Acute Exercise with Moderate Hypoxia Reduces Arterial Oxygen Saturation and Cerebral Oxygenation without Affecting Hemodynamics in Physically Active Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
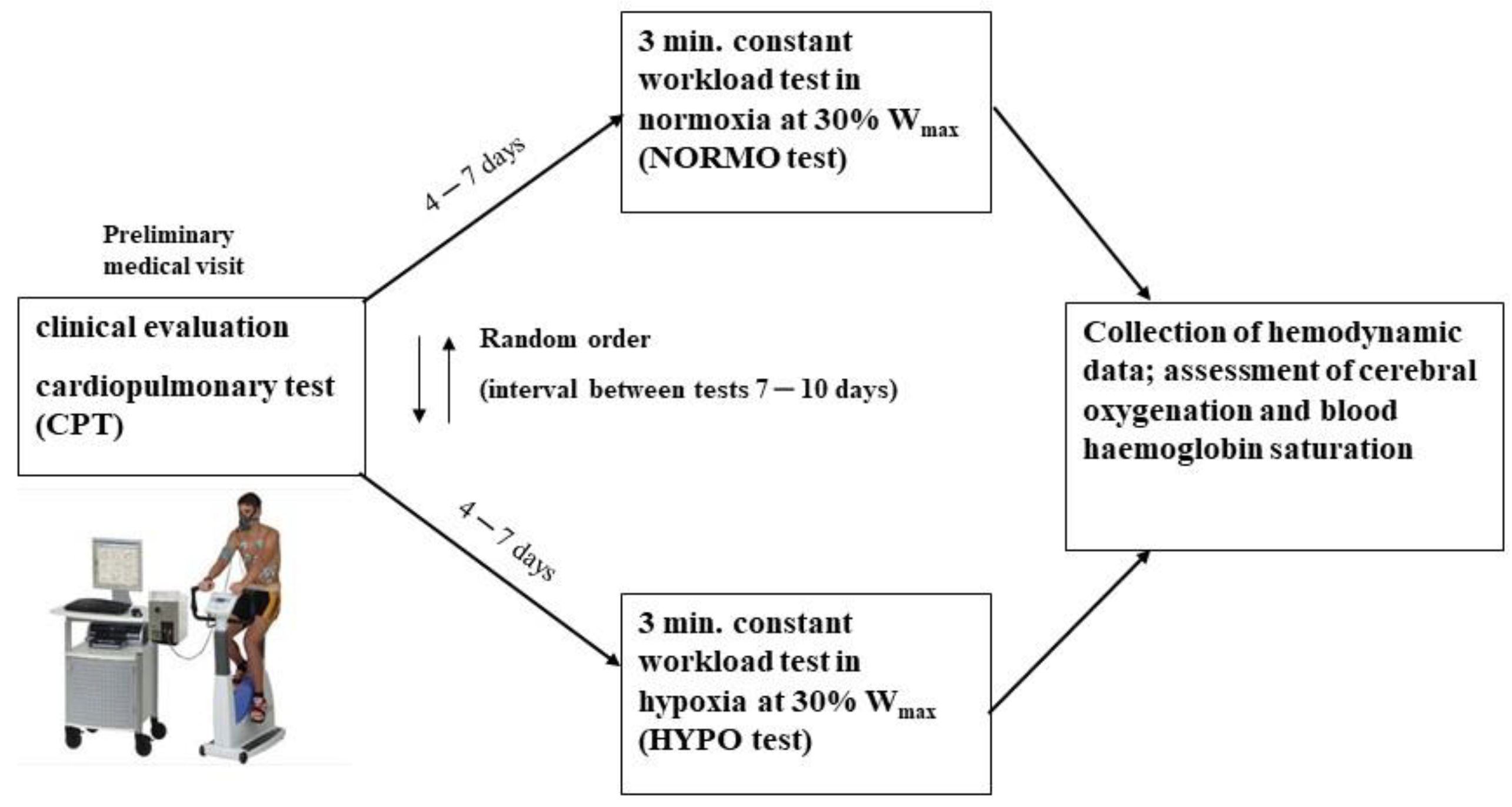
2.2. Experimental Protocol
Preliminary Test
2.3. Exercise Test to Study the Hemodynamic Response in Normoxia and Hypoxia
2.4. Hemodynamic Assessment
2.5. Data Analysis
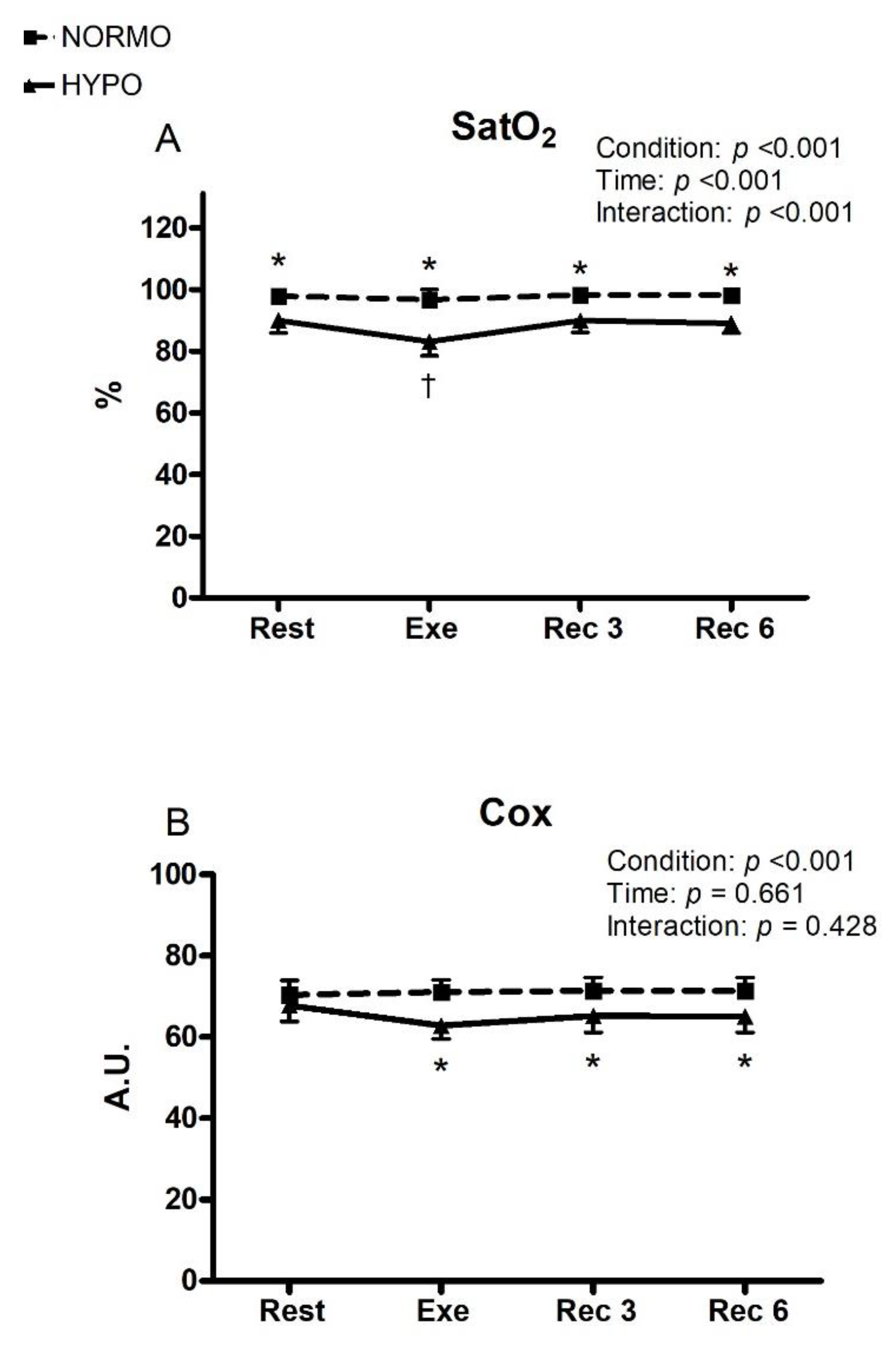
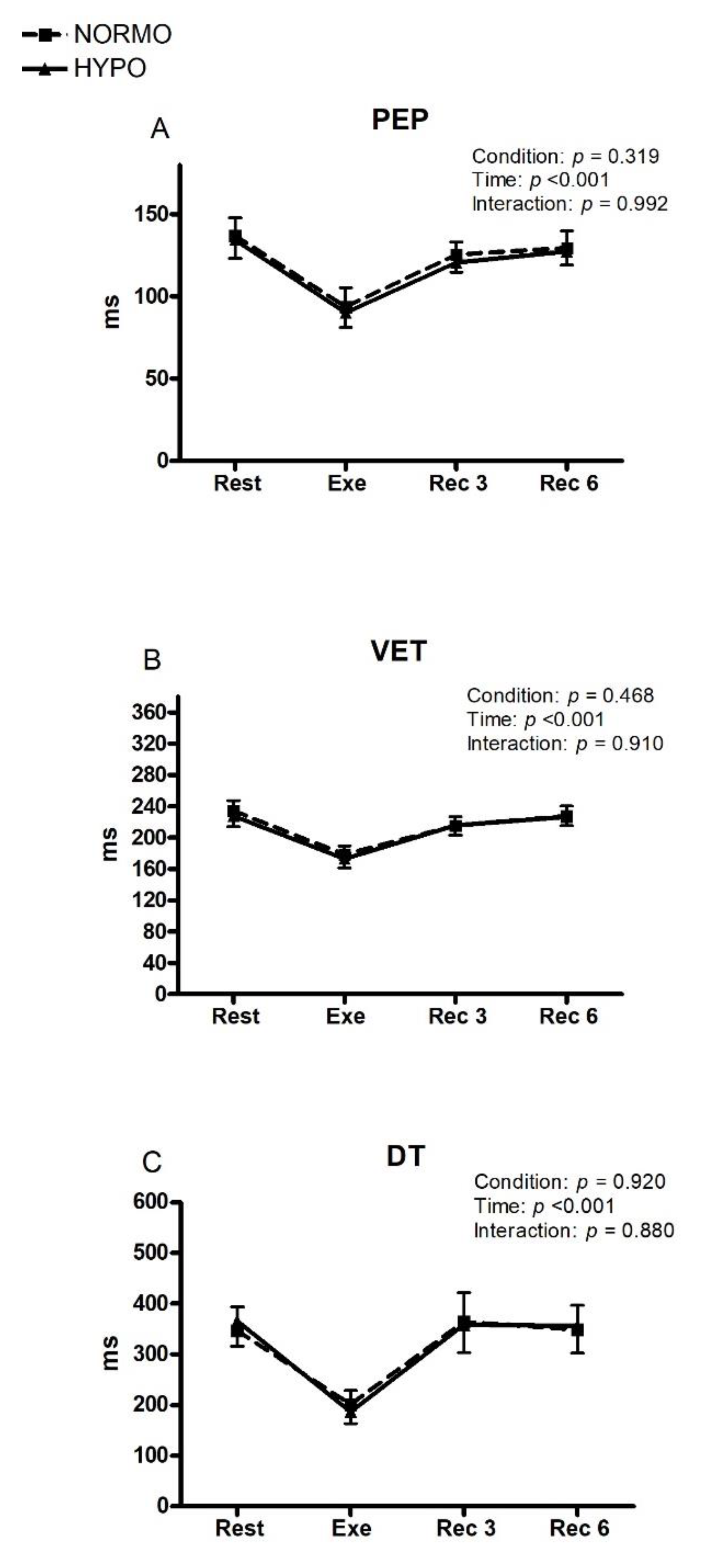
3. Results
4. Discussion
Limitations of Our Study
5. Conclusions
Practical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapman, R.F.; Stray-Gundersen, J.; Levine, B.D. Individual variation in response to altitude training. J. Appl. Physiol. 1998, 4, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Cornolo, J.; Fouillot, J.P.; Schmitt, L.; Povea, C.; Robach, P.; Richalet, J.P. Interactions between exposure to hypoxia and the training-induced autonomic adaptations in a "live high-train low" session. Eur. J. Appl. Physiol. 2006, 96, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.P.; Roles, B.; Schmitt, L.; Woorons, X.; Richalet, J.P. Combining hypoxic methods for peak performance. Sports Med. 2010, 40, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Peeling, P.; Garvican-Lewis, L.A.; Hall, R.; Koivisto, A.E.; Heikura, I.A.; Burke, L.M. Nutrition and Altitude: Strategies to Enhance Adaptation, Improve Performance and Maintain Health: A Narrative Review. Sports Med. 2019, 49 (Suppl. S2), 169–184. [Google Scholar] [CrossRef]
- Naeije, R.; Dedobbeleer, C. Pulmonary hypertension and the right ventricle in hypoxia. Exp. Physiol. 2013, 98, 1247–1256. [Google Scholar] [CrossRef]
- Siebenmann, C.; Lundby, C. Regulation of cardiac output in hypoxia. Scand J. Med. Sci. Sports 2015, 25 (Suppl. S4), 53–59. [Google Scholar] [CrossRef]
- Stembridge, M.; Ainslie, P.N.; Shave, R. Mechanisms underlying reductions in stroke volume at rest and during exercise at high altitude. Eur. J. Sport Sci. 2016, 16, 577–584. [Google Scholar] [CrossRef]
