The Emerging Threat of Plasmodium knowlesi Malaria Infection: A Concept Paper on the Vulnerable Factors in Human
Abstract
1. Research Manuscript Section
1.1. Introduction
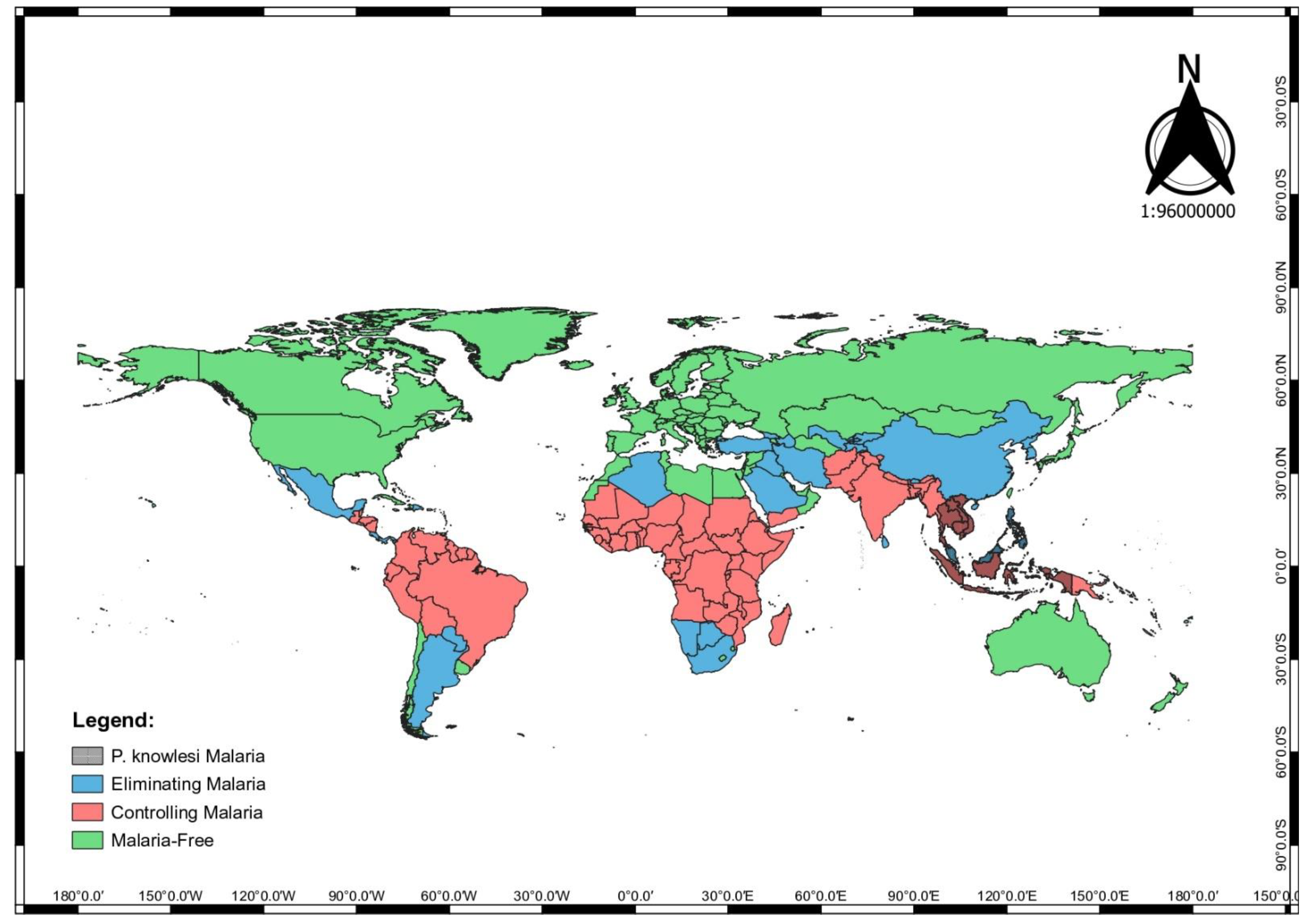
1.2. Vulnerability Factors of P. knowlesi Infection in Human
1.3. Human Genetic
1.4. Human Immune Response
1.5. Sociodemographic
1.6. Socioeconomic
1.7. Environmental Factor
1.8. Social Context and Belief
1.9. Human Behaviour
1.10. Theories and Models to Support the Concept Paper
1.11. Social Determinant of Health Framework
1.12. Theory of Planned Behaviour
1.13. Social Cognitive Theory
1.14. The Protection Motivation Theory
1.15. The Ideation Model
1.16. Murdock’s Model: The Aetiology of Illness (1978)
1.17. The Cytokine Theory
1.18. The Explanatory Model
- How can you get malaria infection?
