Assessment and Implication of PAHs and Compound-Specific δ13C Compositions in a Dated Marine Sediment Core from Daya Bay, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Strategy
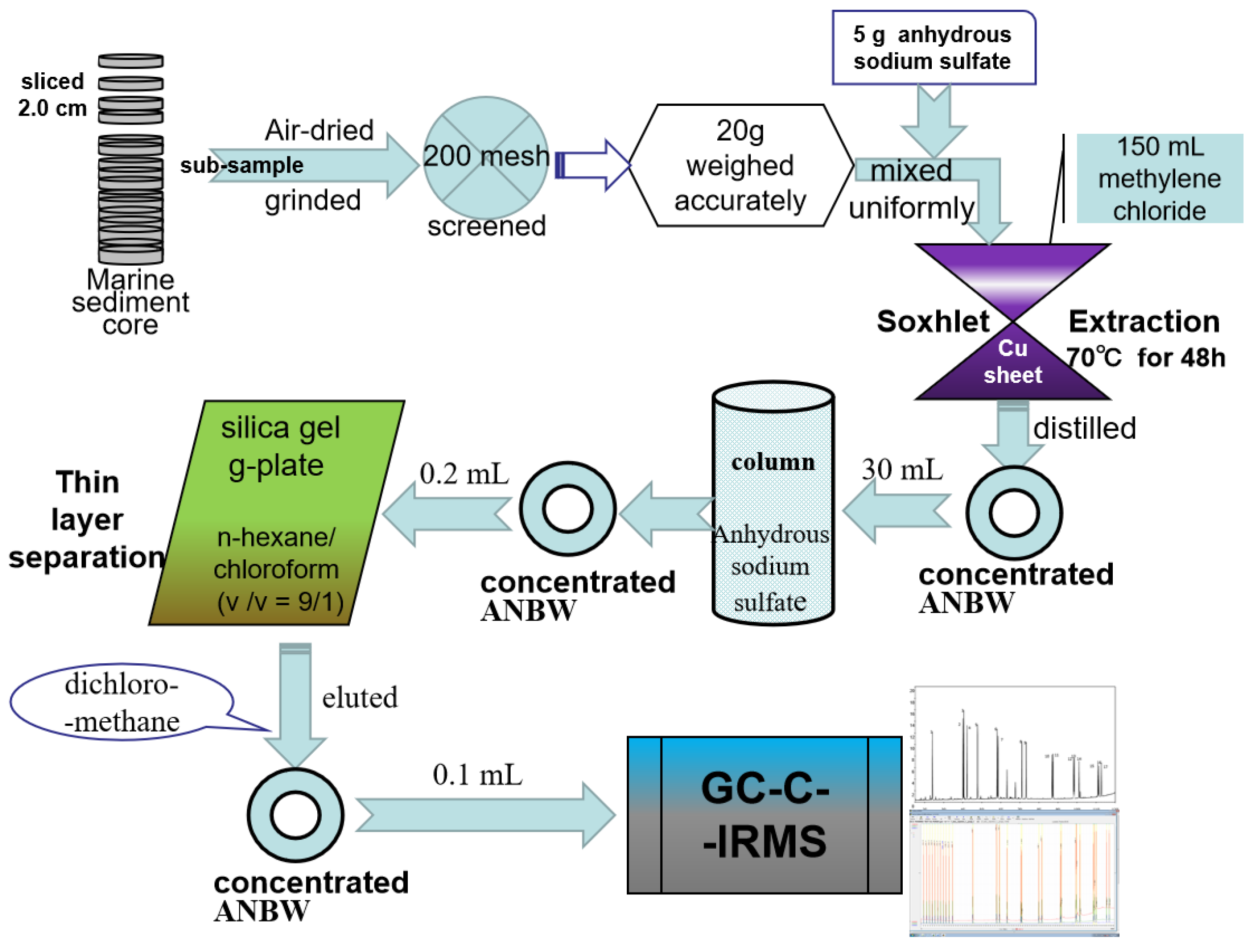
2.2. Treatment and Separation Procedures
2.2.1. Extraction of PAHs
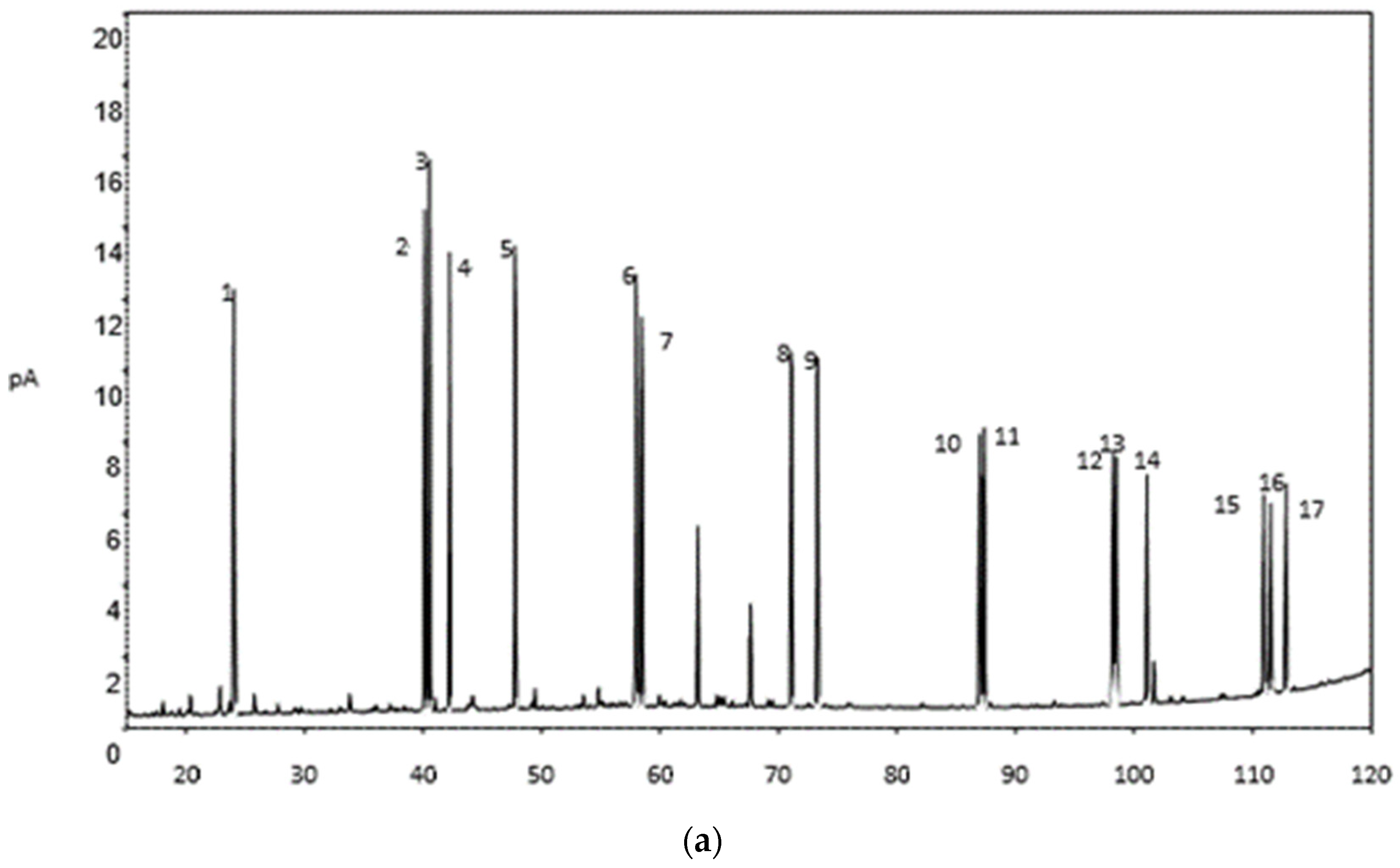
2.2.2. Determination of GC-C-IRMS
2.3. Quality Control
3. Results and Discussion
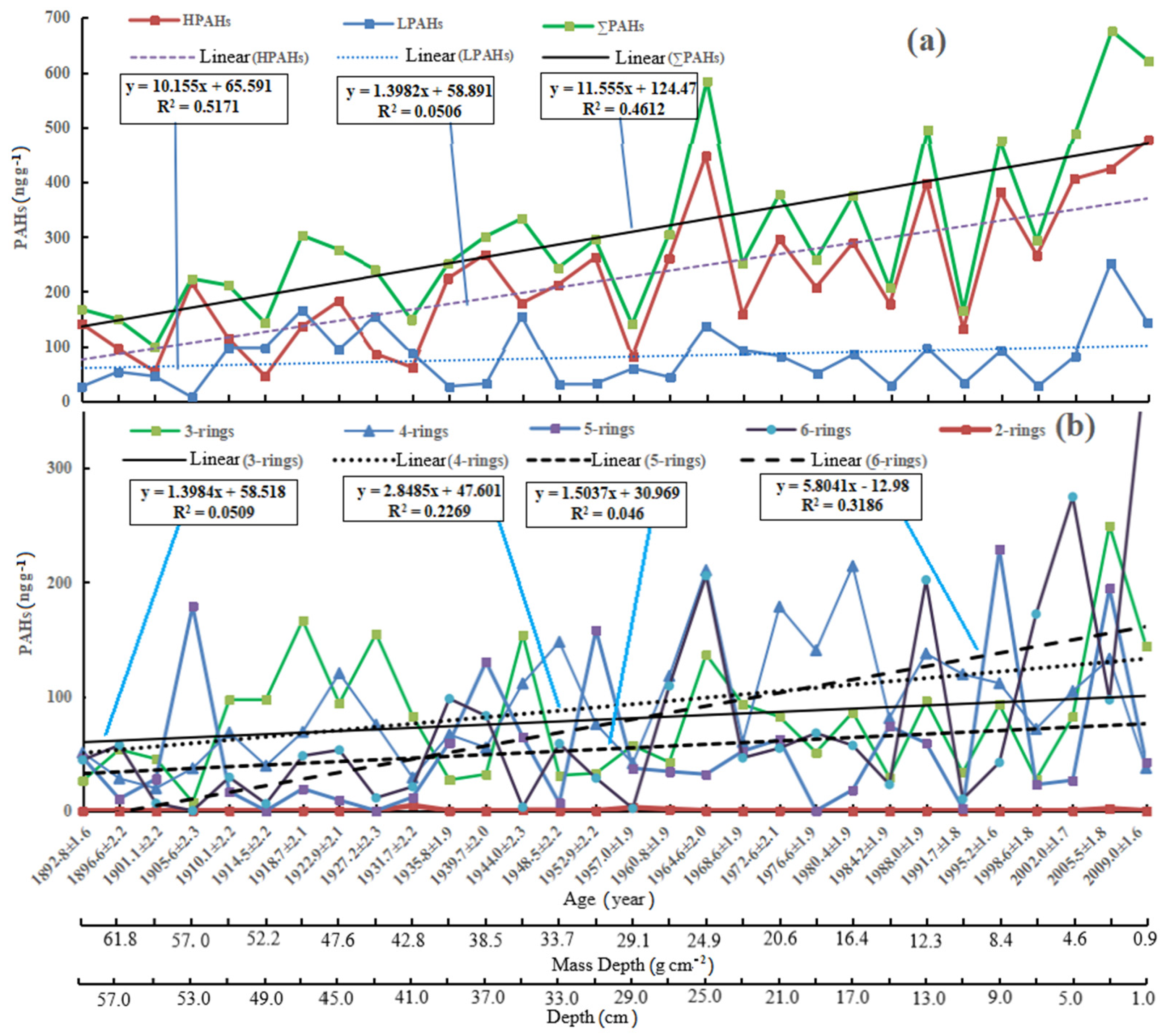
3.1. General Trends of PAHs in Sediment Core
3.2. Molecular Ratios of Specific Aromatic Compounds and Possible Sources
