Amount and Frequency of Added Sugars Intake and Their Associations with Dental Caries in United States Adults
Abstract
:1. Introduction
2. Methods
2.1. Data Source
2.2. Measures
2.3. Statistical Analysis
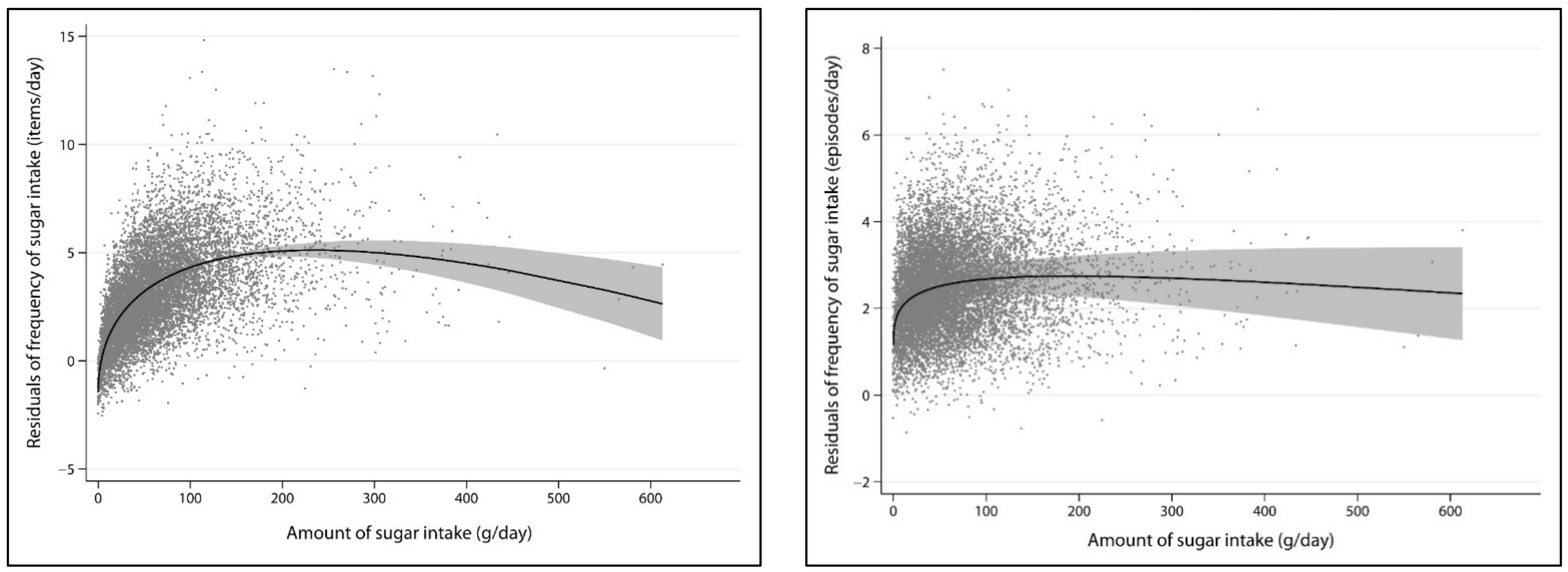
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guideline: Sugar Intake for Adults and Children; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Moynihan, P.J.; Kelly, S.A. Effect on caries of restricting sugars intake: Systematic review to inform WHO guidelines. J. Dent. Res. 2014, 93, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moores, C.J.; Kelly, S.A.M.; Moynihan, P.J. Systematic Review of the Effect on Caries of Sugars Intake: Ten-Year Update. J. Dent. Res. 2022, 18, 220345221082918. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2015; Volume 2022. [Google Scholar]
- Mela, D.J.; Woolner, E.M. Perspective: Total, Added, or Free? What Kind of Sugars Should We Be Talking About? Adv. Nutr. 2018, 9, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Livingstone, M.B. Associations between Meal and Snack Frequency and Diet Quality in US Adults: National Health and Nutrition Examination Survey 2003–2012. J. Acad. Nutr. Diet. 2016, 116, 1101–1113. [Google Scholar] [CrossRef]
- Leech, R.M.; Worsley, A.; Timperio, A.; McNaughton, S.A. The role of energy intake and energy misreporting in the associations between eating patterns and adiposity. Eur. J. Clin. Nutr. 2018, 72, 142–147. [Google Scholar] [CrossRef]
- Al-Zwaylif, L.H.; O’Toole, S.; Bernabé, E. Type and timing of dietary acid intake and tooth wear among American adults. J. Public Health Dent. 2018, 78, 214–220. [Google Scholar] [CrossRef]
- Van Loveren, C. Sugar Restriction for Caries Prevention: Amount and Frequency. Which Is More Important? Caries Res. 2019, 53, 168–175. [Google Scholar] [CrossRef]
- Lagerweij, M.; van Loveren, C. Chapter 7: Sugar and Dental Caries. Monogr. Oral Sci. 2020, 28, 68–76. [Google Scholar] [CrossRef]
- Stephan, R.M.; Miller, B.F. A quantitative method for evaluating physical and chemical agents which modify production of acids in bacterial plaques on human teeth. J. Dental Res. 1943, 22, 45–51. [Google Scholar] [CrossRef]
- Bernabé, E.; Vehkalahti, M.M.; Sheiham, A.; Lundqvist, A.; Suominen, A.L. The Shape of the Dose-Response Relationship between Sugars and Caries in Adults. J. Dent. Res. 2016, 95, 167–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dye, B.A.; Afful, J.; Thornton-Evans, G.; Iafolla, T. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2011–2014. BMC Oral Health 2019, 19, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, S.A.; Clemens, J.C.; Shimizu, M.; Friday, J.E.; Moshfegh, A.J. Food Patterns Equivalents Database 2015–2016: Methodology and User Guide; Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture: Beltsville, MD, USA, 2018. [Google Scholar]
- Bowman, S.A. Added sugars: Definition and estimation in the USDA Food Patterns Equivalents Databases. J. Food Compos. Anal. 2017, 64, 64–67. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Dwyer, J.; Terry, A.; Moshfegh, A.; Johnson, C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016, 7, 121–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulgoni, V.L., 3rd; Gaine, P.C.; Scott, M.O. Comparison of Various Methods to Determine Added Sugars Intake to Assess the Association of Added Sugars Intake and Micronutrient Adequacy. Nutrients 2020, 12, 2816. [Google Scholar] [CrossRef]