- Amann, M.; Romer, L.M.; Pegelow, D.F.; Jacques, A.J.; Hess, C.J.; Dempsey, J.A. Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J. Appl. Physiol. 2006, 101, 119–127. [Google Scholar] [CrossRef]
- Owen, J.; Patterson, S.D.; Waldron, M. The effect of severe and moderate hypoxia on exercise at a fixed level of perceived exertion. Eur. J. Appl. Physiol. 2019, 119, 1213–1224. [Google Scholar]
- Romer, L.M.; Haverkamp, H.C.; Amann, M.; Lovering, A.T.; Pegelow, D.F.; Dempsey, J.A. Effect of acute severe hypoxia on peripheral fatigue and endurance capacity in healthy humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R598–R606. [Google Scholar] [CrossRef]
- Yan, B.; Hu, Y.; Ji, H.; Bao, D. The effect of acute hypoxia on left ventricular function during exercise. Eur. J. Appl. Physiol. 2007, 100, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Heunks, L.M.; Machiels, H.A.; Ennen, L.; Dekhuijzen, P.N. Effects of modulation of nitric oxide on rat diaphragm isotonic contractility during hypoxia. J. Appl. Physiol. 2003, 94, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Heunks, L.M.; Versteeg, E.M.; van der Heijden, H.F.; Ennen, L.; van Kuppevelt, T.H.; Vina, J.; Dekhuijzen, P.N. Hpoxia-induced dysfunction of rat diaphragm: Role of peroxynitrite. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L16–L26. [Google Scholar] [CrossRef] [PubMed]
- Dedobbeleer, C.; Hadefi, A.; Naeije, R.; Unger, P. Left ventricular adaptation to acute hypoxia: A speckle-tracking echocardiography study. J. Am. Soc. Echocardiogr. 2013, 26, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Goebel, B.; Handrick, V.; Lauten, A.; Fritzenwanger, M.; Schütze, J.; Otto, S.; Figulla, H.R.; Edvardsen, T.; Poerner, T.C.; Jung, C. Impact of acute normobaric hypoxia on regional and global myocardial function: A speckle tracking echocardiography study. Int. J. Cardiovasc. Imaging 2013, 29, 561–570. [Google Scholar] [CrossRef]
