Antiretroviral Therapy in Sub-Saharan Africa. JAIDS J. Acquir. Immune Defic. Syndr. 2009, 52, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Darling, K.; Hachfeld, A.; Cavassini, M.; Kirk, O.; Furrer, H.; Wandeler, G. Late presentation to HIV care despite good access to health services: Current epidemiological trends and how to do better. Swiss Med. Wkly. 2016, 146, w14348. [Google Scholar] [CrossRef]
- Kiertiburanakul, S.; Boettiger, D.; Lee, M.P.; Omar, S.F.; Tanuma, J.; Ng, O.T.; Durier, N.; Phanuphak, P.; Ditangco, R.; Chaiwarith, R.; et al. Trends of CD4 cell count levels at the initiation of antiretroviral therapy over time and factors associated with late initiation of antiretroviral therapy among Asian HIV-positive patients. J. Int. AIDS Soc. 2014, 17, 18804. [Google Scholar] [CrossRef]
- Belay, H.; Alemseged, F.; Angesom, T.; Hintsa, S.; Abay, M. Effect of late HIV diagnosis on HIV-related mortality among adults in general hospitals of Central Zone Tigray, northern Ethiopia: A retrospective cohort study. HIV/AIDS-Res. Palliat. Care 2017, 9, 187–192. [Google Scholar] [CrossRef]
- Zannou, D.M.; Gandaho, P.B.; Azon-Kouanou, A.; Ahouada, C.; Agbodande, K.A.; Wanvoegbe, A.; Akakpo, J.; Houngbe, F. Late Presentation to Care among People Living with HIV in Cotonou, Benin: A Retrospective Analysis from 2003 to 2014. Open J. Intern. Med. 2017, 07, 123–134. [Google Scholar] [CrossRef][Green Version]
- Ministério da Saúde (MISAU) Direcção Nacional de Saúde Pública (DNSP). Engajamento dos Homens nos Cuidados de Saúde; MISAU: Maputo, Mozambique, 2018.
- Amin, A.; Boothe, M.; Cassimo, M.N.; Duce, P.; Fazito, E.; Gobet, B.; Horth, R.; Nhantumbo, I.; Lara, J.; Manembe, L.; et al. Distribuição da incidência de infecções por HIV na população de 15 a 49 anos em Moçambique por modo de transmissão; ONUSIDA: Geneva, Switzerland, 2013. [Google Scholar]
- Diaz, A.; Del Romero, J.; Rodriguez, C.; Alastrue, I.; Belda, J.; Bru, F.; Cámara, M.M.; Junquera, M.L.; Sanz, I.; Viloria, L.J.; et al. Effects of region of birth, educational level and age on late presentation among men who have sex with men newly diagnosed with HIV in a network of STI/HIV counselling and testing clinics in Spain. Eurosurveillance 2015, 20, 21088. [Google Scholar] [CrossRef]