- Royston, P.; Ambler, G.; Sauerbrei, W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int. J. Epidemiol. 1999, 28, 964–974. [Google Scholar] [CrossRef] [Green Version]
- Royston, P.; Sauerbrei, W. Building multivariable regression models with continuous covariates in clinical epidemiology. Methods Inf. Med. 2005, 44, 561–571. [Google Scholar]
- Sauerbrei, W.; Royston, P.; Binder, H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat. Med. 2007, 26, 5512–5528. [Google Scholar] [CrossRef]
- Royston, P.; Sauerbrei, W. Multivariable Model-Building: A Pragmatic Approach to Regression Anaylsis Based on Fractional Polynomials for Modelling Continuous Variables; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Royston, P. Model selection for univariable fractional polynomials. Stata J. 2017, 17, 619–629. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S, discussion 1229S–1231S. [Google Scholar] [CrossRef]
- Powell, E.S.; Smith-Taillie, L.P.; Popkin, B.M. Added Sugars Intake Across the Distribution of US Children and Adult Consumers: 1977–2012. J. Acad. Nutr. Diet. 2016, 116, 1543–1550.e1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moynihan, P. Sugars and Dental Caries: Evidence for Setting a Recommended Threshold for Intake. Adv. Nutr. 2016, 7, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Head, D.; Devine, D.A.; Marsh, P.D. In silico modelling to differentiate the contribution of sugar frequency versus total amount in driving biofilm dysbiosis in dental caries. Sci. Rep. 2017, 7, 17413. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.; Zinn, C.; Schofield, G. The consumption of processed sugar- and starch-containing foods, and dental caries: A systematic review. Eur. J. Oral Sci. 2020, 128, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Halvorsrud, K.; Lewney, J.; Craig, D.; Moynihan, P.J. Effects of Starch on Oral Health: Systematic Review to Inform WHO Guideline. J. Dent. Res. 2019, 98, 46–53. [Google Scholar] [CrossRef] [PubMed]
| Categorical Variables | Excluded (n = 1107) | Study Sample (n = 10,514) | p-Value a | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | 0.823 | ||||
| Men | 563 | 49.1 | 5011 | 48.6 | |
| Women | 544 | 50.9 | 5503 | 51.4 | |
| Race/ethnicity | <0.001 | ||||
| White | 255 | 45.2 | 4358 | 67.5 | |
| Black | 298 | 17.8 | 2300 | 10.5 | |
| Hispanic | 374 | 24.8 | 2390 | 14.0 | |
| Mixed/Other | 180 | 12.2 | 1466 | 8.1 | |
| Education | <0.001 | ||||
| Less than high school | 330 | 24.0 | 1848 | 11.8 | |
| High school | 242 | 22.2 | 2263 | 20.3 | |
| More than high school | 529 | 53.9 | 6403 | 67.8 | |
| Poverty income ratio | <0.001 | ||||
| 0–100% | 89 | 37.3 | 2185 | 14.4 | |
| 101–200% | 69 | 34.5 | 2643 | 20.3 | |
| 201–300% | 24 | 12.3 | 1583 | 14.9 | |
| 301–400% | 13 | 9.0 | 1207 | 12.6 | |
| >400% | 12 | 7.0 | 2896 | 37.8 | |
| Dental attendance pattern | 0.708 | ||||
| Regular | 335 | 45.0 | 4211 | 46.2 | |
| Irregular | 547 | 55.0 | 6303 | 53.8 | |
| Numerical Variables | Mean | (SD) | Mean | (SD) | p-Value a |