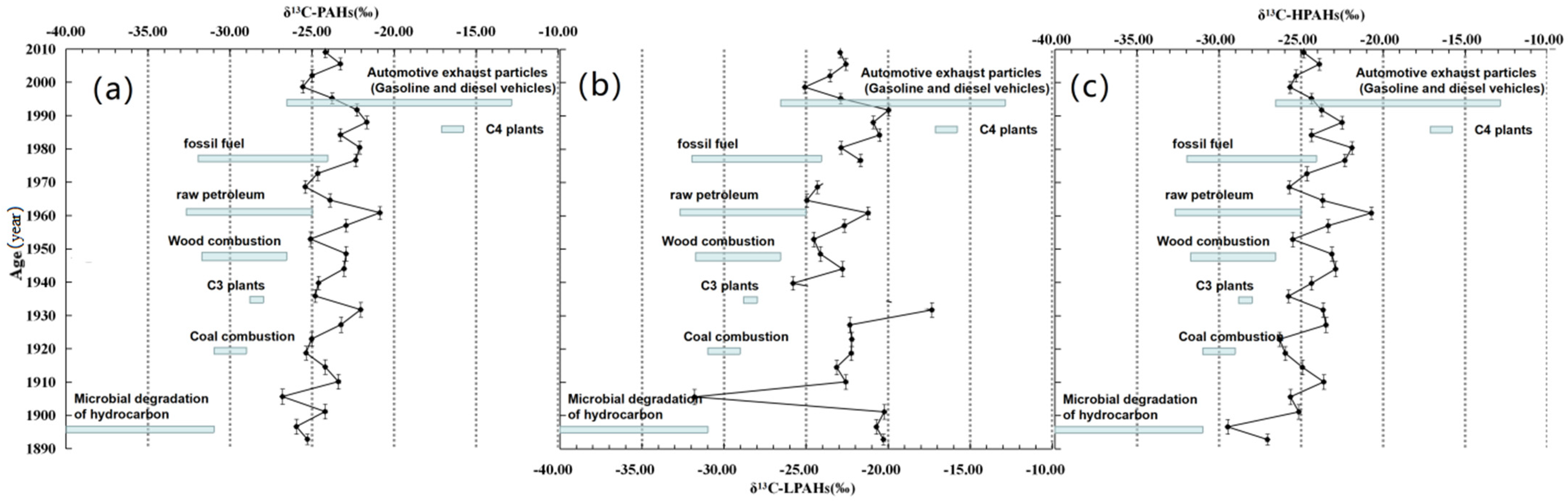
3.3. Molecular and Isotopic Compositions (Compound-Specific δ13C Compositions)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelboin, H.V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: Role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev. 1980, 60, 1107–1166. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fernández, A.C.; Sprovieri, M.; Piazza, R.; Frignani, M.; Sanchez-Cabeza, J.-A.; Feo, M.L.; Bellucci, L.G.; Marco, V.; Pérez-Bernal, L.H.; Páez-Osuna, F. 210Pb-derived history of PAH and PCB accumulation in sediments of a tropical inner lagoon (Las Matas, Gulf of Mexico) near a major oil refinery. Geochim. Cosmochim. Acta 2012, 82, 136–153. [Google Scholar] [CrossRef]
- Stark, A.; Abrajano, T., Jr.; Hellou, J.; Metcalf-Smith, J.L. Molecular and Isotopic Characterization of Polycyclic Aromatic Hydrocarbon Distribution and Sources at the International Segment of the St. Lawrence River. Org. Geochem. 2003, 34, 225–237. [Google Scholar] [CrossRef]
- USEPA. Methods for Chemical Analysis of Water and Wastes; EPA: Cincinnati, OH, USA, 1982.