- Koirala, S.; Deuba, K.; Nampaisan, O.; Marrone, G.; Ekström, A.M. For the CAT-S group Facilitators and barriers for retention in HIV care between testing and treatment in Asia—A study in Bangladesh, Indonesia, Lao, Nepal, Pakistan, Philippines and Vietnam. PLoS ONE 2017, 12, e0176914. [Google Scholar] [CrossRef]
- Takah, N.F.; Awungafac, G.; Aminde, L.N.; Ali, I.; Ndasi, J.; Njukeng, P. Delayed entry into HIV care after diagnosis in two specialized care and treatment centres in Cameroon: The influence of CD4 count and WHO staging. BMC Public Health 2016, 16, 529. [Google Scholar] [CrossRef]
- Beyene, M.B.; Beyene, H.B. Predictors of Late HIV Diagnosis among Adult People Living with HIV/AIDS Who Undertake an Initial CD4 T Cell Evaluation, Northern Ethiopia: A Case-Control Study. PLoS ONE 2015, 10, e0140004. [Google Scholar] [CrossRef]
- Assen, A.; Molla, F.; Wondimu, A.; Abrha, S.; Melkam, W.; Tadesse, E.; Yilma, Z.; Eticha, T.; Abrha, H.; Workneh, B.D. Late presentation for diagnosis of HIV infection among HIV positive patients in South Tigray Zone, Ethiopia. BMC Public Health 2016, 16, 558. [Google Scholar] [CrossRef] [PubMed]
- Abaynew, Y.; Deribew, A.; Deribe, K. Factors associated with late presentation to HIV/AIDS care in South Wollo Zone Ethiopia: A case-control study. AIDS Res. Ther. 2011, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Barrington, C.; Knudston, K.; Bailey, O.A.P.; Aguilar, J.M.; Loya-Montiel, M.I.; Morales-Miranda, S. HIV Diagnosis, Linkage to Care, and Retention among Men Who Have Sex with Men and Transgender Women in Guatemala City. J. Health Care Poor Underserved 2016, 27, 1745–1760. [Google Scholar] [CrossRef]
- Manirankunda, L.; Loos, J.; Alou, T.A.; Colebunders, R.; Nöstlinger, C. “It’s Better Not To know’: Perceived Barriers to HIV Voluntary Counseling and Testing among Sub-Saharan African Migrants in Belgium. AIDS Educ. Prev. 2009, 21, 582–593. [Google Scholar] [CrossRef]