- Magnani, S.; Mulliri, G.; Roberto, S.; Sechi, F.; Ghiani, G.; Sainas, G.; Nughedu, G.; Vargiu, R.; Bassareo, P.P.; Crisafulli, A. Systolic and Diastolic Functions After a Brief Acute Bout of Mild Exercise in Normobaric Hypoxia. Front. Physiol. 2021, 12, 650696. [Google Scholar] [CrossRef]
- Mulliri, G.; Sainas, G.; Magnani, S.; Roberto, S.; Ghiani, G.; Mannoni, M.; Pinna, V.; Willis, S.J.; Millet, G.P.; Doneddu, A.; et al. Effects of exercise in normobaric hypoxia on hemodynamics during muscle metaboreflex activation in normoxia. Eur. J. Appl. Physiol. 2019, 119, 1137–1148. [Google Scholar] [CrossRef]
- Mulliri, G.; Magnani, S.; Roberto, S.; Sechi, F.; Ghiani, G.; Sainas, G.; Nughedu, G.; Hosseini Kakhak, S.A.; Bassareo, P.P.; Crisafulli, A. A brief bout of exercise in hypoxia reduces ventricular filling rate and stroke volume response during muscle metaboreflex activation. Eur. J. Appl. Physiol. 2020, 120, 2115–2126. [Google Scholar] [CrossRef]
- Howley, E.T.; Bassett, D.R.; Welch, H.G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc. 1995, 27, 1292–1301. [Google Scholar] [CrossRef]
- Płoszczyca, K.; Czuba, M.; Chalimoniuk, M.; Gajda, R.; Baranowski, M. Red Blood Cell 2,3-Diphosphoglycerate Decreases in Response to a 30 km Time Trial Under Hypoxia in Cyclists. Front. Physiol. 2021, 12, 670977. [Google Scholar] [CrossRef]
- Michael, S.; Graham, K.S.; Oam, G.M.D. Cardiac Autonomic Responses during Exercise and Post-exercise Recovery Using Heart Rate Variability and Systolic Time Intervals-A Review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, A.; Melis, F.; Orrù, V.; Lener, R.; Lai, C.; Concu, A. Hemodynamic during a postexertional asystolia in a healthy athlete: A case study. Med. Sci. Sports Exerc. 2000, 32, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Marongiu, E.; Piepoli, M.F.; Milia, R.; Angius, L.; Pinna, M.; Bassareo, P.P.; Roberto, S.; Tocco, F.; Concu, A.; Crisafulli, A. Effects of acute vasodilation on the hemodynamic response to muscle metaboreflex. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H1387–H1396. [Google Scholar] [CrossRef] [PubMed]