- Buczyńska, A.J.; Geypens, B.; Van Grieken, R.; De Wael, K. Stable carbon isotopic ratio measurement of polycyclic aromatic hydrocarbons as a tool for source identification and apportionment—A review of analytical methodologies. Talanta 2013, 105, 435–450. [Google Scholar] [CrossRef]
- O’Malley, P.; Abrajano, T.A., Jr.; Hellou, J. Determination of the 13C/12C ratios of individual PAH from environmental samples, can PAH sources be apportioned? Org. Geochem. 1994, 21, 809–822. [Google Scholar] [CrossRef]
- O’Malley, V.P.; Abrajano, T.A.; Hellou, J. Stable carbon isotopic apportionment of individual polycyclic aromatic hydrocarbons in St. John’s Harbour, Newfoundland. Environ. Sci. Technol. 1996, 30, 634–639. [Google Scholar] [CrossRef]
- O’Malley, V.P.; Burke, R.A.; Schlotzhauer, W.S. Using GC-MS/combustion/IRMS to determine the 13C/12C ratios of individual hydrocarbons produced from the combustion of biomass materials-application to biomass burning. Org. Geochem. 1997, 27, 567–581. [Google Scholar] [CrossRef]
- Walker, S.; Dickhut, R.; Chisholmbrause, C.; Sylva, S.; Reddy, C. Molecular and Isotopic Identification of PAH Sources in a highly industrialized urban estuary. Org. Geochem. 2005, 36, 619–632. [Google Scholar] [CrossRef]
- Bekaert, C.; Rast, C.; Ferrier, V.; Bispo, A.; Jourdain, M.J.; Vasseur, P. Use of in vitro (Ames and Mutatox tests) and in vivo (Amphibian Micronucleus test) assays to assess the genotoxicity of leachates from a contaminated soil. Org. Geochem. 1999, 30, 953–962. [Google Scholar] [CrossRef]
- Bispo, A.; Jourdain, M.J.; Jauzein, M. Toxicity and genotoxicity of industrial soils polluted by polycyclic aromatic hydrocarbons (PAHs). Org. Geochem. 1999, 30, 947–952. [Google Scholar] [CrossRef]
- Khalili, N.R.; Scheff, P.A.; Holsen, T.M. PAH source fifingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 533–542. [Google Scholar] [CrossRef]
- Bi, X.H.; Sheng, G.Y.; Peng, P.A.; Chen, Y.J.; Zhang, Z.Q.; Fu, J.M. Distribution of particulate- and vapor-phase n-alkanes and polycyclic aromatic hydrocarbons in urban atmosphere of Guangzhou, China. Atmos. Environ. 2003, 37, 289–298. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, Y.J.; Kang, C.H. Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmos. Environ. 2002, 36, 2917–2924. [Google Scholar] [CrossRef]
- McRae, C.; Snape, C.E.; Sun, C.G.; Fabbri, D.; Tartari, D.; Trombini, C.; Fallick, A.E. Use of Compound-Specific Stable Isotope Analysis to Source Anthropogenic Natural Gas-Derived Polycyclic Aromatic Hydrocarbons in a Lagoon Sediment. Environ. Sci. Technol. 2000, 34, 4684–4686. [Google Scholar] [CrossRef]
- McRae, C.; Sun, C.G.; Mc Millan, C.F.; Snape, C.E.; Fallick, A.E. Sourcing of Fossil Fuel-Derived PAH in the Environment. Polycycl. Aromat. Compd. 2000, 20, 90–97. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namiesnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Freeman, K.H.; Hayes, J.M. Fractionation of carbon isotopes by phytoplankton and estimates of ancient CO2 levels. Glob. Biogeochem. Cycles 1992, 6, 185–198. [Google Scholar] [CrossRef]
- Freeman, K.H.; Hayes, J.M.; Trendel, J.; Albrecht, P. Evidence from carbon isotope measurements for diverse origin of sedimentary hydrocarbons. Nature 1990, 343, 254–256. [Google Scholar] [CrossRef]
- Hayes, J.M.; Freeman, K.H.; Popp, B.N.; Hoham, C.H. Compound-specific isotopic analyses: A novel tool for reconstruction of ancient biogeochemical processes. Org. Geochem. 1990, 16, 1115–1128. [Google Scholar] [CrossRef]
- Hayes, J.M. Factors controlling 13C contents of sedimentary organic compounds: Principles and evidence. Mar. Geol. 1993, 113, 111–112. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Budzinski, H.; Garrigues, P.; Eglinton, T.I. Ancient polycyclic aromatic hydrocarbons in modern soils: 13C, 14C and biomarker evidence. Org. Geochem. 1997, 26, 353–359. [Google Scholar] [CrossRef]
- McRae, C.; Snape, C.E.; Fallick, A.E. Variations in the stable isotope ratios of specific aromatic and aliphatic hydrocarbons from coal conversion processes. Analyst 1998, 7, 1519–1523. [Google Scholar] [CrossRef]
- Ballentine, D.C.; Macko, S.A.; Turekian, V.C.; Gilhooly, W.P.; Martincigh, B. Compound specific isotope analysis of fatty acids and polycyclic aromatic hydrocarbons in aerosols: Implications for biomass burning. Org. Geochem. 1996, 25, 97–104. [Google Scholar] [CrossRef]