- Sayles, J.N.; Hays, R.D.; Sarkisian, C.A.; Mahajan, A.P.; Spritzer, K.L.; Cunningham, W.E. Development and Psychometric Assessment of a Multidimensional Measure of Internalized HIV Stigma in a sample of HIV-positive Adults Jennifer. AIDS Behav. 2008, 12, 748–758. [Google Scholar] [CrossRef]
- Horino, T.; Sato, F.; Kato, T.; Hosaka, Y.; Shimizu, A.; Kawano, S.; Hoshina, T.; Nakaharai, K.; Nakazawa, Y.; Yoshikawa, K.; et al. Associations of HIV testing and late diagnosis at a Japanese university hospital. Clinics 2016, 70, 73–77. [Google Scholar] [CrossRef]
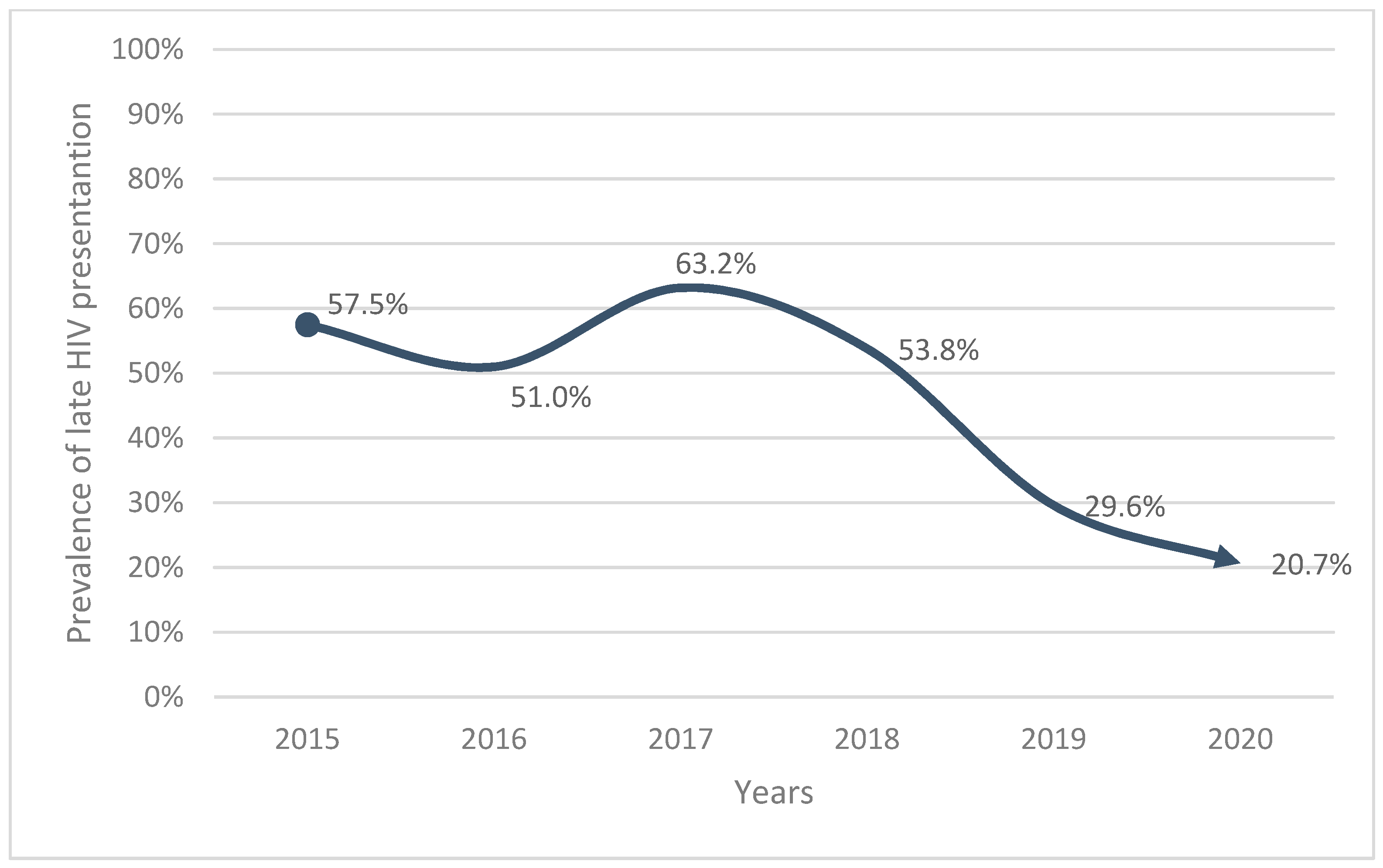
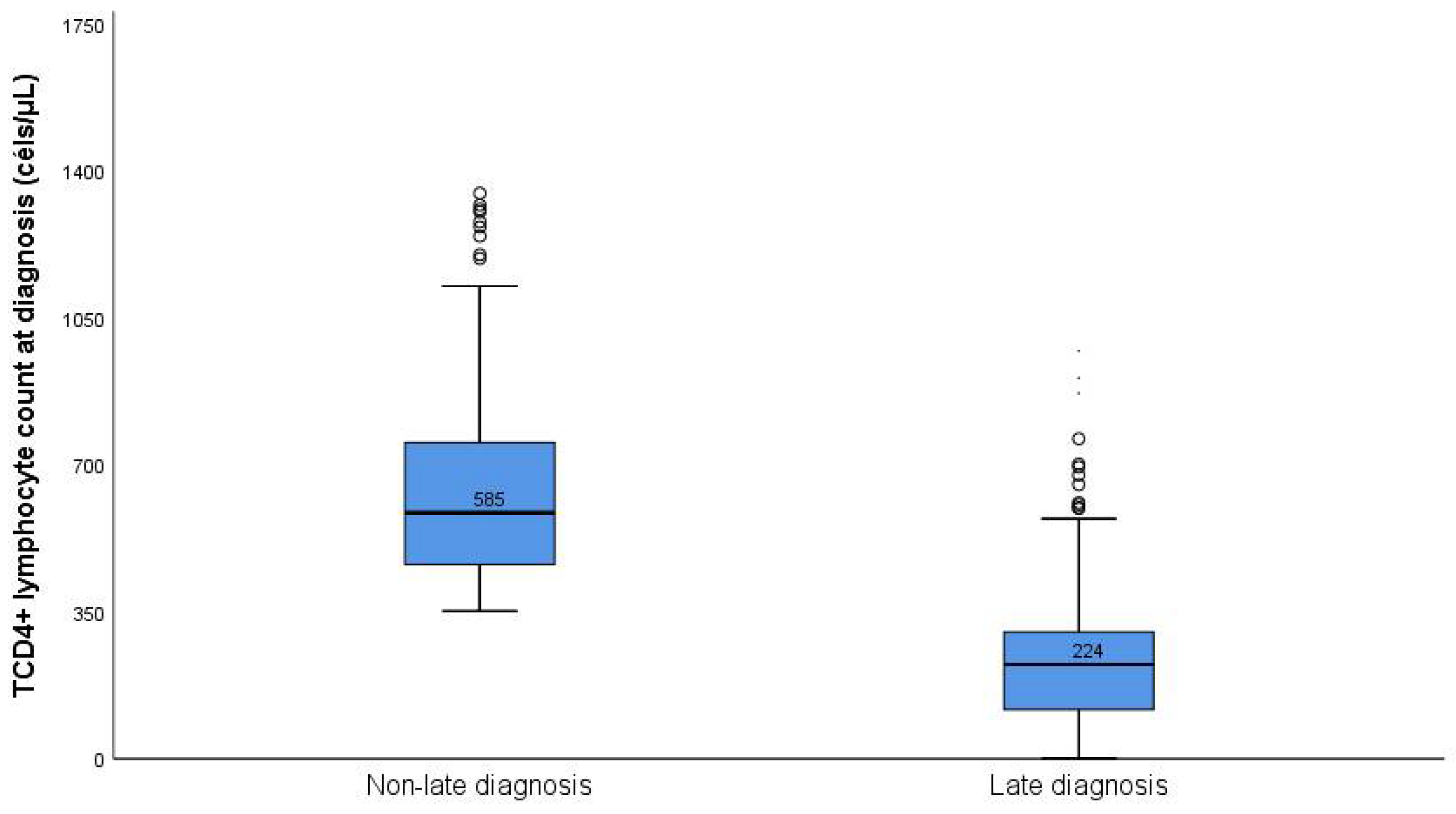
| Variables | Total | Late Presentation | Non-Late Presentation | p Value * | |||
|---|---|---|---|---|---|---|---|
| n 519 | (%) 100 | n 242 | (%) 46.6 | n 277 | (%) 53.4 | ||
| Year of diagnosis | <0.001 | ||||||
| 2015 | 80 | 15.4 | 46 | 57.5 | 34 | 42.5 | |
| 2016 | 82 | 15.8 | 42 | 51 | 40 | 49 | |
| 2017 | 98 | 18.9 | 62 | 63.2 | 36 | 36.8 | |
| 2018 | 91 | 17.5 | 49 | 53.8 | 42 | 46.2 | |
| 2019 | 91 | 17.5 | 27 | 29.6 | 64 | 23.1 | |
| 2020 | 77 | 14.8 | 16 | 20.7 | 61 | 22 | |
| Gender | 0.009 | ||||||
| Female | 398 | 76.7 | 173 | 71.5 | 252 | 81.2 | |
| Male | 121 | 23.3 | 69 | 28.5 | 52 | 18.8 | |
| Age | 0.001 | ||||||
| 18–24 | 80 | 15.4 | 22 | 9.1 | 58 | 20.9 | |
| 25–34 | 176 | 33.9 | 79 | 32.6 | 97 | 35 | |
| 35–49 | 211 | 40.7 | 113 | 46.7 | 98 | 35.4 | |
| +50 | 52 | 10 | 28 | 11.6 | 24 | 8.7 | |
| Education level | 0.632 | ||||||
| No education | 72 | 13.9 | 36 | 14.9 | 36 | 13 | |
| Primary | 263 | 50.7 | 125 | 51.7 | 138 | 49.8 | |
| Secondary or higher | 184 | 35.5 | 81 | 33.5 | 103 | 37.2 | |
| Marital status at the time of diagnosis | 0.008 | ||||||
| Married or living with a partner | 295 | 56.8 | 133 | 55 | 162 | 58.5 | |
| Previously married or lived with a partner | 75 | 14.5 | 47 | 19.4 | 28 | 10.1 | |
| Never married or lived with a partner | 149 | 28.7 | 62 | 25.6 | 87 | 31.4 | |
| Variables | Total | Late Presentation | Not Late Presentation | p Value * | |||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | ||
| Some symptoms suggestive of HIV before the diagnosis | <0.001 | ||||||
| No | 328 | 63.2 | 110 | 45.5 | 218 | 78.7 | |
| Yes | 191 | 36.8 | 132 | 54.5 | 59 | 21.3 | |