- Tocco, F.; Marongiu, E.; Pinna, M.; Roberto, S.; Pusceddu, M.; Angius, L.; Migliaccio, G.; Milia, R.; Concu, A.; Crisafulli, A. Assessment of circulatory adjustments during underwater apnoea in elite divers by means of a portable device. Acta Physiol. 2013, 207, 290–298. [Google Scholar] [CrossRef]
- Bernstein, D.P. A new stroke volume equation for Thoracic Electrical Bioimpedance: Theory and rationale. Crit. Care Med. 1986, 14, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, N.; Cox, D.; Jamnik, R. Endurance athletes’ stroke volume does not plateau: Major advantage is diastolic function. Med. Sci. Sports Exerc. 1994, 26, 1116–1121. [Google Scholar] [CrossRef]
- Milia, R.; Roberto, S.; Mulliri, G.; Loi, A.; Marcelli, M.; Sainas, G.; Milia, N.; Marongiu, E.; Crisafulli, A. Effect of aging on hemodynamic response to metaboreflex activation. Eur. J. Appl. Physiol. 2015, 115, 1693–1703. [Google Scholar] [CrossRef]
- Sanna, I.; Pinna, V.; Milia, R.; Roberto, S.; Olla, S.; Mulliri, G.; Crisafulli, A. Hemodynamic Responses during Enduro-Motorcycling Performance. Front. Physiol. 2017, 8, 1062. [Google Scholar] [CrossRef]
- Sainas, G.; Marongiu, E.; Milia, R.; Palazzolo, G.; Ibba, G.; Roberto, S.; Ghiani, G.; Tocco, F.; Crisafulli, A. Mean blood pressure assessment during post-exercise: Results from two different methods of calculation. J. Sports Sci. Med. 2016, 15, 424–433. [Google Scholar]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Morgan, B.J. Humans In Hypoxia: A Conspiracy Of Maladaptation? Physiology 2015, 30, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.P.; Debevec, T.; Brocherie, F.; Malatesta, D.; Girard, O. Therapeutic use of exercising in hypoxia: Promises and limitations. Front. Physiol. 2016, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Wilber, R.L. Current trends in altitude training. Sports Med. 2001, 31, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.P.; Joyner, M.J. Compensatory vasodilatation during hypoxic exercise: Mechanisms responsible for matching oxygen supply to demand. J. Physiol. 2012, 590, 6321–6326. [Google Scholar] [CrossRef]