- Why do you think you have been exposed to malaria infection?
- Why is malaria infection serious?
- How are your symptoms before you were diagnosed with malaria? [prompt: Do they get worst?]
- If you have re-infection, how serious can it get?
- Why is early treatment necessary?
- How to prevent malaria infection?
2. Discussion
3. Conclusions
4. Study Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Singh, B.; Sung, L.K.; Matusop, A.; Radhakrishnan, A.; Shamsul, S.S.; Cox-Singh, J.; Thomas, A.; Conway, D.J. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2004, 363, 1017–1024. [Google Scholar] [CrossRef]
- Amir, A.; Cheong, F.W.; de Silva, J.R.; Liew, J.W.K.; Lau, Y.L. Plasmodium knowlesi malaria: Current research perspectives. Infect. Drug Resist. 2018, 11, 1145. [Google Scholar] [CrossRef]
- Singh, B.; Daneshvar, C. Human infections and detection of Plasmodium knowlesi. Clin. Microbiol. Rev. 2013, 26, 165–184. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report: 20 Years of Global Progress and Challenges; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Chin, A.Z.; Maluda, M.C.M.; Jelip, J.; Jeffree, M.S.; Culleton, R.; Ahmed, K. Malaria elimination in Malaysia and the rising threat of Plasmodium knowlesi. J. Physiol. Anthropol. 2020, 39, 36. [Google Scholar] [CrossRef] [PubMed]
- Grigg, M.J.; Cox, J.; William, T.; Jelip, J.; Fornace, K.M.; Brock, P.M.; von Seidlein, L.; Barber, B.E.; Anstey, N.M.; Yeo, T.W.; et al. Individual-level factors associated with the risk of acquiring human Plasmodium knowlesi malaria in Malaysia: A case-control study. Lancet Planet. Health 2017, 1, 97–104. [Google Scholar] [CrossRef]
- Rajahram, G.S.; Cooper, D.J.; William, T.; Grigg, M.J.; Anstey, N.M.; Barber, B.E. Deaths from Plasmodium knowlesi malaria: Case series and systematic review. Clin. Infect. Dis. 2019, 69, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Barber, B.E.; William, T.; Jikal, M.; Jilip, J.; Dhararaj, P.; Menon, J.; Yeo, T.W.; Anstey, N.M. Plasmodium knowlesi malaria in children. Emerg. Infect. Dis. 2011, 17, 814. [Google Scholar] [CrossRef]
- Muller, M.; Schlagenhauf, P. Plasmodium knowlesi in travellers, update 2014. Int. J. Infect. Dis. 2014, 22, 55–64. [Google Scholar] [CrossRef][Green Version]
- Bird, E.M.; Parameswaran, U.; William, T.; Khoo, T.M.; Grigg, M.J.; Aziz, A.; Marfurt, J.; Yeo, T.W.; Auburn, S.; Anstey, N.M.; et al. Transfusion-transmitted severe Plasmodium knowlesi malaria in a splenectomised patient with Beta-Thalassaemia Major in Sabah, Malaysia: A case report. Malar. J. 2016, 15, 357. [Google Scholar] [CrossRef]
- Van den Eede, P.; Van, H.N.; Van Overmeir, C.; Vythilingam, I.; Duc, T.N.; Hung, L.X.; Manh, H.N.; Anne, J.; D’Alessandro, U.; Erhart, A. Human Plasmodium knowlesi infections in young children in Central Vietnam. Malar. J. 2009, 8, 249. [Google Scholar] [CrossRef]