| Reason for HIV testing | 0.018 | ||||||
| VCT | 314 | 60.5 | 150 | 62 | 164 | 59.2 | |
| PITC | 205 | 39.5 | 92 | 38 | 113 | 40.8 | |
| Number of sexual partners at diagnosis | 0.302 | ||||||
| None | 51 | 9.8 | 27 | 11.2 | 24 | 8.7 | |
| Only one | 412 | 79.4 | 185 | 76.4 | 227 | 81.9 | |
| More than one | 56 | 10.8 | 30 | 12.4 | 26 | 9.4 | |
| Prior screened for HIV | 0.009 | ||||||
| No | 337 | 64.9 | 167 | 69 | 170 | 61.4 | |
| Yes | 182 | 35.1 | 75 | 31 | 107 | 38.6 | |
| Used illicit drug at the time of diagnosis | 0.482 ψ | ||||||
| No | 511 | 98.5 | 237 | 97.9 | 274 | 98.9 | |
| Yes | 8 | 1.5 | 5 | 2.1 | 3 | 1.1 | |
| Regularly used condoms, before the positive result | 0.668 | ||||||
| No | 414 | 79.8 | 195 | 80.6 | 219 | 79.1 | |
| Yes | 105 | 20.2 | 47 | 19.4 | 58 | 20.9 | |
| Fear of stigma | 0.029 | ||||||
| No | 296 | 57 | 149 | 61.6 | 147 | 53.1 | |
| Yes | 223 | 43 | 93 | 38.4 | 130 | 46.9 | |
| Prior information about HIV | 0.017 | ||||||
| No | 30 | 5.8 | 14 | 5.8 | 16 | 5.8 | |
| Yes | 489 | 94.2 | 228 | 94.2 | 261 | 94.2 | |
| Before the diagnosis performed regular check-ups | 0.023 | ||||||
| No | 383 | 73.8 | 190 | 78.5 | 193 | 69.7 | |
| Yes | 136 | 26.2 | 52 | 21.5 | 84 | 30.3 | |
| Visited a traditional medicene | |||||||
| No | 502 | 96.7 | 232 | 95.9 | 270 | 97.5 | |
| Yes | 17 | 3.3 | 10 | 4.1 | 7 | 2.5 | |
| Explanatory Variables | OR (95% CI) | p-Value | AOR (95% CI) | p-Value |
|---|---|---|---|---|
| Year of diagnosis | ||||
| 2015 | 1 | 1 | ||
| 2016 | 1.776 (1.516–1.942) | 0.023 | 2.717 (1.362–3.421) | 0.041 |
| 2017 | 1.273 (1.017–2.329) | 0.034 | 1.182 (1.009–2.295) | 0.002 |
| 2018 | 2.862 (1.471–3.580) | 0.013 | 1.857 (0.937–2.682) | 0.055 |
| 2019 | 0.312 (0.166–0.586) | 0.001 | 0.279 (0.139–0.563) | 0.001 |
| 2020 | 0.194 (0.096–0.393) | 0.001 | 0.169 (0.076–0.375) | 0.001 |
| Gender | ||||
| Feminine | 1 | 1 | ||
| Male | 1.726 (1.144–2.603) | 0.009 | 2.417 (1.853–4.355) | 0.028 |
| Age | ||||
| 18–24 | 1 | 1 | ||
| 25–34 | 2.147 (1.210–3.811) | 0.009 | 1.738 (0.989–3.362) | 0.101 |
| 35–49 | 3.040 (1.736–5.324) | <0.001 | 1.499 (1.054–2.978) | <0.001 |