- Ballentine, D.C.; Macko, S.A.; Turekian, V.C. Variability of stable carbon isotopic s in individual fatty acids from combustion of Ca and C3 plants: Implications for biomass burning. Chem. Geol. 1998, 152, 151–161. [Google Scholar] [CrossRef]
- Okuda, T.; Kumata, H.; Zakaria, M.P.; Naraoka, H.; Ishiwatari, R.; Takada, H. Source identification of malaysian atmospheric polycyclic aromatic hydrocarbons nearby forest fires using molecular and isotopic compositions. Org. Geochem. 2002, 36, 611–618. [Google Scholar] [CrossRef]
- Peng, L.; Bai, Z.P.; Zhu, T.; Xu, Y.C.; Li, J.; Feng, Y.C. Origin of atmospheric polycyclic aromatic hydrocarbons (PAHs) in two Chinese cities using compound-specific stable carbon isotopic analysis. Environ. Sci. 2004, 25, 16–20, (In Chinese with English Abstract). [Google Scholar]
- Peng, L.; Li, J.; Zhu, T.; Bai, Z.P.; Xu, Y.C.; Feng, Y.C. Carbon isotope characteristics of polycyclic aromatic hydrocarbons in atmospheric particles and their source analysis in urban area of Zhengzhou City. China Environ. Sci. 2005, 25, 106–109, (In Chinese with English Abstract). [Google Scholar]
- Mcrae, C.; Sun, C.; Snape, C.E.; Fallick, A.E.; Taylor, D. δ13C values of coal-derived PAHs from different processes and their application to source apportionment. Org. Geochem. 1999, 30, 881–889. [Google Scholar] [CrossRef]
- Qiu, Y.W. The characteristics of nutrients variation in the Daya Bay. Acta Oceanol. Sin. 2001, 23, 85–93, (In Chinese with English Abstract). [Google Scholar]
- Xu, G.Z. Environment and Resource in Daya Bay; Anhui Science and Technology Press: Hefei, China, 1989. (In Chinese) [Google Scholar]
- Wang, X.P.; Cai, W.G.; Lin, Q.; Jia, X.P.; Zhou, G.J.; Gan, J.L.; Lu, X.Y. The distribution variation of the nutrition salts in the waters of Daya Bay. Trans. Ocean. Limnol. 1996, 4, 20–27, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wang, Y.S.; Lou, Z.P.; Sun, C.C.; Sun, S. Ecological environment changes in Daya Bay, China, from 1982 to 2004. Mar. Pollut. Bull. 2008, 56, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Lou, Z.P.; Sun, C.C.; Wu, M.L.; Han, S.H. Multivariate statistical analysis of water quality and phytoplankton characteristics in Daya Bay, China, from 1999 to 2002. Oceanologia 2006, 48, 193–211. [Google Scholar]
- Wang, Y.S.; Wang, Z.D.; Huang, L.M. Environment changes and trends in Daya Bay in recent 20 years. J. Trop. Oceanogr. 2004, 23, 85–95, (In Chinese with English Abstract). [Google Scholar]
- Wang, Z.Y.; Yan, W.; Chi, J.S.; Zhang, G. Spatial and vertical distribution of organochlorine pesticides in sediments from Daya Bay, South China. Mar. Pollut. Bull. 2008, 56, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.L.; Chen, S.Y. Petroleum pollution in surface sediments of Daya Bay, South China, revealed by chemical fingerprinting of aliphatic and alicyclic hydrocarbons. Estuar. Coast. Shelf Sci. 2008, 80, 95–102. [Google Scholar] [CrossRef]
- Song, X.Y.; Huang, L.M.; Zhang, J.L.; Huang, X.P.; Zhang, J.B.; Yin, J.Q.; Tan, Y.H.; Liu, S. Variation of phytoplankton biomass and primary production in Daya Bay during spring and summer. Mar. Pollut. Bull. 2004, 49, 1036–1044. [Google Scholar] [CrossRef]
- Zheng, B.J. The Damage of Petroleum Pollution to the Sea Waters of Daya Bay and Protective Measures. J. Huizhou Univ. (Nat. Sci.) 2002, 22, 78–81, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Chi, J.S.; Yan, W.; Zhang, G.; Guo, L.L.; Liu, G.Q.; Liu, X.; Zou, S.C. High-resolution sedimentary record of PAHs and OCPs in Daya Bay, China. J. Trop. Oceanogr. 2005, 24, 44–52, (In Chinese with English Abstract). [Google Scholar]
- Qiu, Y.W.; Zhou, J.L.; Maskaoui, K.; Hong, H.S.; Wang, Z.D. Distribution of polycyclic aromatic hydrocarbons in water and sediments from Daya Bay and their ecological hazard assessment. China. J. Trop. Oceanogr. 2004, 23, 72–80, (In Chinese with English Abstract). [Google Scholar]