- Shimizu, S.; Chotirat, S.; Dokkulab, N.; Hongchad, I.; Khowsroy, K.; Kiattibutr, K.; Maneechai, N.; Manopwisedjaroen, K.; Petchvijit, P.; Phumchuea, K.; et al. Malaria cross-sectional surveys identified asymptomatic infections of Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi in Surat Thani, a southern province of Thailand. Int. J. Infect. Dis. 2020, 96, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.E. History of the discovery of the malaria parasites and their vectors. Parasites Vectors 2010, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Monroe, A.; Moore, S.; Koenker, H.; Lynch, M.; Ricotta, E. Measuring and characterizing night time human behaviour as it relates to residual malaria transmission in Sub-Saharan Africa: A review of the published literature. Malar. J. 2019, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Naserrudin, N.A.; Hod, R.; Jeffree, M.S.; Ahmed, K.; Culleton, R.; Hassan, M.R. The role of human behavior in Plasmodium knowlesi malaria infection: A systematic review. Int. J. Environ. Res. Public Health 2022, 19, 3675. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.H.; Manin, B.O.; Vythilingam, I.; Fornace, K.; Drakeley, C.J. Effect of different habitat types on abundance and biting times of Anopheles balabacensis baisas (Diptera: Culicidae) in Kudat district of Sabah, Malaysia. Parasites Vectors 2019, 12, 364. [Google Scholar] [CrossRef]
- Mackinnon, M.J.; Mwangi, T.W.; Snow, R.W.; Marsh, K.; Williams, T.N. Heritability of malaria in Africa. PLoS Med. 2005, 2, 340. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.-A. What is a host? attributes of individual susceptibility. Infect. Immun. 2018, 86, e00636-17. [Google Scholar] [CrossRef]
- Weatherall, D. Genetic variation and susceptibility to infection: The red cell and malaria. Br. J. Haematol. 2008, 141, 276–286. [Google Scholar] [CrossRef]
- Janet, C.S.; Balbir, S.; Cyrus, D.; Timotrhy, P.; John, P.W.; Sanjeev, K. Anti-Inflammatory Cytokines Predominate in Acute Human Plasmodium knowlesi Infections. PLoS ONE 2011, 6, e20541. [Google Scholar]
- Clark, I.; Rockett, K. The cytokine theory of human cerebral malaria. Parasitol. Today 1994, 10, 410–412. [Google Scholar] [CrossRef]
- Frimpong, A.; Amponsah, J.; Adjokatseh, A.S.; Agyemang, D.; Bentum-Ennin, L.; Ofori, E.A.; Kyei-Baafour, E.; Akyea-Mensah, K.; Adu, B.; Mensah, G.I.; et al. Asymptomatic malaria infection is maintained by a balanced pro-and anti-inflammatory response. Front. Microbiol. 2020, 11, 2832. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, I.; Sharp, B. Patterns in age-species malaria incidence in a population exposed to low levels of malaria transmission intensity. Trop. Med. Int. Health 2001, 6, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Fornace, K.M.; Herman, L.S.; Abidin, T.R.; Chua, T.H.; Daim, S.; Lorenzo, P.J.; Grignard, L.; Nuin, N.A.; Ying, L.T.; Grigg, M.J.; et al. Exposure and infection to Plasmodium knowlesi in case study communities in Northern Sabah, Malaysia and Palawan, the Philippines. PLoS Negl. Trop. Dis. 2018, 12, 0006432. [Google Scholar] [CrossRef]