| Age, years | 46.5 | (20.8) | 46.7 | (16.4) | 0.796 |
| Energy intake, kcal/day | 1950.4 | (911.5) | 2107.7 | (772.7) | <0.001 |
| Amount of intake b, g/day | 61.9 | (57.1) | 68.8 | (54.5) | 0.026 |
| Frequency of intake b, items/day | 4.8 | (2.4) | 5.4 | (2.3) | <0.001 |
| Frequency of intake b, episodes/day | 3.9 | (1.3) | 4.1 | (1.1) | 0.003 |
| DMFS, surfaces | 31.5 | (36.5) | 34.2 | (30.1) | 0.116 |
| DS, surfaces | 2.7 | (8.5) | 2.4 | (7.5) | 0.340 |
| Outcome | Models Compared a | df | Deviance | Deviance Difference | p-Value | Powers |
|---|---|---|---|---|---|---|
| Frequency of added | Omitted | 2 | 44,092.7 | 3474.5 | <0.001 | |
| sugars intake | Linear | 2 | 42,297.1 | 1678.9 | <0.001 | 1 |
| items/day | FP1 | 1 | 40,774.2 | 156.0 | <0.001 | 0 |
| FP2 | 0 | 40,618.2 | 0.0 | 0.5; 1 | ||
| Frequency of added | Omitted | 2 | 30,610.3 | 387.9 | <0.001 | |
| sugars intake | Linear | 2 | 30,461.6 | 239.2 | <0.001 | 1 |
| episodes/day | FP1 | 1 | 30,247.4 | 25.0 | <0.001 | 0 |
| FP2 | 0 | 30,222.5 | 0.0 | 0.5; 0.5 |
| Outcome | Indicator of Added Sugars Intake | Estimate | Model 1 a | Model 2A a | Model 2B a |
|---|---|---|---|---|---|
| DMFS | 1-SD change in amount | Rate Ratio | 1.12 | 1.11 | 1.11 |
| (54.5 g/day) | [95% CI] | [1.08–1.16] | [1.08–1.15] | [1.07–1.15] | |
| p-value | <0.001 | <0.001 | <0.001 | ||
| 1-SD change in frequency | Rate Ratio | 1.06 | 1.02 | ||
| (2.3 items/day) | [95% CI] | [1.03–1.09] | [0.99–1.04] | ||
| p-value | <0.001 | 0.301 | |||
| 1-SD change in frequency | Rate Ratio | 1.07 | 1.05 | ||
| (1.1 episodes/day) | [95% CI] | [1.04–1.09] | [1.02–1.08] | ||
| p-value | <0.001 | 0.001 | |||
| DS | 1-SD change in amount | Rate Ratio | 1.41 | 1.49 | 1.43 |
| (54.5 g/day) | [95% CI] | [1.32–1.52] | [1.38–1.61] | [1.33–1.54] | |
| p-value | <0.001 | <0.001 | <0.001 | ||
| 1-SD change in frequency | Rate Ratio | 1.09 | 0.91 | ||
| (2.3 items/day) | [95% CI] | [0.97–1.22] | [0.81–1.02] | ||
| p-value | 0.135 | 0.101 | |||
| 1-SD change in frequency | Rate Ratio | 1.01 | 0.95 | ||
| [95% CI] | [0.92–1.11] | [0.86–1.04] | |||
| p-value | 0.867 | 0.269 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alosaimi, N.; Bernabé, E. Amount and Frequency of Added Sugars Intake and Their Associations with Dental Caries in United States Adults. Int. J. Environ. Res. Public Health 2022, 19, 4511. https://doi.org/10.3390/ijerph19084511
Alosaimi N, Bernabé E. Amount and Frequency of Added Sugars Intake and Their Associations with Dental Caries in United States Adults. International Journal of Environmental Research and Public Health. 2022; 19(8):4511. https://doi.org/10.3390/ijerph19084511
Chicago/Turabian StyleAlosaimi, Norah, and Eduardo Bernabé. 2022. "Amount and Frequency of Added Sugars Intake and Their Associations with Dental Caries in United States Adults" International Journal of Environmental Research and Public Health 19, no. 8: 4511. https://doi.org/10.3390/ijerph19084511
APA StyleAlosaimi, N., & Bernabé, E. (2022). Amount and Frequency of Added Sugars Intake and Their Associations with Dental Caries in United States Adults. International Journal of Environmental Research and Public Health, 19(8), 4511. https://doi.org/10.3390/ijerph19084511