- Sun, R.X.; Ke, C.L.; Gu, Y.G.; Lu, T.T.; Du, F.Y.; Ma, S.W.; Lin, Q. Residues and risk assessment of polycyclic aromatic hydrocarbons in the surface sediments and marine organisms from Dapeng Bay, Shenzhen. Chin. J. Environ. Sci. 2013, 34, 3832–3839, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Tang, J.Y.; Liu, X.D.; Zhou, L.M.; Zhou, K.; Zhao, Z.Y. Risk assessment research of polycyclic aromatic hydrocarbons in marine surface sediments of Shenzhen nearshore. Mar. Environ. Sci. 2017, 36, 838–843, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Wang, P.; Lin, Q.; Ke, C.L. Characteristics and ecological risk of polycyclic aromatic hydrocarbons in surface sediments from Dapeng’ao Bay of Daya Bay. Guizhou Agric. Sci. 2010, 38, 208–212, (In Chinese with English Abstract). [Google Scholar]
- Yan, W.; Chi, J.S.; Wang, Z.Y.; Huang, W.X.; Zhang, G. Spatial and temporal distribution of polycyclic aromatic hydrocarbons (PAHs) in sediments from Daya Bay, South China. Environ. Pollut. 2009, 157, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Maskaoui, K.; Qiu, Y.W.; Hong, H.S.; Wang, Z.D. Polychlorinated biphenyl congeners and organochlorine insecticides in the water column and sediments of Daya Bay, China. Environ. Pollut. 2001, 113, 373–384. [Google Scholar] [CrossRef]
- Zhou, J.L.; Maskaoui, K. Distribution of polycyclic aromatic hydrocarbons in water and surface sediments from Daya Bay, China. Environ. Pollut. 2003, 121, 269–281. [Google Scholar] [CrossRef]
- Zhou, P.; Li, D.M.; Li, H.T.; Fang, H.D.; Huang, C.G. Distribution of radionuclides in a marine sediment core off the waterspout of the nuclear power plants in Daya Bay, northeastern South China Sea. J. Environ. Radioact. 2015, 145, 102–112. [Google Scholar] [CrossRef]
- Zhou, P.; Li, D.M.; Zhao, L.; Li, H.T.; Ni, Z.X.; Zhao, F.; Yu, H.S.; Li, X.M. A 120-year sedimentary record and its environmental implications, in a dated marine sediment core from Daya Bay in the northeastern South China Sea. Mar. Pollut. Bull. 2019, 145, 248–253. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.Y.; Cao, J.P. Compound-specific carbon stable isotope analysis of 16 polycyclic aromatic hydrocarbons in sediments by Gas Chromatography- Combustion-Isotope Ratio Mass Spectrometry (GC-C-IRMS). Pet. Geol. Exp. 2018, 40, 532–537, (In Chinese with English Abstract). [Google Scholar]
- Wang, X.Y. Determination of Compound-Specific Carbon Isotope of Polycyclic Aromatic Hydrocarbons in Marine Sediments from the South China Sea. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2016. (In Chinese with English Abstract). [Google Scholar]
- SY/T5118-1995; Standard of Petroleum and Natural Gas Industry of the People’s REPUBLIC of China. Determination of Bitumen from Rocks by Chloroform Extraction. China National Petroleum Corporation: Beijing, China, 1995.
- Lu, T.T.; Lin, Q.; Ke, C.L.; Sun, R.X. Polycyclic aromatic hydrocarbons and risk assessment in the surface sediments from Lingdingyang, Pearl River Estuary. J. Fish. Sci. China 2012, 19, 336–347, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Zhang, J.D.; Wang, Y.H.; Cheng, H.; Jiang, Z.Y.; Sun, C.C.; Wu, M.L. Distribution and sources of the polycyclic aromatic hydrocarbons in6 the sediments of the Pearl River estuary, China. Ecotoxicology 2015, 24, 1643–1649. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, X.W.; Lin, L.; Zou, S.C.; Li, Y.; Yang, Q.S.; Luan, T.G. Characterizing the parent and alkyl polycyclic aromatic hydrocarbons in the Pearl River Estuary, Daya Bay and northern South China Sea: Influence of riverine input. Environ. Pollut. 2015, 199, 66–72. [Google Scholar] [CrossRef]
- Lu, T.T. Study on the Polycyclic Aromatic Hydrocarbons and Its Ecological Risk in Sediments from the Coast of Guangdong Province. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2012; pp. 1–78. (In Chinese). [Google Scholar]
- Arcos, J.C.; Argus, M.G. Chemical Induction of Cancer, Structural Bases and Biological Mechanisms; Academic Press: New York, NY, USA, 1975; Volume IIA. [Google Scholar]
- Denissenko, M.F.; Pao, A.; Tang, M.; Pfeifer, G.P. Preferential formation of benzo(a)pyrene adducts at lung cancer mutational hotspots in P53. Science 1996, 274, 430–432. [Google Scholar] [CrossRef]
- Peng, Y.H.; Sun, L.H.; Chen, H.R.; Wang, Z.D. Study on Eutrophication and Change of Nutrients in the Daya Bay. Mar. Sci. Bull. 2002, 21, 44–49, (In Chinese with English Abstract). [Google Scholar]
- Wang, Z.H.; Matsuoka, K.; Qi, Y.Z.; Chen, J.F.; Lu, S.H. Dinoflagellate cyst records in recent sediments from Daya Bay, South China Sea. Phycol. Res. 2004, 52, 396–407. [Google Scholar] [CrossRef]
