Abstract
A central aspect to the management of type 2 Diabetes Mellitus (T2DM) and hypertension is promoting a healthy lifestyle, and nutritional therapy (NT) can support patients achieving glycemic control and blood pressure targets. This systematic review aimed to evaluate the effectiveness of NT in the management of patients with T2DM and/or hypertension in primary care. Primary outcomes were HbA1c, systolic blood pressure (SBP) and diastolic blood pressure (DBP). Thirty-nine studies were included, thirty on T2DM and nine on hypertension. With a moderate quality of evidence, educational/counseling programs and food replacement programs in primary care likely reduce HbA1c on patients with T2DM (mean difference (MD): −0.37, 95% CI: −0.57 to −0.17, 7437 patients, 27 studies; MD: −0.54, 95% CI: −0.75 to −0.32, 440 patients, 2 studies, respectively). Mediterranean diet for T2DM was accessed by one study, and no difference between the groups was found. Educational and counseling programs likely reduce DBP in patients with hypertension (MD: −1.79, 95% CI: −3.46, −0.12, 2840 patients, 9 studies, moderate quality of the evidence), but the effect in SBP was unclear due to risk of bias and imprecision. Nutritional therapy strategies (i.e., educational/counseling programs and food replacement programs) in primary care improved HbA1c in patients with T2DM and DBP in individuals with hypertension.
1. Introduction
Hypertension and diabetes mellitus (DM) are leading causes of cardiovascular disease and premature death. The total number of patients with diabetes mellitus has quadrupled in the past three decades, and it now affects approximately 1 in 11 adults worldwide [1], while the prevalence of hypertension is estimated at 31.1% among adults [2]. Besides, 11.3% of global deaths in 2019 among adults between 20 and 79 years-old were attributed to diabetes [3], and 14% of deaths globally to hypertension, according to data from 2015 [4].
In addition to the high mortality, the morbidity associated with hypertension and diabetes represent a significant economic burden for patients, caretakers, and health systems. The estimated global cost of DM in 2015 was USD 1.31 trillion or 1.8% of global gross domestic product, and 34% of this was attributed to indirect costs such as loss of productivity [5]. Moreover, hypertension and DM are the health conditions with the highest absolute increase in annual US healthcare expenditure over the past three decades [6].
Despite the increasing investments on chronic disease management, an expressive number of patients do not reach treatment targets. A multicenter, cross-sectional, questionnaire-based study conducted in 9 Latin American countries showed that 56.8% of patients with type 2 DM (T2DM) had poor glycemic control (i.e., HbA1c ≥ 7%) [7]. The lowest treatment success was identified in Peru, where only 7.5% achieved metabolic and blood pressure levels as recommended by the American Diabetes Association (ADA) [8]. Likewise, only 13.8% of adults with hypertension in 2010 had their BP controlled worldwide [9]. Although the management of hypertension and T2DM are well stablished, there is a gap between knowledge and attitude that hinders the implementation of successful management strategies [10]. Hence, the coordination of care and patient self-management are the two utmost important aspects that can be promoted in primary care [11].
A central aspect to the management of T2DM and hypertension is providing healthy lifestyle education. Nutrition therapy (NT) consists of education and support to help patients adopt healthy eating pattern, what plays a fundamental role in the management of T2DM and hypertension, and its complications [12,13,14]. The ADA recommends that NT should generally promote dietary quality and energy restriction and combine patient preferences and metabolic needs [12]. With regard to dietary quality, several approaches have been studied, such as the Mediterranean diet, the Dietary Approaches to Stop Hypertension (DASH), a low carbohydrate diet and a vegetarian diet [12]. A network meta-analysis has shown that all dietary approaches are effective to improve glycemic control, but the Mediterranean diet had the more significant effect [15]. The DASH and Mediterranean diets also contribute to BP reduction [16].
Systematic reviews have demonstrated the effectiveness of NT and lifestyle education on the management of T2DM [17,18,19,20] and hypertension [21,22]. These systematic reviews either restricted the inclusion criteria to group-based educational programs [20]; or did not consider antihypertensive medications as part of the standard care [22]; or were conducted on specific populations, such as young adults [21], obese patients [17], patients at risk for T2DM [19]. However, none of them exclusively focused on the primary care setting. Hence, the objective of this systematic review was to evaluate the effectiveness of NT programs delivered exclusively in the primary care setting in the management of adult patients with T2DM and/or hypertension.
2. Materials and Methods
This systematic review was conducted following the Cochrane collaboration handbook [23], and is reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) Statement [24]. The protocol was published elsewhere [25], and had been previously registered with the international Prospective register of Systematic Reviews (PROSPERO) under registration number CRD42018118117.
2.1. Inclusion and Exclusion Criteria
We sought to include randomized controlled trials that met the following inclusion criteria (PICO):
Participants (P): adult patients (i.e., aged ≥ 18) diagnosed with T2DM and or hypertension. The diagnosis of T2DM should have been established according to the ADA criteria (i.e., fasting glycaemia > 200 mg/dL associated with classic T2Dm symptoms; glycaemia 2 h after overload with 75 g of glucose ≥ 200 mg/dL; HbA1c ≥ 6.5%) [12]. Hypertension should be characterized by persistent systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg [26].
Types of intervention (I): Nutritional therapy (NT) programs delivered in a primary care setting which focused on stimulating healthy nutrition for a minimum duration of four months. We considered the nutritional strategies that were provided in addition to, and not instead of, the regular pharmacological treatment to T2DM and/or hypertension. We included: (i) educational or counseling programs addressing nutritional recommendations to reduce calories and dietary fat and lifestyle healthy behaviors, (ii) food replacement program followed by stepped reintroduction of meals; (iii) Mediterranean diet, (iv) DASH diet, (v) low carbohydrate diet, (vi) vegetarian diet, (vii) low glycemic index diet, (viii) high protein diet. The educational and counseling interventions could be delivered by any health care professional, such as dieticians, physical educators, nurses, psychologists, health educators, physicians, and peer-supporters (i.e., trained in some way in the context of the intervention, although having no formal professional or paraprofessional certificated or degree tertiary education).
Comparison (C): Conventional treatment for T2DM and hypertension, which consisted of pharmacological treatment and general healthy lifestyle advice. An episodic consultation with a dietician, nurse, physical trainer, or educator in diabetes for general healthy lifestyle advice or general nutritional orientation were considered conventional treatment if the patients were not provided subsequent follow up.
Outcomes (O): Primary outcomes were glycemic control and blood pressure control, which were measured by HbA1c (%) and SBP/DBP (mmHg), respectively. The secondary outcomes were frequency of cardiovascular events (acute myocardial infarction and stroke), weight loss (measured as change in weight or in BMI) and death. Outcomes were evaluated at 6, 12 and more than 12 months.
We excluded studies that included patients aged <18 years old, pregnant women, diagnosis of secondary hypertension. Further exclusion criteria were if the intervention was based exclusively on dietary supplements, too low energy diets (less than 600 kcal/day), if the intervention was not delivered in a primary care setting (i.e., studies that recruited patients in the primary setting, but delivered the intervention elsewhere were excluded), and if there was a cointervention not common to both groups.
2.2. Identification of Studies
General search strategies were developed for the main electronic health databases: Embase (1980–2019), Medline though PubMed (1966–2019), LILACS (through Virtual Health Library, 1982–2019) and CENTRAL (Cochrane Collaboration Controlled Trials Register, 1982–2019). A second search on the previously mentioned databases was conducted on 27 December 2021. Search strategies included the following descriptors and synonyms: T2DM OR hypertension—AND—primary health care OR community health planning—AND—nutritional therapy OR lifestyle—AND randomized controlled trial. In PubMed and Embase a filter for randomized controlled trials were applied. There was no language restriction. The full search strategy is on the Supplementary Material (Table S1).
The databases searched for eligible studies included: Trip database, SCOPUS, Web of Science, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Australasian Medical Index, and Chinese Biomedical Literature Database. Furthermore, we searched for studies on ClinicalTrials.gov, the Brazilian Registry of Clinical Trials (Rebec) and grey literature, through abstracts published in annals and lectures. References of relevant primary or secondary studies were screened to identify additional eligible studies. Endnote software was used to download references and remove duplicates.
Two reviewers (RGOFL and JSCG) independently performed the initial screening of titles and abstracts using the software Rayyan QCRI [27]. The studies selected for full-text review were subsequently assessed for adequacy to the proposed PICO. In case of disagreement, there was a consensus meeting between the reviewers and the project coordinator (VdSN-N) for a final decision.