- Wolfel, E.E.; Selland, M.A.; Cymerman, A.; Brooks, G.A.; Butterfield, G.E.; Mazzeo, R.S.; Grover, R.F.; Reeves, J.T. O2 extraction maintains O2 uptake during submaximal exercise with beta-adrenergic blockade at 4300 m. J. Appl. Physiol. 1998, 85, 1092–1102. [Google Scholar] [CrossRef][Green Version]
- Calbet, J.A.; Robach, P.; Lundby, C. The exercising heart at altitude. Cell. Mol. Life Sci. 2009, 66, 3601–3613. [Google Scholar] [CrossRef]
- Dinenno, F.A. Skeletal muscle vasodilation during systemic hypoxia in humans. J. Appl. Physiol. 2016, 120, 216–225. [Google Scholar] [CrossRef]
- Marshall, J.M. Interactions between local dilator and sympathetic vasoconstrictor influences in skeletal muscle in acute and chronic hypoxia. Exp. Physiol. 2015, 100, 1400–1411. [Google Scholar] [CrossRef]
- Fu, Q.; Townsend, N.E.; Shiller, S.M.; Martini, E.R.; Okazaki, K.; Shibata, S.; Truijens, M.J.; Rodríguez, F.A.; Gore, C.J.; Stray-Gundersen, J.; et al. Intermittent hypobaric hypoxia exposure does not cause sustained alterations in autonomic control of blood pressure in young athletes. Am. J. Physiol. Regul. Integr. Comp Physiol. 2007, 292, R1977–R1984. [Google Scholar] [CrossRef]
- Bouak, F.; Vartanian, O.; Hofer, K.; Cheung, B. Acute mild hypoxic hypoxia effects on cognitive and simulated aircraft pilot performance. Aerosp. Med. Hum. Perform. 2018, 89, 526–535. [Google Scholar] [CrossRef]
- Amann, M.; Romer, L.M.; Subudhi, A.W.; Pegelow, D.F.; Dempsey, J.A. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J. Physiol. 2007, 581 Pt 1, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Halliwill, J.R.; Minson, C.T. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J. Appl. Physiol. 2002, 93, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Mourot, L. Limitation of Maximal Heart Rate in Hypoxia: Mechanisms and Clinical Importance. Front. Physiol. 2018, 9, 972. [Google Scholar] [CrossRef] [PubMed]