- Baumard, P.; Budzinski, H.; Michon, Q.; Garrigues, P.; Burgeot, T.; Bellocq, J. Origin and bioavailability of PAHs in the Mediterranean Sea from mussel and sediment records. Estuar. Coast. Shelf Sci. 1998, 47, 77–90. [Google Scholar] [CrossRef]
- Benlahcen, K.T.; Chaoui, A.; Budzinski, H.; Garrigues, H. Distribution and sources of polycyclic aromatic hydrocarbons in some Mediterranean coastal sediments. Mar. Pollut. Bull. 1997, 34, 298–305. [Google Scholar] [CrossRef]
- Cheng, X.H.; Forsythe, J.; Peterkin, E. Some factors affecting SPME analysis and PAHs in Philadelphia’s urban waterways. Water Res. 2013, 47, 2331–2340. [Google Scholar] [CrossRef]
- Gschwend, P.M.; Hites, R.A. Fluxes of polycyclic aromatic hydrocarbons to marine and lacustrine sediments in the northeastern United States. Geochim. Cosmochim. Acta 1981, 45, 2359–2367. [Google Scholar] [CrossRef]
- Garrigues, P.; Budzinski, H.; Manitz, M.P.; Wise, S.A. Pyrolytic and petrogenic inputs in recent sediments: A definitive signature through phenanthrene and chysene compound distribution. Polycycl. Aromat. Compd. 1995, 7, 275–284. [Google Scholar] [CrossRef]
- Klamer, H.J.C.; Fomsgaard, L. Geographical distribution of chlorinated biphenyls (CBs) and polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the Humber Plume, North Sea. Mar. Pollut. Bull. 1993, 26, 201–206. [Google Scholar] [CrossRef]
- Liu, L.Y.; Wang, J.Z.; Wei, G.L.; Guan, Y.F.; Wong, C.S.; Zeng, E.Y. Sediment records of polycyclic aromatic hydrocarbons (PAHs) in the continental shelf of China: Implications for evolving anthropogenic impacts. Environ. Sci. Technol. 2012, 46, 6497–6504. [Google Scholar] [CrossRef] [PubMed]
- Sicre, M.A.; Marty, J.C.; Saliot, A.; Aparicio, X.; Grimalt, J.; Albaiges, J. Aliphatic and aromatic hydrocarbons in different sized aerosols over the mediterranean sea: Occurrence and origin. Atmos. Environ. 1987, 21, 2247–2259. [Google Scholar] [CrossRef]
- Soclo, H. Etude De La Distribution Des Hydrocarbures Aromatiques Polycycliques Dans Les Seediments Marins Reecents, Identifucation Des Sources. Ph.D. Thesis, University Bourdeaux I, Bourdeaux, France, 1986; p. 158. [Google Scholar]
- Readman, J.W.; Mantoura, R.F.C.; Rhead, M.M. A record of polycyclic aromatic hydrocarbon (PAH) pollution obtained from accreting sediments of the Tamar Estuary, U.K.: Evidence for non-equilibrium behaviour of PAH. Sci. Total Environ. 1987, 66, 73–94. [Google Scholar] [CrossRef]
- Readman, J.W.; Fillmann, G.; Tolosa, L. Petroleum and PAHs contamination of the Black Sea. Mar. Pollut. Bull. 2002, 44, 48–62. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R. PAHs in the Fraser River Basin: Acritical appraisal of PAH ratios as in dicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Budzinski, H.; Jones, I.; Bellocq, J. Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde Estuary. Mar. Chem. 1997, 58, 85–97. [Google Scholar] [CrossRef]
- Marr, L.C.; Kirchstetter, T.W.; Harley, R.A.; Miguel, A.H.; Hering, S.V.; Hammond, S.K. Characterization of polycyclic aromatic hydrocarbons in motor vehicles fuels and exhaust emissions. Environ. Sci. Technol. 1999, 33, 3091–3099. [Google Scholar] [CrossRef]
- Nielsen, T. Traffic contributions of polycyclic aromatic hydrocarbons in the centre of a large city. Atmos. Environ. 1996, 30, 3481–3490. [Google Scholar] [CrossRef]
- Dickhut, R.M.; Canuel, E.A.; Gustafson, K.E.; Liu, K.; Arzayus, K.M.; Walker, S.E.; Edgecombe, G.; Gaylor, M.O.; MacDonald, E.H. Automatic sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay region. Environ. Sci. Technol. 2000, 34, 4635–4640. [Google Scholar] [CrossRef]
- Okuda, T.; Kumata, H.; Naraoka, H.; Takada, H. Origin of atmospheric polycyclic aromatic hydrocarbons (PAHs) in Chinese cities solved by compound-specific stable carbon isotopic analyses. Org. Geochem. 2002, 33, 1737–1745. [Google Scholar] [CrossRef]
- Okuda, T.; Takada, H.; Naraoka, H. Thermodynamic Behavior of Stable Carbon Isotopic s of Individual Polycyclic Aromatic Hydrocarbons Derived from Automobiles. Polycycl. Aromat. Compd. 2003, 23, 219–236. [Google Scholar] [CrossRef]
- Okuda, T.; Kumata, H.; Naraoka, H.; Takada, H. Molecular and compound-specific stable carbon isotope ratio of polycyclic aromatic hydrocarbons (PAHs) in the atmosphere in suburban areas. Geochem. J. 2004, 38, 89–100. [Google Scholar] [CrossRef]