2.3. Data Extraction and Risk of Bias of Included Studies
Two reviewers (LRB and JSCG) used a standardized form to extract relevant data of the included studies (i.e., study identification, publication date, country, sample size, follow up, type of intervention and control, baseline characteristics of patients, and outcome results). To assess the risk of bias of included studies, the same two reviewers used the revised version of the Cochrane tool (RoB 2), which considers bias arising from five domains: (i) the randomization process; (ii) due to deviations from intended interventions (including blinding of patients and personnel, balanced baseline characteristics, and report of measurement of compliance with the intended intervention); (iii) due to missing outcome data; (iv) in measurement of the outcome; and (v) in selection of the reported result. To ensure consistency between reviewers, a calibration exercise was performed and in the case of disagreement, a consensus meeting with the project coordinator (VdSN-N) was made. For domain (iii), while we considered a loss of follow up of more than 20% as high risk of bias for RCTs, we allowed a loss of follow up of 30% for pragmatic trials. It was also considered high risk if the loss of follow up was unbalanced between the two groups. For domain (iv) it was considered high risk of bias when the data collection on the outcomes of interest was performed based on patient records instead of a standardized data collection. Finally, for domain (v) we also tracked the published protocols and trials registrations, when available.
2.4. Synthesis and Analysis of Data
The unit of analysis was the data published in the included studies. Similar outcomes in at least 2 studies were plotted in random-effect meta-analyses using the Stata Statistical Software 17 (StataCorp LLC, College Station, TX, USA). The random effects model was chosen as the analytic model for the meta-analysis. Continuous data were expressed as means and SD and the differences between means with 95% CIs were used as estimate of intervention effect. The mean adjusted differences (MAD) were preferred when available. For studies that reported median and interquartile range, the estimated mean of the sample and SD were obtained from Hozo et al. [28].
For the cluster randomized trials, we used a formula suggested by the Cochrane handbook to find the trial’s effective sample size, which is its original sample size divided by the “design effect.” The design effect can be calculated by:
1 + (M − 1) × ICC, where M is the average cluster size and ICC is the intracluster correlation coefficient [29].
Inconsistencies between the results of the included studies were ascertained by visual inspection of forest plots (no overlap of CIs around the effect estimates of the individual studies) and by Higgins or I2 statistic, in which I2 > 50% indicates a moderate probability of heterogeneity, and by χ2 tests, where p < 0.10 indicates heterogeneity. In the case of statistical heterogeneity, we used meta-regression to explore the causes of the inconsistency. Meta-regression models (with random effects) were adjusted with the MD as a dependent variable; and months of follow-up, type of intervention, risk of bias, if MAD was used and characteristics of the baseline participants as moderator variables (mean age and mean HbA1c). The Knapp–Hartung correction was used to calculate the significance of the meta-regression coefficients.
2.5. Quality of Evidence
The quality of the evidence of the intervention’s effect estimate was assessed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodological guideline [30]. To assess the domain imprecision the optimal information size (OIS) was calculated using the Stata Statistical Software 17 (StataCorp LLC, College Station, TX, USA). A change of 0.5% for the outcome HbA1c, 4 mmHg for the outcome SBP, and 3 mmHg for the outcome DBP were considered to be clinically significant. For all outcomes, 0.05 alfa error and 0.20 beta were assumed. The quality of the evidence was rated down for imprecision if the OIS criterion was not met [30].
3. Results
We identified 6363 references on the first search and 1099 references on the second search, following the removal of duplicates. After reading the title and abstract, 124 references were selected for full reading. The 85 excluded references, and the reasons for exclusion are detailed on Table S2 on the supplementary material. The main reasons for exclusion were non-randomized trial (3 studies), patients did not meet eligibility criteria (8 studies), wrong intervention (10 studies) or intervention inferior to 4 months (14 studies), intervention not delivered in primary care setting (36 studies), wrong comparator (7 studies), wrong outcome (3 studies), cointervention not common to both groups (3 studies) or study protocol (1 study). Figure 1 shows the study selection process.
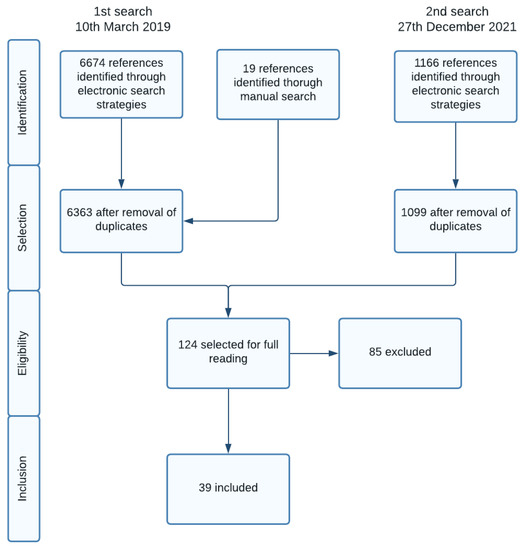
Figure 1.
Study selection process.
3.1. Characteristics of Included Studies
Thirty-nine studies met our eligibility criteria and were therefore included [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Among the included studies, nine evaluated NT primarily for hypertension [61,62,63,64,65,66,67,68,69], 30 evaluated NT primarily for T2DM [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. The characteristics of included studies according to the inclusion criteria are detailed in Appendix A and Appendix B.
Nine studies assessed the effect of NT on patients primarily diagnosed with hypertension [61,62,63,64,65,66,67,68,69]. While most studies excluded patients with T2DM, 2 of these studies allowed for patients with concomitant diagnosis of hypertension and T2DM [65,67]. All of the interventions consisted of counseling or educational programs delivered in groups or individually by practice nurses (six studies) [61,62,64,66,68,69], dieticians (two studies) [63,65] and health educators (one study) [67].
Thirty studies included patients primarily diagnosed with T2DM, and in all of the studies a proportion of included patients had concomitant diagnosis of hypertension [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. The interventions studies consisted of educational and counseling programs in 27 studies [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,47,48,49,50,51,52,53,54,55,57,59,60], food substitution program in two studies [46,56], and only one studied assessed the effect of Mediterranean diet [58]. The educational/counseling programs were delivered by multi-professional teams in eight studies [35,36,39,41,47,48,53,54], practice nurse in six studies [37,38,40,42,43,59], dieticians (i.e., nutritionists) in five studies [31,34,44,52,60], pharmacists in two studies [45,50], peer supporters in two studies [49,55] and therapists in one study [51]. In three studies, the intervention was delivered by specialists in diabetes or population health management [32,33,57]. Eight studies were cluster randomized trials [31,33,36,48,49,55,63] and three studies despite the cluster randomization, individual patient data were used for the analysis [32,43,50].
3.2. Risk of Bias
Risk of bias of included studies is shown in Figure 2 and Figure S1 (Supplementary material). For the (ii) domain (i.e., deviation from the intended intervention) there were some concerns for most studies, given the behavioral nature of the educational and counseling interventions and lack of blinding of patients and personnel. Among the studies rated as high risk for the (i) domain (i.e., randomization process), three lacked blinding of allocation [44,45,62], and two had unbalances in baseline characteristics (Höchsmann et al. allocated more diabetic patients to the intervention arm and Tejada Tebayas et al. the intervention group had a lower educational profile) [57,63].
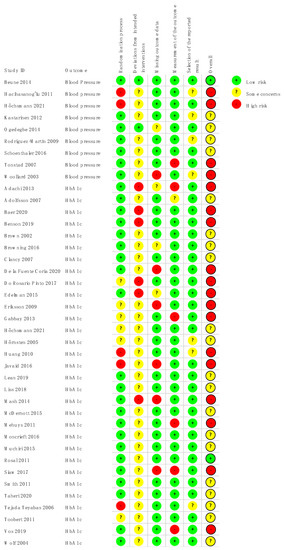
Figure 2.
Risk of bias of included studies according to the main outcomes analyzed.
For the domain missing outcome data, four studies were rated as high risk of bias due to high and unbalanced dropout rates, suggesting that the loss of follow-up was related to the intervention [38,45,48,54].
3.3. Meta-Analysis of NT for Hypertension
The meta-analysis showed an association between NT and reduction in DBP, MD: −1.79, 95% CI: −3.46 to −0.12, I2: 88.1%, 2840 patients, 9 studies, moderate quality of the evidence, Figure 3A, Table 1). However, meta-analysis did not indicate any clear effect of NT in patients primarily diagnosed with hypertension on SBP (MD: −2.17, 95%CI −6.81, 2.46, 4518 patients, 9 studies, I2: 88.8%, low quality of the evidence, Figure 3B, Table 1). After visual inspection of the forest plot, the high heterogeneity in both meta-analyses was attributed to one study that overestimated the effect of the intervention [62]. In a sensitivity analysis without Hacihasanoğlu et al. [62], the intervention effect on DBP remained in favor of the intervention, and the MD in SBP still crossed the line of null effect (Figure S2 Supplementary material).
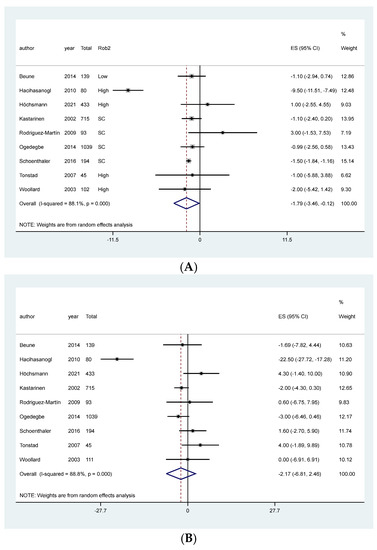
Figure 3.