- Worrall, E.; Basu, S.; Hanson, K. Is malaria a disease of poverty? A review of the literature. Trop. Med. Int. Health 2005, 10, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Fornace, K.M.; Brock, P.M.; Abidin, T.R.; Grignard, L.; Herman, L.S.; Chua, T.H.; Daim, S.; William, T.; Patterson, C.L.; Hall, T.; et al. Environmental risk factors and exposure to the zoonotic malaria parasite Plasmodium knowlesi across Northern Sabah, Malaysia: A population-based cross-sectional survey. Lancet Planet. Health 2019, 3, 179–186. [Google Scholar] [CrossRef]
- Ang, J.X.D.; Yaman, K.; Kadir, K.A.; Matusop, A.; Singh, B. New vectors that are early feeders for Plasmodium knowlesi and other simian malaria parasites in Sarawak, Malaysian Borneo. Sci. Rep. 2021, 11, 7739. [Google Scholar] [CrossRef]
- Ekawati, L.L.; Johnson, K.C.; Jacobson, J.O.; Cueto, C.A.; Zarlinda, I.; Elyazar, I.R.; Fatah, A.; Sumiwi, M.E.; Noviyanti, R.; Cotter, C.; et al. Defining malaria risks among forest workers in Aceh, Indonesia: A formative assessment. Malar. J. 2020, 19, 441. [Google Scholar] [CrossRef]
- Kahissay, M.H.; Fenta, T.G.; Boon, H. Beliefs and perception of ill-health causation: A sociocultural qualitative study in rural North-Eastern Ethiopia. BMC Public Health 2017, 17, 124. [Google Scholar] [CrossRef]
- Espino, F.; Manderson, L.; Acuin, C.; Domingo, F.; Ventura, E. Perceptions of malaria in a low endemic area in the Philippines: Transmission and prevention of disease. Acta Trop. 1997, 63, 221–239. [Google Scholar] [CrossRef]
- Taek, M.M.; Banilodu, L.; Neonbasu, G.; Watu, Y.V.; Ew, B.P.; Agil, M. Ethnomedicine of Tetun ethnic people in West Timor Indonesia: Philosophy and practice in the treatment of malaria. Integr. Med. Res. 2019, 8, 139–144. [Google Scholar] [CrossRef]
- Manderson, L.; Cartwright, E.; Hardon, A.E. The Routledge Handbook of Medical Anthropology; Routledge: New York, NY, USA, 2016. [Google Scholar]
- WHO. Multi-Sectoral Approach for the Prevention and Control of Vector-Borne Diseases; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ajzen, I. The theory of planned behavior. Organ. Behav. Hum. Decis. Processes 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Kamat, V.R. “I thought it was only ordinary fever!” cultural knowledge and the micropolitics of therapy seeking for childhood febrile illness in Tanzania. Soc. Sci. Med. 2006, 62, 2945–2959. [Google Scholar] [CrossRef]
- Hii, J.; Hustedt, J.; Bangs, M.J. Residual malaria transmission in select countries of Asia-Pacific Region: Old wine in a new barre. J. Infect. Dis. 2021, 223, 111–142. [Google Scholar] [CrossRef] [PubMed]
- Teddlie, C.; Tashakkori, A. Foundations of Mixed Methods Research: Integrating Quantitative and Qualitative Approaches in the Social and Behavioral Sciences; Sage: Thousand Oaks, CA, USA, 2008. [Google Scholar]
- Storey, J.D.; Babalola, S.O.; Ricotta, E.M.; Fox, K.A.; Toso, M.; Lewicky, N.; Koenker, H. Associations between ideational variables and bed net use in Madagaskar, Mali and Nigeria. BMC Public Health 2018, 18, 484. [Google Scholar] [CrossRef] [PubMed]
- Solar, O.; Irwin, A. A Conceptual Framework for Action on the Social Determinants of Health; Social Determinants of Health Discussion Paper 2 (Policy and Practice); WHO Document Production Services: Geneva, Switzerland, 2010. [Google Scholar]