| Wmax (W) | 253.8 (243.6–273.0) |
|---|---|
| VO2max (mL·kg−1·min−1) | 40.29 (38.48–42.1) |
| VO2max (mL·min−1) | 2939 (2759–3119) |
| VCO2max (mL·min−1) | 3504 (3289–3719) |
| RERmax | 1.19 (1.15–1.23) |
| VEmax (L·min−1) | 94.74 (87.57–101.90) |
| HRmax (bpm) | 177.4 (172.9–181.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulliri, G.; Magnani, S.; Roberto, S.; Ghiani, G.; Sechi, F.; Fanni, M.; Marini, E.; Stagi, S.; Lai, Y.; Rinaldi, A.; et al. Acute Exercise with Moderate Hypoxia Reduces Arterial Oxygen Saturation and Cerebral Oxygenation without Affecting Hemodynamics in Physically Active Males. Int. J. Environ. Res. Public Health 2022, 19, 4558. https://doi.org/10.3390/ijerph19084558
Mulliri G, Magnani S, Roberto S, Ghiani G, Sechi F, Fanni M, Marini E, Stagi S, Lai Y, Rinaldi A, et al. Acute Exercise with Moderate Hypoxia Reduces Arterial Oxygen Saturation and Cerebral Oxygenation without Affecting Hemodynamics in Physically Active Males. International Journal of Environmental Research and Public Health. 2022; 19(8):4558. https://doi.org/10.3390/ijerph19084558
Chicago/Turabian StyleMulliri, Gabriele, Sara Magnani, Silvana Roberto, Giovanna Ghiani, Fabio Sechi, Massimo Fanni, Elisabetta Marini, Silvia Stagi, Ylenia Lai, Andrea Rinaldi, and et al. 2022. "Acute Exercise with Moderate Hypoxia Reduces Arterial Oxygen Saturation and Cerebral Oxygenation without Affecting Hemodynamics in Physically Active Males" International Journal of Environmental Research and Public Health 19, no. 8: 4558. https://doi.org/10.3390/ijerph19084558
APA StyleMulliri, G., Magnani, S., Roberto, S., Ghiani, G., Sechi, F., Fanni, M., Marini, E., Stagi, S., Lai, Y., Rinaldi, A., Isola, R., Vargiu, R., Spranger, M. D., & Crisafulli, A. (2022). Acute Exercise with Moderate Hypoxia Reduces Arterial Oxygen Saturation and Cerebral Oxygenation without Affecting Hemodynamics in Physically Active Males. International Journal of Environmental Research and Public Health, 19(8), 4558. https://doi.org/10.3390/ijerph19084558