Meta-analysis of the effect of NT on blood pressure in patients primarily diagnosed with hypertension. (A) Diastolic blood pressure, (B) Systolic blood pressure.

Table 1.
Summary of findings and quality of the evidence.
Subgroup analyses performed according to length of follow-up and sensitivity analyses separating studies that presented the results as MAD or post-intervention values, and according to risk of bias did not change the effect on blood pressure (Figures S3–S5 on the Supplementary material, respectively).
3.4. Meta-Analysis of NT for T2DM
Meta-analysis of studies assessing the effect of counseling and educational programs on HBA1c favored the intervention (MD: −0.37, 95% CI: −0.57, −0.17, I2: 85.8%, 7437 patients, 27 studies, moderate quality of the evidence, Figure 4A). We investigated heterogeneity using meta-regression. The joint and individual tests for categorical and no categorical covariates gave us a p-value higher than 0.05, indicating no association between these covariates and the size of the treatment effect (Figures S6 and S7 on the Supplementary material). However, through visual inspection of the forest plot, we asserted that two studies overestimated the effect of the intervention [43,45]. A sensitivity meta-analysis excluding these two studies resolved the heterogeneity and still favored the intervention (MD: −0.25, 95% CI: −0.35, −0.16, I2: 24%, 7094 patients, 25 studies, Figure S8 on the Supplementary material).
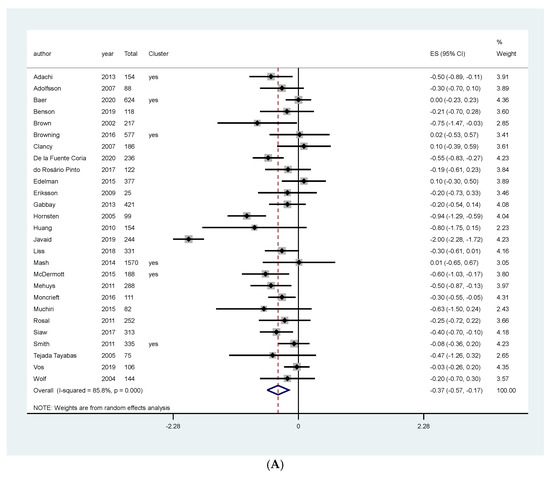
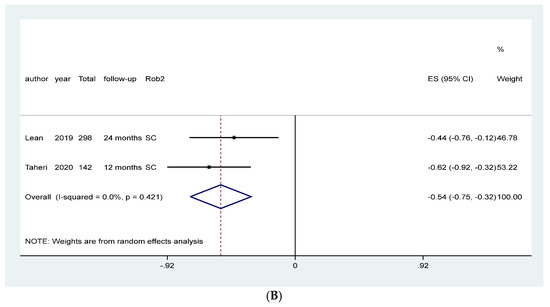
Figure 4.
Meta-analysis of the effect of NT on HbA1C in patients primarily diagnosed with type 2 Diabetes Mellitus according to the type of intervention. (A) Counseling and educational programs, (B) Food replacement.
Subgroup analyses performed according to length of follow-up and sensitivity analyses separating studies that presented the results as MAD or post-intervention values, and according to risk of bias did not change the effect on HbA1c (Figures S9–S11 on the supplementary material, respectively).
Meta-analysis of studies assessing the effect of food replacement on HBA1c favored the intervention (MD: −0.54, 95% CI: −0.75, −0.32, I2: 0%, 440 patients, 2 studies, Figure 4B, moderate quality of the evidence, Table 1).
Secondary Outcomes
Among studies assessing educational and counseling NT for T2DM, the meta-analyses of DBP and BMI did not show a statistical difference between groups (MD: −0.84, 95% CI: −1.85, 0.16, I2: 44.8%, 5508 patients, 14 studies, Figure S12 on the Supplementary Material; MD: 0.07 95%CI: −0.19, 0.05, I2: 24.5%, 2378 patients, 11 studies, Figure S13 on the Supplementary Material, respectively). For SBP, a trend of no effect was identified (MD: −2.56, 95% CI: −5.5 to 0.38, I2: 83.6%, 5508 patients, 15 studies, Figure S14 on the Supplementary Material), but in a sensitivity analysis without Javaid et al. [45] (a study which overestimated the intervention), the heterogeneity was resolved and the reduction in SBP showed to be significant (MD: −1.68, 95% CI: −2.98, −0.38, I2: 0%, 5264 patients, 14 studies, Figure S15 on the Supplementary Material).
3.5. Publication Bias
Publication bias was investigated for HbA1c outcome. Since asymmetries in the funnel plot was not observed, the Egger test was performed (p: 0.988, Figure S16 on the Supplementary Material), indicating no publication bias.
3.6. Studies Not Included in the Meta-Analysis
Only 1 study assessed the effect of Mediterranean diet on T2DM, and therefore it was not possible to perform a meta-analysis [58]. Toobert et al. investigated the effect of a nutritional therapy based on Mediterranean diet on 280 patients aged ≥30 and <75 and with Latino ethnicity and the primary outcomes were self-efficacy and behaviour change and HbA1c as secondary outcome. Patients in the intervention groups presented mean HbA1c of 8.4 (standard error (SE): 0.003), which dropped to 7.8 (SE: 0.03) after 6 months, but it was not maintained (i.e., mean HbA1c at 12 and 24 months were both 8.4, SE: 0.03).
The secondary outcomes mortality and frequency of cardiovascular events were not evaluated by the primary studies included in this review, and therefore, were not assessed.
4. Discussion
In this systematic review, we identified three NT strategies evaluated in primary care: counseling and educational programs, food replacement programs and Mediterranean diet. Only one study on Mediterranean diet in primary care was identified [58], and it was not included in a meta-analysis. The included studies reported the metabolic targets as main outcomes, such as glycemic control, blood pressure reduction and BMI. However, no study reported the outcomes mortality or frequency of cardiovascular events.
According to the meta-analyses, NT in primary care likely reduces HbA1c in patients with T2DM. With moderate quality of the evidence, counseling and educational programs were associated with a mean change of −0.37 in HbA1c and food replacement programs with a mean change in HbA1c of −0.54, both when compared to usual care. In addition, counseling and educational programs slightly reduce DBP (MD: −1.79) in patients with hypertension when compared to usual care. It was not possible to ascertain an association of NT with change in SBP.
The effectiveness of NT in the management of T2DM and hypertension have been investigated in systematic reviews, although none had focused exclusively on the primary care setting. Patients with T2DM and hypertension are mostly managed in the primary healthcare level, and access to specialized medical nutritional therapy is limited [18]. The lack of training on nutritional advice and patient overload often makes lifestyle interventions challenging for primary care nurses and physicians [11].
The systematic review by Kalyoncu et al. investigated the effectiveness of nutrition-based practices in prevention of hypertension but focused on healthy young adults [21]. Nicolson et al. performed a meta-analysis of five studies comparing antihypertensive medication versus lifestyle interventions (without antihypertensive), and likewise had inconclusive results on SBP reduction [22]. However, distinct from the review form Nicolson et al., in our review NT was often offered in combination to antihypertensive medications as part of the standard care at the primary care clinic. Besides, our review included more studies published after 2004.
Previous systematic reviews investigating the effectiveness of NT in T2DM had focused mainly on weight loss among obese patients [17], or T2DM prevention among healthy patients at risk for T2DM [19]. In addition, the benefit of group-based self-management education in reducing HbA1c had been previously indicated in a systematic review [20]. However, the inclusion criteria of this previous systematic review were restricted to group-based educational programs [20]. Moreover, two robust studies were published after this systematic review was carried out, which were now included in our analyses [34,47]. Furthermore, García-Molina et al. conducted a meta-analysis of studies assessing the effectiveness of lifestyle intervention (educational programs) in T2DM management [18]. García-Molina et al. found a weighted mean difference of −0.51 in HbA1c, a reduction that is more pronounced than the results from our meta-analysis. Although the inclusion criteria were similar to those used in our review, our review excluded studies conducted in settings other than the primary care, assessed separately patients with hypertension and applied the GRADE methodology to assess the quality of the evidence.
Among the studies included in our meta-analyses, three studies had clustered samples (i.e., subjects were randomized at a group level), but the results were analyzed at an individual level [32,43,50]. By not taking clustering into account, the results may show significance where none exists [70]. Moreover, in one study there was no allocation concealment (i.e., subjects with even numbers were allocated to the intervention arm, while odd number subjects to the control arm) [45]. The individual-level analysis in a clustered sample in Hörnsten et al. [43], and the lack of allocation concealment in Javaid et al. [45], may explain the overestimated effect on HbA1c in both studies identified in the meta-analysis. Hence, we conducted sensitivity analysis, and the effect in favor of the intervention was maintained.