- Wang, D.R. Stable Isotope Geochemistry in Oil and Gas; Petroleum Industry Press: Beijing, China, 2000; pp. 191–192. (In Chinese) [Google Scholar]
- Yang, J.D.; Xu, S.J. Isotopes and Global Environmental Change; Geology Press: Beijing, China, 2007; pp. 32–33. (In Chinese) [Google Scholar]
- Xu, J.J.; Xu, S.N.; Li, C.H.; Liu, Y.; Lin, L. Ecological Environment Quality Assessment on Petrochemical Sewage Discharge Waters of Daya Bay in Winter. J. Agro-Environ. Sci. 2013, 32, 1456–1466, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Xu, S.N.; Chen, Z.Z.; Lin, L.; Xu, J.J.; Li, C.H. Ecosystem health assessment of the petrochemical sewage waters in Daya Bay. Acta Ecol. Sin. 2016, 36, 1421–1430, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Yang, W.C.; Huang, D.J.; Chen, J.X.; Chen, X.Y.; Liu, W.; Wang, Y.S. Research on ecological environment quality in the sea area near the second petrochemical sewage pipeline discharge outlet in Daya Bay. J. Mar. Sci. 2019, 37, 85–91, (In Chinese with English Abstract). [Google Scholar] [CrossRef]




| Sea Area | Rang | Mean | Reference |
|---|---|---|---|
| Sediment core W2(2) from Daya Bay | 99.3~676.5 | 303.6 | In the study |
| Sediment from Daya Bay | 115~1134 | - | [41,46,47] |
| Surfance sediment from Daya Bay | 42.5~158.2 | 126.2 | [45] |
| Surfance sediment in Dapeng’ao bay | 237.3~1139 | - | [44] |
| 256.7~744.1 | - | ||
| Sediment from Daya Bay | 140~491 | 310 ± 92.4 | [55] |
| Sediment from Dapeng Bay | 216.6~1314 | 572.5 | [42] |
| Sediment core from Daya Bay | 77.0~306.0 | 192.0 | [40,45] |
| Sediments of Shenzhen nearshore including Daya Bay | 227.5~3897 | 870. 6 | [43] |
| Sediment core No. 10 from Daya Bay | 118.1~319.9 | 210.2 | [45] |
| Sediment from the Lingdingyang of the Pearl River estuary | 143.9~522.7 | 287.05 | [53] |
| Sediment from thePearl River Estuary | 144.0~1289 | 430 ± 216 | [55] |
| 126.1~3829 | 563.5 | [54] | |
| Sediment from the northern South China sea | 274~335 | 304 | [55] |
| Sediment from the middle area in South China Sea | 276.4~792.2 | 430.6 | [56] |
| Molecular Ratios | Phe/Ant | Antt/(Ant + Phe) | Fluo/(Fluo + Pyr) | BgP/InP | InP/(InP + BaP) | BaA/Chr | BaA/(BaA + Chr) | |
|---|---|---|---|---|---|---|---|---|
| possible sources | >10 or 15 petrogenic source | <0.10 petrogenic source | <0.50 petroleum | Ratio >1 is relatively high in automotive exhaust particles | <0.20 petroleum | ≤0.40 Petrogenic origin | <0.20 petroleum | |
| 0.4–0.5 * liquid fossil fuel combustion | 0.2–0.5 * liquid fossil fuel combustion | 0.2–0.35 ** petroleum combustion | ||||||
| <10 pyrolytic sources | >0.10 pyrolytic source | >0.50 grass, wood, and coal combustion | >0.50 grass, wood, and coal combustion | >0.90 pyrolytic origin | >0.35 grass, wood, and coal combustion | |||
| our study | Mean Range # | 9.19 0.02–74.4 | 0.48 0.01–0.98 | 0.27 0.13–0.47 | 4.57 0.06–28.6 | 0.57 0.03–0.94 | 7.96 0.36–69.1 | 0.55 0.27–0.99 |
| reference | [64,69,72,73] | [68,72,73] | [74,75] | [72] | [64,76] | [71,72] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Li, D.; Wang, X.; Cao, J.; Huang, S.; Zhou, P. Assessment and Implication of PAHs and Compound-Specific δ13C Compositions in a Dated Marine Sediment Core from Daya Bay, China. Int. J. Environ. Res. Public Health 2022, 19, 4527. https://doi.org/10.3390/ijerph19084527
Lu Y, Li D, Wang X, Cao J, Huang S, Zhou P. Assessment and Implication of PAHs and Compound-Specific δ13C Compositions in a Dated Marine Sediment Core from Daya Bay, China. International Journal of Environmental Research and Public Health. 2022; 19(8):4527. https://doi.org/10.3390/ijerph19084527
Chicago/Turabian StyleLu, Yan, Dongmei Li, Xiaoyun Wang, Jianping Cao, Sheng Huang, and Peng Zhou. 2022. "Assessment and Implication of PAHs and Compound-Specific δ13C Compositions in a Dated Marine Sediment Core from Daya Bay, China" International Journal of Environmental Research and Public Health 19, no. 8: 4527. https://doi.org/10.3390/ijerph19084527
APA StyleLu, Y., Li, D., Wang, X., Cao, J., Huang, S., & Zhou, P. (2022). Assessment and Implication of PAHs and Compound-Specific δ13C Compositions in a Dated Marine Sediment Core from Daya Bay, China. International Journal of Environmental Research and Public Health, 19(8), 4527. https://doi.org/10.3390/ijerph19084527