- Bandura, A. Social cognitive theory. In Annals of Child Development. Vol. 6. Six Theories of Child Development; Vasta, R., Ed.; JAI Press: Greenwich, CT, USA, 1989; pp. 1–60. [Google Scholar]
- Senkowski, V.; Gannon, C.; Branscum, P. Behavior change techniques used in theory of planned behavior physical activity interventions among older adults: A systematic review. J. Aging Phys. Act. 2019, 27, 746–754. [Google Scholar] [CrossRef]
- Rogers, R.W. A protection motivation theory of fear appeals and attitude change1. J. Psychol. 1975, 91, 93–114. [Google Scholar] [CrossRef]
- Kincaid, D.L. Social networks, ideation, and contraceptive behavior in bangladesh: A longitudinal analysis. Soc. Sci. Med. 2000, 50, 215–231. [Google Scholar] [CrossRef]
- Murdock, G.P.; Wilson, S.F.; Frederick, V. World distribution of theories of illness. Transcult. Psychiatr. Res. Rev. 1980, 17, 37–64. [Google Scholar] [CrossRef]
- Heggenhougen, H.K.; Hackethal, V.; Vivek, P. The Behavioural and Social Aspects of Malaria and Its Control: An Introduction and Nnnotated Biliography Vol. TDR/STR/SEB/VOL/03.1; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Kleinman, A. Concepts and a model for the comparison of medical systems as cultural systems. Soc. Sci. Med. Part B Med. Anthropol. 1978, 12, 85–93. [Google Scholar] [CrossRef]
- Wong, Y.P.; Lau, Y.L.; Radu, S.; Chee, H.Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018, 124, 626–643. [Google Scholar] [CrossRef]
- Lau, Y.L.; Lai, M.Y.; Fong, M.Y.; Jelip, J.; Mahmud, R. Loop-mediated isothermal amplification assay for identification of five human Plasmodium species in Malaysia. Am. J. Trop. Med. Hygeine 2016, 94, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Peng, W.K.; Srivastava, S. Multi-Omics Advancements towards Plasmodium vivax Malaria Diagnosis. Diagnostics 2021, 11, 2–17. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naserrudin, N.A.; Hod, R.; Jeffree, M.S.; Ahmed, K.; Hassan, M.R. The Emerging Threat of Plasmodium knowlesi Malaria Infection: A Concept Paper on the Vulnerable Factors in Human. Int. J. Environ. Res. Public Health 2022, 19, 4419. https://doi.org/10.3390/ijerph19074419
Naserrudin NA, Hod R, Jeffree MS, Ahmed K, Hassan MR. The Emerging Threat of Plasmodium knowlesi Malaria Infection: A Concept Paper on the Vulnerable Factors in Human. International Journal of Environmental Research and Public Health. 2022; 19(7):4419. https://doi.org/10.3390/ijerph19074419
Chicago/Turabian StyleNaserrudin, Nurul Athirah, Rozita Hod, Mohammad Saffree Jeffree, Kamruddin Ahmed, and Mohd Rohaizat Hassan. 2022. "The Emerging Threat of Plasmodium knowlesi Malaria Infection: A Concept Paper on the Vulnerable Factors in Human" International Journal of Environmental Research and Public Health 19, no. 7: 4419. https://doi.org/10.3390/ijerph19074419
APA StyleNaserrudin, N. A., Hod, R., Jeffree, M. S., Ahmed, K., & Hassan, M. R. (2022). The Emerging Threat of Plasmodium knowlesi Malaria Infection: A Concept Paper on the Vulnerable Factors in Human. International Journal of Environmental Research and Public Health, 19(7), 4419. https://doi.org/10.3390/ijerph19074419