Some limitations to our results must be acknowledged. First, we could not evaluate the effect of NT on hard outcomes such as mortality and frequency of cardiovascular events. The surrogate continuous outcomes evaluated allow a limited interpretation of the real clinical impact of the interventions. For HbA1c, a change of 0.5% is considered as clinically significant [71,72]. For blood pressure, a reduction of 10 mmHg is associated with a reduction of 17% in coronary artery disease incidence, 27% in stroke, 28% in heart failure and 13% in all-cause mortality [73]. When considering these cutoffs to extrapolate the correlation of blood pressure and HbA1c reduction on long term complications and mortality, the effect of NT is arguable. For instance, the mean change in DBP in patients with hypertension identified in our meta-analysis, although statistically significant, was notably lower than what could be regarded as clinically significant. Second, during data extraction, it was not possible to ensure that the studies considered equivalent variables for the calculation of the MAD.
Third, there was noticeable variability among the educational programs provided by the included studies, regarding intensity of the intervention, the professional responsible for delivering the intervention and the content of the intervention. While some educational interventions followed a nutrition education curriculum [48,52], others were based on motivational interviewing [42], or culturally tailored self-management interventions [35,53,58]. Moreover, some programs provided broad lifestyle modification advice, entailing promotion of regular physical activity. The choice of the best suited intervention might be influenced by previous local practices, logistic capacities, and cultural aspects, although the common aspect of these educational and counseling programs was that the NT was offered as part of a coordinated care in a primary setting [11].
Lifestyle modification is a central aspect to the management of patients with T2DM and hypertension [12]. Although the implementation of lifestyle and nutritional interventions in the primary care setting is challenging, the effect of educational programs on the management of hypertension (i.e., reduction in DBP) and T2DM, and food replacement for the management of T2DM have been extensively studied, although future studies could help establish the effect of NT on SBP. These results may contribute to further implementing NT in the primary care setting. Moreover, NT programs tailored for the primary care should be encouraged.
5. Conclusions
Nutritional therapy strategies (i.e., educational/counseling programs and food replacement programs) in primary care improved the glycemic control in patients with T2DM and educational programs in primary care improved DBP in individuals with hypertension. NT programs tailored for the primary care should be encouraged.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19074243/s1, Table S1 Search Strategy PubMed; Table S2 Excluded studies and main reason for exclusion; Figure S1 Summary of risk of bias; Figure S2 Meta-analysis of nutritional therapy for hypertension, sensitivity analysis without Hacihasanoğlu. A. effect on systolic blood pression, B. effect on diastolic blood pressure; Figure S3 Meta-analysis of nutritional therapy for hypertension for primary outcome (systolic blood pressure), subgroup analysis according to length of follow-up; Figure S4 Meta-analysis of nutritional therapy for hypertension for primary outcome (systolic blood pressure), subgroup analysis according to risk of bias. Abbreviations: SC: some concerns; Figure S5 Meta-analysis of nutritional therapy for hypertension for primary outcome (systolic blood pressure) separating studies that presented the results as mean adjusted difference (i.e., change score) or post-intervention values; Figure S6 Meta-regression assessing follow up as modifier of the effect of nutritional therapy on HbA1c; Figure S7 Meta-regression assessing mean age and mean HbA1cs as modifiers of the effect of nutritional therapy on HbA1c; Figure S8 Meta-analysis of nutritional therapy for diabetes mellitus for primary outcome (HbA1c) without Javaid et al. and Hörnstein et al.; Figure S9 Meta-analysis of nutritional therapy for diabetes mellitus for primary outcome (HbA1c), subgroup analysis according to length of follow up; Figure S10 Meta-analysis of nutritional therapy for diabetes mellitus for primary outcome (HbA1c), subgroup analysis separating studies that presented the results as mean adjusted difference (i.e., change score) or post-intervention values; Figure S11 Meta-analysis of nutritional therapy for diabetes mellitus for primary outcome (HbA1c), subgroup analysis according to risk of bias; Figure S12 Meta-analysis of nutritional therapy for diabetes mellitus. Secondary outcome: systolic blood pressure; Figure S13 Meta-analysis of nutritional therapy for diabetes mellitus without Javaid et al. Secondary outcome: systolic blood pressure; Figure S14 Meta-analysis of nutritional therapy for diabetes mellitus. Secondary outcome: diastolic blood pressure; Figure S15 Meta-analysis of nutritional therapy for diabetes mellitus. Secondary outcome: body mass index; Figure S16 Funnel plot assessing publication bias on the meta-analysis of nutritional therapy for diabetes mellitus for the primary outcome (i.e., HbA1c).
Author Contributions
Conceptualization, V.d.S.N.-N.; methodology, V.d.S.N.-N. and J.S.C.G.; formal analysis, V.d.S.N.-N., L.R.B., R.G.O.F.L. and J.S.C.G.; resources, V.d.S.N.-N.; writing—original draft preparation, V.d.S.N.-N. and J.S.C.G.; writing—review and editing, V.d.S.N.-N., L.R.B., R.G.O.F.L. and J.S.C.G.; supervision, V.d.S.N.-N.; project administration, V.d.S.N.-N.; funding acquisition, V.d.S.N.-N. and R.G.O.F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project was partially funded by the Call for Financial Support for Studies in Health Technology Assessment of German Hospital Oswaldo Cruz in partnership with the Brazilian Health Ministry through the Program of Support for the Institutional Development of the Unified Health System (PROADI-SUS) (DECIT/SCTIE/MS-PROADI-SUS, 2018), Grant number 25000.009785/2018-44. The São Paulo Research Foundation, FAPESP, supported Luiza Rocco Banzato during the conduction of this study (Undergraduate research project, process 2018/25035-5).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated during this study is provided in the main manuscript and its supplementary material.
Acknowledgments
The authors would like to thank Lehana Tabane for the support during the conduction of this study.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Characteristics of included studies according to the eligibility criteria for studies offering nutritional therapy primarily for hypertension.
Table A1.
Characteristics of included studies according to the eligibility criteria for studies offering nutritional therapy primarily for hypertension.
| Study/Year | Study Design | Country | Setting | Inclusion Criteria a | Intervention | Intensity of Intervention b | Control | Outcomes | Follow Up |
|---|---|---|---|---|---|---|---|---|---|
| Beune 2014 | Cluster RCT | The Netherlands | 4 PHCC | Aged ≥ 20, SBP ≥ 140 mmHg at the last office visit, Ghanaian or Surinamese origin | Counseling program delivered by a trained practice nurse | Three 30-min sessions over 5 months | Usual care | (i) Proportion of patients with a SBP reductionin at least 10 mmHg; (ii) mean between-group differences in changes in SBP and DBP; (iii) adherence to lifestyle and medication recommendations (MMAS-8 score) | 6 months |
| Hacihasanoğlu 2011 | RCT | Turkey | 3 PHCC | Aged ≥ 35, had blood pressure ≥ 140/90 mmHg, were prescribed antihypertensive medication | Counseling program delivered by a practice nurse | Six monthly sessions for 6 months | Usual Care (Monthly monitoring of blood pressure) | (i) mean between-group differences in changes in SBP and DBP; (ii) medication compliance (MASES score) and (iii) lifestyle behaviours (HPLP score). | 6 months |
| Höchsmann 2021 | RCT | USA | 18 PHCC | Aged ≥ 20 and <75, BMI ≥ 30–50 kg/m2 | Educational program delivered by dieticians, physical education | Sixteen face-to-face and six telephone sessions over 6 months, monthly sessions for 18 months | Usual care, printed educational material | (i)changes in fasting glucose and lipid profile; (ii) blood pressure | 24 months |
| Kastarinen 2002 | RCT | Finland | 10 PHCC | Aged ≥ 25 and <74, had SBP ≥ 140 and <179, DBP ≥ 90 and <109 mmHg, were prescribed antihypertensive medication | Educational program delivered by trained practice nurses | Four sessions over 12 months followed by 3 sessions in the 2nd year. | Usual care | (i) mean between-group differences in changes in SBP and DBP; (ii) changes in BMI, waist and hip circumference, lipid profile, alcohol consumption, urinary sodium and potassium excretion | 24 months |
| Ogedegbe 2014 | RCT | USA | 30 PHCC | Aged ≥ 18, HTN diagnosed at least 6 months prior to inclusion, blood pressure ≥ 140/90 mmHg, were prescribed antihypertensive medication | Educational program delivered by dieticians and health educators | Six monthly sessions for 6 months | Printed educational material and four group educational sessions about mineral and vitamin replacement | (i) Proportion of patients with a BP reduction to at least 140/90 mmHg (or to 130/80 mmHg for those with DM and KD); (ii) mean within-patient changes in SBP and DBP; (iii) cost-effectiveness | 12 months |
| Rodriguez-Martín 2009 | RCT | Spain | 1 PHCC | HTN diagnosed at least 6 months prior to inclusion | Educational program delivered by trained practice nurses in groups | Twelve monthly sessions for 12 months | Usual care | (i) mean between-group differences in changes in SBP and DBP; (ii) level of physical activity; (iii) Quality of life (SF-36 questionnaire); (iv) Cardiovascular risk (Framingham score) | 24 months |
| Schoenthaler 2016 | RCT | USA | 1 PHCC | Aged ≥ 18, blood pressure ≥ 140/90 mmHg (or to 130/80 mmHg for those with DM and KD), identified as Black of African Americans | Counseling program delivered by trained health educators | Ten weekly group sessions for 3 months, followed by monthly telephone sessions for 3 more months. | Usual care and printed educational material | (i)within patient change in SBP and DBP; (ii) adherence to lifestyle and medication recommendations (MMAS-8 score) | 6 months |
| Tonstad 2007 | RCT | Norway | 1 PHCC | Aged ≥ 30 and <69, SBP > 140 or DBP > 90 mmHg | Counseling program delivered by trained practice nurses | Monthly sessions | Usual care and one session for general healthy lifestyle advice | (i)within patient change in SBP and DBP, waist circumference, lipid profile; (ii) Cardiovascular risk (Framingham score) | 6 months |
| Woollard 2003 | RCT | Australia | 4 PHCC | Aged ≥ 20 and <75, SBP ≥ 140 or DBP ≥ 90 mmHg or were prescribed antihypertensive medication | Counseling program delivered by trained practice nurses | Twelve monthly sessions for 12 months | Usual care | (i) mean between-group differences in changes in SBP and DBP, weight, BMI, urinary sodium and potassium (ii) Proportion of patients with a BP reduction to at least 130/85 mmHg | 12 and 18 months |
a Inclusion criteria besides having a diagnosis of hypertension. b Interventions were offered individually, unless stated otherwise. Abbreviations. RCT: randomized controlled trial; PHCC: primary health care center; HTN: hypertension; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; KD: kidney disease; MMAS-8: Morisky medication adherence scale; MASES: Medication adherence and self-efficacy scale; HPLP: health promotion life-style profile scale.