| +50 | 3.076 (1.477–6.405) | 0.003 | 1.620 (0.646–4.064) | 0.303 |
| Marital status at the time of diagnosis | ||||
| Married or living with a partner | 1 | 1 | ||
| Previously married or lived with a partner | 2.045 (1.214–3.443) | 0.007 | 1.542 (0.835–2.846) | 0.067 |
| Never married or lived with a partner | 0.868 (0.583–0.996) | 0.036 | 0.318 (0.132–0.893) | 0.042 |
| Some symptoms suggestive of HIV before the diagnosis | ||||
| No | 1 | 1 | ||
| Yes | 5.434 (3.023–6.504) | <0.001 | 4.033 (2.604–6.155) | <0.001 |
| Reason for HIV testing | ||||
| VCT | 1 | |||
| PITC | 3.890 (1.625–4.267) | 0.018 | 2.190 (1.875–3.043) | 0.002 |
| Prior screened for HIV | ||||
| No | 1 | 1 | ||
| Yes | 0.396 (0.184–0.714) | 0.009 | 0.235 (0.142–0.654) | 0.041 |
| Fear of stigma | ||||
| No | 1 | 1 | ||
| Yes | 4.417 (2.998–6.011) | 0.029 | 2.808 (1.477–3.369) | 0.042 |
| Prior information about HIV | ||||
| No | 1 | 1 | ||
| Yes | 0.302 (0.278–0.697) | 0.017 | 0.421 (0.276–0.854) | 0.039 |
| Before the diagnosis, performed regular checkups | ||||
| No | 1 | 1 | ||
| Yes | 0.490 (0.159–0.984) | 0.023 | 0.585 (0.153–0.826) | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chone, J.S.; Abecasis, A.B.; Varandas, L. Determinants of Late HIV Presentation at Ndlavela Health Center in Mozambique. Int. J. Environ. Res. Public Health 2022, 19, 4568. https://doi.org/10.3390/ijerph19084568
Chone JS, Abecasis AB, Varandas L. Determinants of Late HIV Presentation at Ndlavela Health Center in Mozambique. International Journal of Environmental Research and Public Health. 2022; 19(8):4568. https://doi.org/10.3390/ijerph19084568
Chicago/Turabian StyleChone, Jeremias Salomão, Ana Barroso Abecasis, and Luís Varandas. 2022. "Determinants of Late HIV Presentation at Ndlavela Health Center in Mozambique" International Journal of Environmental Research and Public Health 19, no. 8: 4568. https://doi.org/10.3390/ijerph19084568
APA StyleChone, J. S., Abecasis, A. B., & Varandas, L. (2022). Determinants of Late HIV Presentation at Ndlavela Health Center in Mozambique. International Journal of Environmental Research and Public Health, 19(8), 4568. https://doi.org/10.3390/ijerph19084568