Appendix B

Table A2.
Characteristics of included studies according to the eligibility criteria for studies offering nutritional therapy primarily for type 2 diabetes mellitus.
Table A2.
Characteristics of included studies according to the eligibility criteria for studies offering nutritional therapy primarily for type 2 diabetes mellitus.
| Study/Year | Study Design | Country | Setting | Inclusion Criteria a | Intervention | Intensity of Intervention | Control | Outcomes | Follow Up |
|---|---|---|---|---|---|---|---|---|---|
| Adachi 2013 | Cluster RCT | Japan | 11 PHCC | Aged ≥ 20 and <79, with clinically documented diagnosis of T2DM (i.e., HbA1c ≥ 6.5%) | Educational program delivered by dieticians | Three to 4 sessions over 6 months | Usual care and one session with dietician for general nutritional advice | (i) changes in HbA1c; (ii) changes in weight, BMI, confidence in diabetes knowledge, satisfaction with daily life | 6 months |
| Adolfsson 2007 | Cluster RCT | Sweden | 18 PHCC | Aged < 75, HBA1c between 6.5% and 10%, diagnosed with T2DM at least 1 year before inclusion | Educational program delivered by physicians and diabetes specialists | Four to 5 sessions over 7 months | Usual care | (i) changes in HbA1c; (ii) changes in blood pressure, BMI, fasting plasma glucose, lipid profile, dietary intake | 12 months |
| Baer 2020 | Cluster RCT | USA | 15 PHCC | Aged ≥ 20 and <70, BMI between 27 and 40, diagnosed with either HTN or T2DM | Educational program delivered by a non-clinical populational health manager | Access to an online weight management program, monthly check in calls for 12 months and one consultation with a dietician at month 6 | Usual care | (i) weight change; (ii) proportion of patients with weight loss > 5%, changes in SBP and DBP, lipid profile, HbA1C, quality of life, level of physical activity and confidence in ability to lose weight. | 12 months |
| Benson 2019 | RCT | USA | 2 PHCC | Aged ≥ 45 and <75, meeting three or less optimal care measures (HBA1c < 8%, blood pressure < 140/90 mmHg, not using tobacco, taking a statin and aspirin as appropriate) | Educational program delivered by a dietician | Monthly telephone coaching for 12 months | Usual care | (i) composite number of diabetes optimal care goals met; (ii) changes in BMI, LDL, HbA1c | 12 months |
| Brown 2002 | RCT | USA and Mexico | Community spaces | Aged ≥ 35 and <70, diagnosis of T2DM after 35 years old | Educational program delivered by nurses, dieticians, and community workers | Weekly two-hour individual sessions for three months followed by biweekly group sessions for six months | Usual care (wait list) | (i) diabetes-related knowledge, changes in HbA1c, fasting blood glucose, lipid profile, BMI | 12 months |
| Browning 2016 | Pragmatic cluster RCT | China | 39 PHCC | Aged ≥ 50 registered in one of the participating PHCC | Educational program delivered by community physicians, nurses and psychologists | Monthly telephone and in person sessions for 12 months | Usual care | (i) changes in HBA1c; (ii) changes in SBP and DBP, BMI, waist and hip circumference, fasting plasma glucose, lipid profile, psychosocial and selfcare behavior outcomes | 12 months |
| Clancy 2007 | RCT | USA | 1 PHCC | Aged ≥ 18 and HBA1c > 8% | Educational program delivered by internal medicine physicians and practice nurses | Monthly group sessions for 12 months | Usual care | (i) changes in HBA1c; (ii) changes in SBP and DBP, lipid profile | 12 months |
| De la Fuente Coria 2020 | RCT | Spain | 1 PHCC | Aged ≥ 18 and <80 | Educational program delivered by a trained practice nurse | Six 30-min sessions over 6 months, followed by two sessions at month 12 and month 18 | Usual care | (i) changes in HbA1c, SBP, DBP, lipid profile; (ii) achievement of T2DM control targets (i.e., HbA1c < 7%, fasting glucose < 130 mg/dL) | 12 months and 24 months |
| do Rosário Pinto 2017 | RCT | Portugal | 1 CHC | Middle-aged patients with HbA1c > 7.5% | Educational program delivered by doctors, nurses, psychologists, dieticians, pharmacists | Six sessions of individual or in group over 6 months | Usual care | (i) Changes in HbA1C, BMI and blood pressure, (ii) changes in self-care activities | 6 months |
| Edelman 2015 | RCT | USA | 9 PHCC | Aged ≥ 18, diagnosis of T2DM (and HbA1C > 7.5%) concomitant to HTN, in use of hypertensive medication | Educational program delivered by a trained practice nurse | Twelve telephone sessions every two months for 24 months | Usual care, printed educational material | (i) changes in HBA1c and changes in SBP; (ii) changes in DBP, weight, lipid profile | 24 months |
| Eriksson 2009 | RCT | Sweden | 1 PHCC | Aged ≥ 18 and <65, with clinically documented diagnosis of HTN, T2DM, obesity dyslipidaemia or any combination of the beforementioned | Educational program delivered by dieticians and physical educators in groups | Five sessions over 3 months, followed by 6 sessions within the 1st year, 4 sessions over the 2nd year and 2 over the 3rd year. | Usual care and printed educational material | (i) changes in anthropometry (BMI, weight, waist, waist-to-hip ratio), self-reported physical activity, blood pressure, lipid profile, fasting blood glucose, glucose tolerance, and HbA1c | 3 years |
| Gabbay 2013 | Pragmatic RCT | USA | 12 PHCC | Aged ≥ 18 and <75, with HbA1c: 8.5% or blood pressure: 140/90 mmHg or LDL:103 | Counseling program delivered by trained practice nurses | Six sessions over 12 months | Usual care | (i) changes in HbA1c, LDL and blood pressure, satisfaction with diabetes regimen (DTSQ), quality of life (ADDQoL), depression symptoms (CES-D), diabetes self-management (SDSCA) | 24 months |
| Hörnsten 2005 | Cluster RCT | Sweden | 4 PHCC | Aged ≥ 40 and <80, diagnosed with T2DM within the last 2 years | Educational program delivered by a diabetes expert and practice nurses | Ten group sessions over 9 months | Usual care | (i) changes in HbA1c; (ii) overall well-being, treatment satisfaction, lipid profile, BMI, blood pressure | 12 months |
| Huang 2010 | Cluster RCT | Taiwan | 5 PHCC | Aged ≥ 30 and <70, registered at one of the participating centers | Educational program delivered by a dietician | Sessions every 3 months for 12 months | Usual care | (i) changes in HbA1c; (ii) changes in fasting plasma glucose, SBP, DBP, BMI, lipid profile | 12 months |
| Javaid 2019 | RCT | Pakistan | 1 PHCC | Aged ≥ 18 and HbA1c > 8% | Educational program delivered by a pharmacist | Monthly sessions form 15 to 30 min duration | Usual care | (i) changes in fasting plasma glucose and HbA1c; (ii) changes in blood pressure, lipid profile | 9 months |
| Lean 2019 | Cluster RCT | United Kingdom | 49 PHCC | Aged ≥ 20 and <65, diagnosed with T2DM within the last 6 years, BMI of 27–45 kg/m2 | Food substitution program followed by gradual reintroduction of meals | Formula diet for 3–5 months, stepped food reintroduction for 6–8 weeks, support for weight maintenance for 24 months | Usual care | (i) reduction in body weight of 15 kg or more, HbA1c < 6.5%; (ii) changes in weight and HbA1cand number of antihypertensive drugs and antidiabetic drugs | 24 months |
| Liss 2018 | Cluster RCT | USA | 2 CHC | Aged ≥ 18 and BMI ≥ 24 kg/m2 | Educational program delivered by wellness instructors | Weekly group sessions over 6 months, followed by 24 sessions over the second year | Usual care | (i) change in body weight; (i) changes in HbA1c, SBP, total cholesterol and HDL cholesterol | 12 months |
| Mash 2014 | Pragmatic cluster RCT | South Africa | 34 CHC | Adult patients registered at the participating community centers | Educational program delivered by health educators | Four group sessions during between 60–120 min | Usual care | (i) improved diabetes self-care activities, 5% weight loss and 1% reduction in HbA1c; (ii) changes in blood pressure, weight, waist circumference, HbA1c, lipid profile and quality of life | 12 months |
| McDermott 2015 | Pragmatic cluster RCT | Australia | 12 Indigenous communities | Aged ≥ 18, HbA1c ≥ 8.5% and at least one major comorbidity | Educational program delivered to case workers from the community | Home visits and out-of-clinic care according to the patients’ preferences | Usual care | (i)changes in HbA1c; (ii) Changes in blood pressure, weight, height, lipid profile; (iii) Quality of life | 18 months |
| Mehuys 2011 | Cluster RCT | Belgium | 66 community pharmacies | Aged ≥ 45 and <74, BMI ≥ 25 kg/m2, in use of hypoglycaemic medication for at least 12 months | Counseling program delivered by pharmacists | Monthly sessions over 6 months for healthy lifestyle advice including nutrition advice | Usual pharmacist care | (i) changes in fasting plasma glucose, HbA1c; (ii) Adherence to oral hypoglycaemic agents, self-management and knowledge about diabetes | 6 months |
| Moncrieft 2016 | RCT | USA | CHC | Aged ≥ 18 and <70, BMI ≥ 27 kg/m2 and significant depressive symptoms | Educational program delivered by therapists | Seventeen sessions over 12 months including nutrition advice (first 6 months) and behavioral maintenance strategies | Usual care | (i) Changes in weight, HbA1c and depressive symptoms; (ii) Glomerular filtration rate | 6 and 12 months |
| Muchiri 2015 | RCT | South Africa | 2 CHC | Aged ≥ 40 and <70, diagnosed with T2DM at least 1 year before inclusion, without insulin therapy | Educational program delivered by dieticians | Eight weekly sessions (2 h duration), followed by four monthly and two bimonthly groups sessions | Usual care, printed educational material | (i) Change in HbA1c; (ii)change in BI, blod pressure and lipid profile; (iii) dietary behaviors | 6 and 12 months |
| Rosal 2011 | RCT | CHC | Aged ≥ 18 and registered in the participating clinics | Educational program delivered by nutricionists and health educators | 12 month | Usual care | (i) changes in HbA1c and BMI | 4 and 12 months | |
| Siaw 2017 | RCT | Singapure | 4 outpatient healthcare institutions | Aged ≥ 21, with HbA1c > 7%, polypharmacy (taking > 5 medications) and multiple comorbidities | Educational program delivered by pharmacists, nurse educators and dieticians | 30 min sessions monthly over 6 month | Usual care | (i) changes in HbA1c, SBP, lipid profile; (ii) Diabetes treatment satisfaction questionnaire | 3 and 6 months |
| Smith 2011 | Cluster RCT | Ireland | 20 PHCC | Aged ≥ 18 and registered in the participating clinics | Educational program delivered by peer supporters | Nine sessions over two years | Usual care | (i) changes in HbA1c, blood pressure, cholesterol concentration and well-being; (ii)BMI, diabetes self-care activities, self-efficacy, adherence to medications | 24 months |
| Taheri 2020 | RCT | Qatar | 1 PHCC | Aged ≥ 18 and <50, diagnosed at most three years before inclusion, BMI > 27 kg/m2 | Food substitution program followed by gradual reintroduction of meals | 12 week meal replacement, followed by 12-week stepped food reintroduction and maintenance counseling for 6 months | Usual care, standard diet and activity advice | (i) changes in weight; (ii) changes in HbA1c and proportion of patients in diabetes remission (HbA1c < 6.5% without antidiabetic medication) | 12 months |
| Tejada Tayabas 2006 | RCT | Mexico | 1 PHCC | Aged ≥ 18, diagnosed at most four years before inclusion, without comorbidities | Educational program delivered by the investigators | Five group monthly sessions followed by four monthly individual counseling sessions | Usual Care | (i) changes in HbA1c | 9 months |
| Toobert 2011 | RCT | USA | 9 PHCC and 1 CHC | Aged ≥ 30 and <75, Latino ethnicity, diagnosed at least six months before inclusion | Mediterranean Diet | 2 1/2 –day retreat followed by weekly meetings over 6 months, that became less frequent until bi-monthly from month 18 to 24 | Usual care | (i) Self-efficacy and behavior change; (ii) BMI, HbA1c, cardiovascular risk | 12 and 24 months |
| Vos 2019 | RCT | The Netherlands | 43 PHCC | Aged ≥ 18 and <75, diagnosed at least 3 months and at most 5 years before inclusion | Educational program delivered by a nurse | Two individual and 5 groups sessions over 12 weeks, followed by a booster session at month 12 | Usual care | (i) change in BMI; change in HbA1c, SBP, lipid profile, self-management behavior, medication adherence, health status, diabetes-related quality of life and cost-effectiveness | 30 months |
| Wolf 2004 | RCT | USA | 1 general practice research center | Aged ≥ 20, BMI ≥ 27 kg/m2, in use of antidiabetic medication | Counseling program delivered by a dietician | Six individual sessions and 1 group session over 12 months | Usual care, printed educational material | (i) changes in weight and waist circumference; (ii) changes in HbA1c, lipid profile, use of prescription medications and quality of life | 6 and 12 months |
a Inclusion criteria besides having a diagnosis of type II diabetes mellitus Abbreviations. RCT: randomized controlled trial; PHCC: primary health care center; CHC: community health center; HTN: hypertension; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; LDL: low-density lipoprotein cholesterol; DTSQ: Diabetes treatment satisfaction questionnaire; ADDQoL: Audit of Diabetes Dependent Quality of Life questionnaire; CES-D: Center for Epidemiologic Studies Depression scale; SDSCA: Summary of Diabetes Self-Care Activities questionnaire.
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Saeedi, P.; Salpea, P.; Karuranga, S.; Petersohn, I.; Malanda, B.; Gregg, E.W.; Unwin, N.; Wild, S.H.; Williams, R. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108086. [Google Scholar] [CrossRef]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA J. Am. Med. Assoc. 2017, 317, 165–182. [Google Scholar] [CrossRef]
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20–79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Yildiz, M.; Esenboğa, K.; Oktay, A.A. Hypertension and diabetes mellitus: Highlights of a complex relationship. Curr. Opin. Cardiol. 2020, 35, 397–404. [Google Scholar] [CrossRef]
- Stewart, G.L.; Tambascia, M.A.; Guzmán, J.R.; Etchegoyen, F.; Carrión, J.O.; Artemenko, S. Control of type 2 diabetes mellitus among general practitioners in private practice in nine countries of Latin America. Rev. Panam. Salud Pública 2007, 22, 12–20. [Google Scholar] [CrossRef]
- Huayanay-Espinoza, I.E.; Guerra-Castañon, F.; Lazo-Porras, M.; Castaneda-Guarderas, A.; Thomas, N.J.; Garcia-Guarniz, A.-L.; Valdivia-Bustamante, A.A.; Málaga, G. Metabolic control in patients with type 2 diabetes mellitus in a public hospital in Peru: A cross-sectional study in a low-middle income country. PeerJ 2016, 4, e2577. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Jacob, S.; Serrano-Gil, M. Engaging and empowering patients to manage their type 2 diabetes, Part II: Initiatives for success. Adv. Ther. 2010, 27, 665–680. [Google Scholar] [CrossRef]
- Lo, C.; Ilic, D.; Teede, H.; Fulcher, G.; Gallagher, M.; Kerr, P.G.; Murphy, K.; Polkinghorne, K.; Russell, G.; Usherwood, T.; et al. Primary and tertiary health professionals’ views on the health-care of patients with co-morbid diabetes and chronic kidney disease—A qualitative study. BMC Nephrol. 2016, 17, 50. [Google Scholar] [CrossRef]
- American Diabetes Association 5. Lifestyle Management: Standards of Medical Care in Diabetes—2019. Diabetes Care 2018, 42, S46–S60. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureThe JNC 7 Report. JAMA 2003, 289, 2560–2571. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Chaimani, A.; Hoffmann, G.; Schwedhelm, C.; Boeing, H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur. J. Epidemiol. 2018, 33, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Franz, M.J.; Boucher, J.L.; Rutten-Ramos, S.; VanWormer, J.J. Lifestyle Weight-Loss Intervention Outcomes in Overweight and Obese Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Acad. Nutr. Diet. 2015, 115, 1447–1463. [Google Scholar] [CrossRef]
- García-Molina, L.; Lewis-Mikhael, A.-M.; Riquelme-Gallego, B.; Cano-Ibáñez, N.; Oliveras-López, M.-J.; Bueno-Cavanillas, A. Improving type 2 diabetes mellitus glycaemic control through lifestyle modification implementing diet intervention: A systematic review and meta-analysis. Eur. J. Nutr. 2019, 59, 1313–1328. [Google Scholar] [CrossRef]
- Schellenberg, E.S.; Dryden, D.M.; VanderMeer, B.; Ha, C.; Korownyk, C. Lifestyle Interventions for Patients With and at Risk for Type 2 Diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 543–551. [Google Scholar] [CrossRef]
- Odgers-Jewell, K.; Ball, L.E.; Kelly, J.T.; Isenring, E.A.; Reidlinger, D.P.; Thomas, R. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: A systematic review with meta-analyses and meta-regression. Diabet. Med. 2017, 34, 1027–1039. [Google Scholar] [CrossRef]
- Kalyoncu, Z.B.; Pars, H.; Bora-Güneş, N.; Karabulut, E.; Aslan, D. A systematic review of nutrition-based practices in prevention of hypertension among healthy youth. Turk. J. Pediatr. 2015, 56, 335–346. [Google Scholar]
- Nicolson, D.J.; Dickinson, O.H.; Campbell, F.; Mason, J.M. Lifestyle interventions or drugs for patients with essential hypertension: A systematic review. J. Hypertens. 2004, 22, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing Risk of Bias in a Randomized Trial. Available online: www.training.cochrane.org/handbook (accessed on 7 December 2021).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Galendi, J.S.C.; Leite, R.G.O.F.; Mendes, A.L.; Nunes-Nogueira, V.D.S. Effectiveness of strategies for nutritional therapy for patients with type 2 diabetes and/or hypertension in primary care: Protocol of a systematic review of randomised controlled trials. BMJ Open 2019, 9, e030450. [Google Scholar] [CrossRef]
- Malachias, M.; Plavnik, F.L.; Machado, C.A.; Malta, D.; Scala, L.C.N.; Fuchs, S. 7th Brazilian Guideline of Arterial Hypertension: Chapter 1—Concept, Epidemiology and Primary Prevention. Arq. Bras. Cardiol. 2016, 107, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Tierney, J.F.; Clarke, M. Chapter 26: Individual Participant Data. Available online: www.training.cochrane.org/handbook (accessed on 22 December 2021).
- Guyatt, G.; Oxman, A.D.; Sultan, S.; Brozek, J.; Glasziou, P.; Alonso-Coello, P.; Atkins, D.; Kunz, R.; Montori, V.; Jaeschke, R.; et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J. Clin. Epidemiol. 2013, 66, 151–157. [Google Scholar] [CrossRef]
- Adachi, M.; Yamaoka, K.; Watanabe, M.; Nishikawa, M.; Kobayashi, I.; Hida, E.; Tango, T. Effects of lifestyle education program for type 2 diabetes patients in clinics: A cluster randomized controlled trial. BMC Public Health 2013, 13, 467. [Google Scholar] [CrossRef]
- Adolfsson, E.T.; Walker-Engström, M.-L.; Smide, B.; Wikblad, K. Patient education in type 2 diabetes—A randomized controlled 1-year follow-up study. Diabetes Res. Clin. Pract. 2007, 76, 341–350. [Google Scholar] [CrossRef]
- Baer, H.J.; Rozenblum, R.; De La Cruz, B.A.; Orav, E.J.; Wien, M.; Nolido, N.V.; Metzler, K.; McManus, K.D.; Halperin, F.; Aronne, L.J.; et al. Effect of an Online Weight Management Program Integrated With Population Health Management on Weight Change: A Randomized Clinical Trial. JAMA 2020, 324, 1737–1746. [Google Scholar] [CrossRef]
- Benson, G.A.; Sidebottom, A.; Hayes, J.; Miedema, M.D.; Boucher, J.; Vacquier, M.; Sillah, A.; Gamam, S.; VanWormer, J.J. Impact of ENHANCED (diEtitiaNs Helping pAtieNts CarE for Diabetes) Telemedicine Randomized Controlled Trial on Diabetes Optimal Care Outcomes in Patients with Type 2 Diabetes. J. Acad. Nutr. Diet. 2019, 119, 585–598. [Google Scholar] [CrossRef]
- Brown, S.A.; Garcia, A.A.; Kouzekanani, K.; Hanis, C.L. Culturally Competent Diabetes Self-Management Education for Mexican Americans: The Starr County border health initiative. Diabetes Care 2002, 25, 259–268. [Google Scholar] [CrossRef]
- Browning, C.; Chapman, A.; Yang, H.; Liu, S.; Zhang, T.; Enticott, J.; Thomas, S. Management of type 2 diabetes in China: The Happy Life Club, a pragmatic cluster randomised controlled trial using health coaches. BMJ Open 2016, 6, e009319. [Google Scholar] [CrossRef]
- Clancy, D.E.; Huang, P.; Okonofua, E.; Yeager, D.; Magruder, K.M. Group Visits: Promoting Adherence to Diabetes Guidelines. J. Gen. Intern. Med. 2007, 22, 620–624. [Google Scholar] [CrossRef]
- De la Fuente Coria, M.d.C.; Cruz-Cobo, C.; Santi-Cano, M. Effectiveness of a primary care nurse delivered educational intervention for patients with type 2 diabetes mellitus in promoting metabolic control and compliance with long-term therapeutic targets: Randomised controlled trial. Int. J. Nurs. Stud. 2019, 101, 103417. [Google Scholar] [CrossRef]
- do Rosário Pinto, M.; Parreira, P.M.D.S.; Basto, M.L.; Dos Santos Mendes Mónico, L. Impact of a structured multicomponent educational intervention program on metabolic control of patients with type 2 diabetes. BMC Endocr. Disord. 2017, 17, 77. [Google Scholar] [CrossRef]
- Edelman, D.; Dolor, R.J.; Coffman, C.J.; Pereira, K.C.; Granger, B.B.; Lindquist, J.H.; Neary, A.M.; Harris, A.J.; Bosworth, H.B. Nurse-Led Behavioral Management of Diabetes and Hypertension in Community Practices: A Randomized Trial. J. Gen. Intern. Med. 2015, 30, 626–633. [Google Scholar] [CrossRef]
- Eriksson, M.K.; Franks, P.W.; Eliasson, M. A 3-Year Randomized Trial of Lifestyle Intervention for Cardiovascular Risk Reduction in the Primary Care Setting: The Swedish Björknäs Study. PLoS ONE 2009, 4, e5195. [Google Scholar] [CrossRef]
- Gabbay, R.A.; Añel-Tiangco, R.M.; Dellasega, C.; Mauger, D.T.; Adelman, A.; Van Horn, D.H. Diabetes nurse case management and motivational interviewing for change (DYNAMIC): Results of a 2-year randomized controlled pragmatic trial. J. Diabetes 2013, 5, 349–357. [Google Scholar] [CrossRef]
- Hörnsten, A.; Lundman, B.; Stenlund, H.; Sandström, H. Metabolic improvement after intervention focusing on personal understanding in type 2 diabetes. Diabetes Res. Clin. Pract. 2005, 68, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-C.; Hsu, C.-C.; Wang, H.-S.; Shin, S.-J. Prospective Randomized Controlled Trial to Evaluate Effectiveness of Registered Dietitian–Led Diabetes Management on Glycemic and Diet Control in a Primary Care Setting in Taiwan. Diabetes Care 2009, 33, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Javaid, Z.; Imtiaz, U.; Khalid, I.; Saeed, H.; Khan, R.Q.; Islam, M.; Saleem, Z.; Sohail, M.F.; Danish, Z.; Batool, F.; et al. A randomized control trial of primary care-based management of type 2 diabetes by a pharmacist in Pakistan. BMC Health Serv. Res. 2019, 19, 409. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef]
- Liss, D.T.; Finch, E.A.; Cooper, A.; Sheth, A.; Tejuosho, A.D.; Lancki, N.; Ackermann, R.T. One-year effects of a group-based lifestyle intervention in adults with type 2 diabetes: A randomized encouragement trial. Diabetes Res. Clin. Pract. 2018, 140, 36–44. [Google Scholar] [CrossRef]
- Mash, R.J.; Rhode, H.; Zwarenstein, M.; Rollnick, S.; Lombard, C.; Steyn, K.; Levitt, N. Effectiveness of a group diabetes education programme in under-served communities in South Africa: A pragmatic cluster randomized controlled trial. Diabet. Med. 2014, 31, 987–993. [Google Scholar] [CrossRef]
- McDermott, R.A.; Schmidt, B.; Preece, C.; Owens, V.; Taylor, S.; Li, M.; Esterman, A. Community health workers improve diabetes care in remote Australian Indigenous communities: Results of a pragmatic cluster randomized controlled trial. BMC Health Serv. Res. 2015, 15, 68. [Google Scholar] [CrossRef]
- Mehuys, E.; Van Bortel, L.; De Bolle, L.; Van Tongelen, I.; Annemans, L.; Remon, J.-P.; Giri, M. Effectiveness of a community pharmacist intervention in diabetes care: A randomized controlled trial. J. Clin. Pharm. Ther. 2010, 36, 602–613. [Google Scholar] [CrossRef]
- Moncrieft, A.E.; Llabre, M.M.; McCalla, J.R.; Gutt, M.; Mendez, A.J.; Gellman, M.D.; Goldberg, R.B.; Schneiderman, N. Effects of a Multicomponent Life-Style Intervention on Weight, Glycemic Control, Depressive Symptoms, and Renal Function in Low-Income, Minority Patients With Type 2 Diabetes: Results of the Community Approach to Lifestyle Modification for Diabetes Randomized Controlled Trial. Psychosom. Med. 2016, 78, 851–860. [Google Scholar] [CrossRef]
- Muchiri, J.W.; Gericke, G.J.; Rheeder, P. Effect of a nutrition education programme on clinical status and dietary behaviours of adults with type 2 diabetes in a resource-limited setting in South Africa: A randomised controlled trial. Public Health Nutr. 2016, 19, 142–155. [Google Scholar] [CrossRef]
- Rosal, M.C.; Ockene, I.S.; Restrepo, A.; White, M.J.; Borg, A.; Olendzki, B.; Scavron, J.; Candib, L.; Welch, G.; Reed, G. Randomized Trial of a Literacy-Sensitive, Culturally Tailored Diabetes Self-Management Intervention for Low-Income Latinos: Latinos en Control. Diabetes Care 2011, 34, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Siaw, M.Y.L.; Ko, Y.; Malone, D.C.; Tsou, K.Y.K.; Lew, Y.-J.; Foo, D.; Tan, E.; Chan, S.C.; Chia, A.; Sinaram, S.S.; et al. Impact of pharmacist-involved collaborative care on the clinical, humanistic and cost outcomes of high-risk patients with type 2 diabetes (IMPACT): A randomized controlled trial. J. Clin. Pharm. Ther. 2017, 42, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Paul, G.; Kelly, A.; Whitford, D.L.; O’Shea, E.; O’Dowd, T. Peer support for patients with type 2 diabetes: Cluster randomised controlled trial. BMJ 2011, 342, d715. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Zaghloul, H.; Chagoury, O.; Elhadad, S.; Ahmed, S.H.; El Khatib, N.; Amona, R.A.; El Nahas, K.; Suleiman, N.; Alnaama, A.; et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): An open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 477–489. [Google Scholar] [CrossRef]
- Tayabas, L.M.T.; Durango, M.d.P.; Enríquez, S.O.G. Efectividad de un programa educativo en el control del enfermo con diabetes. In Investigación y Educación en Enfermería; Medelín, Colombia, 2006; Volume 24, pp. 48–53. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-53072006000200005&lng=en&nrm=iso (accessed on 17 January 2022).
- Toobert, D.J.; Strycker, L.A.; King, D.K.; Barrera, M., Jr.; Osuna, D.; Glasgow, R.E. Long-term outcomes from a multiple-risk-factor diabetes trial for Latinas: Viva Bien. Transl. Behav. Med. 2011, 1, 416–426. [Google Scholar] [CrossRef]
- Vos, R.C.; Van Heusden, L.; Eikelenboom, N.W.D.; Rutten, G.E.H.M. Theory-based diabetes self-management education with pre-selection of participants: A randomized controlled trial with 2.5 years’ follow-up (ELDES Study). Diabet. Med. 2019, 36, 827–835. [Google Scholar] [CrossRef]
- Wolf, A.M.; Conaway, M.R.; Crowther, J.Q.; Hazen, K.Y.; L.Nadler, J.; Oneida, B.; Bovbjerg, V.E. Translating Lifestyle Intervention to Practice in Obese Patients With Type 2 Diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care 2004, 27, 1570–1576. [Google Scholar] [CrossRef]
- Beune, E.J.A.J.; Van Charante, E.P.M.; Beem, L.; Mohrs, J.; Agyemang, C.O.; Ogedegbe, G.; Haafkens, J.A. Culturally Adapted Hypertension Education (CAHE) to Improve Blood Pressure Control and Treatment Adherence in Patients of African Origin with Uncontrolled Hypertension: Cluster-Randomized Trial. PLoS ONE 2014, 9, e90103. [Google Scholar] [CrossRef]
- Hacihasanoğlu, R.; Gözüm, S. The effect of patient education and home monitoring on medication compliance, hypertension management, healthy lifestyle behaviours and BMI in a primary health care setting. J. Clin. Nurs. 2011, 20, 692–705. [Google Scholar] [CrossRef]
- Höchsmann, C.; Dorling, L., Jr.; Martin, C.K.; Newton, J.R.L.; Apolzan, J.W.; Myers, C.A.; Denstel, K.D.; Mire, E.F.; Johnson, W.D.; Zhang, D.; et al. Effects of a 2-Year Primary Care Lifestyle Intervention on Cardiometabolic Risk Factors: A Cluster-Randomized Trial. Circulation 2021, 143, 1202–1214. [Google Scholar] [CrossRef]
- Kastarinen, M.J.; Puska, P.M.; Korhonen, M.H.; Mustonen, J.N.; Salomaa, V.V.; Sundvall, J.E.; Tuomilehto, J.O.; Uusitupa, M.I.; Nissinen, A.M. Non-pharmacological treatment of hypertension in primary health care: A 2-year open randomized controlled trial of lifestyle intervention against. J. Hypertens. 2002, 20, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Ogedegbe, G.; Tobin, J.N.; Fernandez, S.; Cassells, A.; Diaz-Gloster, M.; Khalida, C.; Pickering, T.; Schwartz, J.E. Counseling African Americans to Control Hypertension: Cluster-randomized clinical trial main effects. Circulation 2014, 129, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.R.; Sánchez, C.C.; Ortiz, L.G.; Rodríguez, J.I.R.; Sánchez, Y.C.; Marcos, M.A.G. Efficacy of an educational intervention group on changes in lifestyles in hypertensive patients in primary care: A randomized clinical trial. Rev. Esp. Salud. Publica. 2009, 83, 441–452. [Google Scholar] [CrossRef]
- Schoenthaler, A.; Luerassi, L.; Silver, S.; Odedosu, T.; Kong, J.; Ravenell, J.; Teresi, J.A.; Ogedegbe, G. Comparative Effectiveness of a Practice-Based Comprehensive Lifestyle Intervention vs. Single Session Counseling in Hypertensive Blacks. Am. J. Hypertens. 2016, 29, 280–287. [Google Scholar] [CrossRef][Green Version]
- Tonstad, S.; Alm, C.S.; Sandvik, E. Effect of Nurse Counselling on Metabolic Risk Factors in Patients with Mild Hypertension: A Randomised Controlled Trial. Eur. J. Cardiovasc. Nurs. 2007, 6, 160–164. [Google Scholar] [CrossRef]
- Woollard, J.; Burke, V.; Beilin, L.J. Effects of general practice-based nurse-counselling on ambulatory blood pressure and antihypertensive drug prescription in patients at increased risk of cardiovascular disease. J. Hum. Hypertens. 2003, 17, 689–695. [Google Scholar] [CrossRef]
- Killip, S.; Mahfoud, Z.; Pearce, K. What Is an Intracluster Correlation Coefficient? Crucial Concepts for Primary Care Researchers. Ann. Fam. Med. 2004, 2, 204–208. [Google Scholar] [CrossRef]
- Fralick, M.; Colacci, M.; Odutayo, A.; Siemieniuk, R.; Glynn, R.J. Lowering of hemoglobin A1C and risk of cardiovascular outcomes and all-cause mortality, a meta-regression analysis. J. Diabetes Its Complicat. 2020, 34, 107704. [Google Scholar] [CrossRef]
- Little, R.R.; Rohlfing, C.L. The long and winding road to optimal HbA1c measurement. Clin. Chim. Acta 2013, 418, 63–71. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).