Home-Based High-Intensity Interval Exercise Improves the Postprandial Glucose Response in Young Adults with Postprandial Hyperglycemia
Abstract
:1. Introduction
2. Methods
2.1. Participants and Screening Procedure
2.2. Familiarization Trials
2.3. Experimental Trials
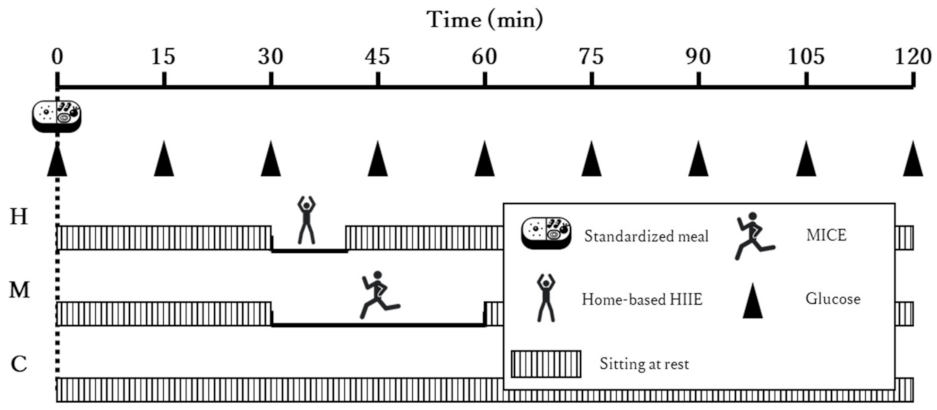
2.4. Trial Conditions
2.5. Measurements
2.6. Food Intake
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Exercise Intensities
3.3. Food Intake
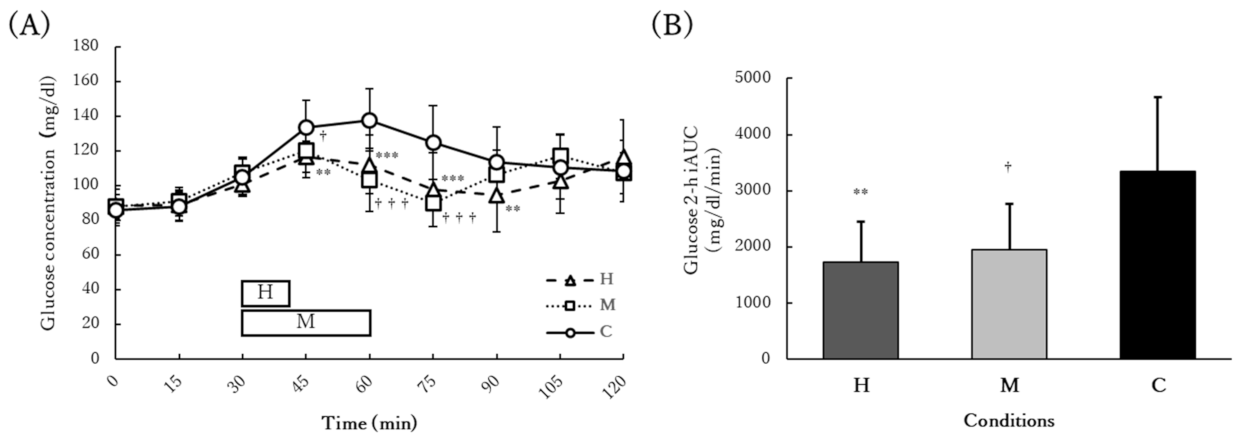
3.4. Acute Glucose Response
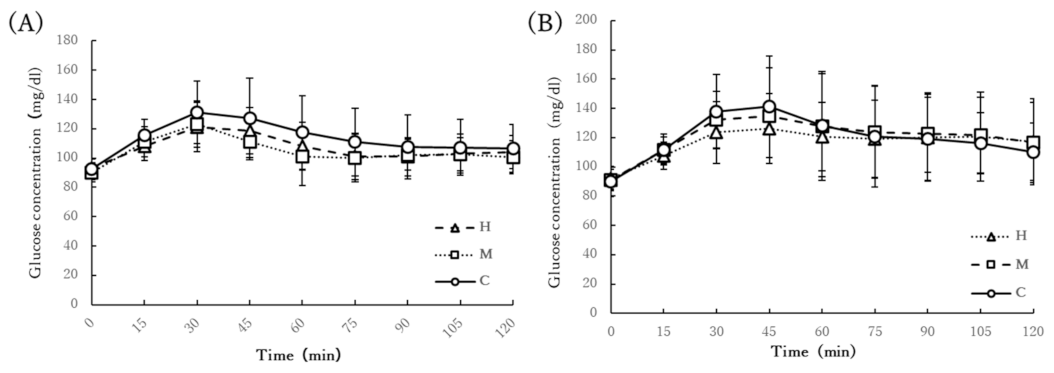
3.5. Chronic Glucose Response
3.6. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lind, M.; Tuomilehto, J.; Uusitupa, M.; Nerman, O.; Eriksson, J.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Peltonen, M.; Pivodic, A.; Lindström, J. The association between HbA1c, fasting glucose, 1-hour glucose and 2-hour glucose during an oral glucose tolerance test and cardiovascular disease in individuals with elevated risk for diabetes. PLoS ONE 2014, 9, e109506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.F.; Xing, G.Q.; Shen, T.; Xu, G.; Peng, Y.; Rao, J.Y.; Shi, R. Postprandial glucose levels are better associated with the risk factors for diabetes compared to fasting glucose and glycosylated hemoglobin (HbA1c) levels in elderly prediabetics: Beneficial effects of polyherbal supplements—A randomized, double-blind, placebo controlled trial. Evid. Based Complement. Altern. Med. 2019, 2019, 7923732. [Google Scholar]
- Cavalot, F.L.; Pagliarino, A.; Valle, M.; Di Martino, L.; Bonomo, K.; Massucco, P.; Anfossi, G.; Trovati, M. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: Lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011, 34, 2237–2243. [Google Scholar] [CrossRef] [Green Version]
- Temelkova-Kurktschiev, T.S.; Koehler, C.; Henkel, E.; Leonhardt, W.; Fuecker, K.; Hanefeld, M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care 2000, 23, 1830–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.A.; Thorpe, J.E.; Testa, R.; Boemi, M.; Giugliano, D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008, 57, 1349–1354. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Hanefeld, M.; Leiter, L.; Monnier, L.; Moses, A.; Owens, D.; Tajima, N.; Tuomilehto, J. Postprandial glucose regulation and diabetic complications. Arch. Intern. Med. 2004, 164, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Colagiuri, S. International Diabetes Federation guideline for management of postmeal glucose: A review of recommendations. Diabet. Med. 2008, 25, 1151–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.J.; Lee, B.C.; Ho, Y.L.; Lin, Y.H.; Chen, C.Y.; Hsu, H.C.; Lin, M.S.; Chien, K.L.; Chen, M.F. Postprandial glucose improves the risk prediction of cardiovascular death beyond the metabolic syndrome in the nondiabetic population. Diabetes Care 2009, 32, 1721–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, T.P.J.; Eves, F.F.; Laye, M.J. Targeting postprandial hyperglycemia with physical activity may reduce cardiovascular disease risk. But what should we do, and when is the right time to move? Front. Cardiovasc. Med. 2018, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Shambrook, P.; Kingsley, M.I.; Taylor, N.F.; Wundersitz, D.W.; Wundersitz, C.E.; Paton, C.D.; Gordon, B.A. A comparison of acute glycaemic responses to accumulated or single bout walking exercise in apparently healthy, insufficiently active adults. J. Sci. Med. Sport 2020, 23, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Salmons, H.; Vinoskey, C.; Kressler, J. A single one-minute, comfortable paced, stair-climbing bout reduces postprandial glucose following a mixed meal. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1967–1972. [Google Scholar] [CrossRef]
- Trost, S.G.; Owen, N.; Bauman, A.E.; Sallis, J.F.; Brown, W. Correlates of adults’ participation in physical activity: Review and update. Med. Sci. Sports Exerc. 2002, 34, 1996–2001. [Google Scholar] [CrossRef]
- Sojka, A.; Machniak, M.; Andrzejewski, W.; Kosendiak, A.; Chwałczyńska, A. Changes in physical activity and the occurrence of specific symptoms of “long-COVID syndrome” in men aged 18–25. Int. J. Environ. Res. Public Health 2022, 19, 1199. [Google Scholar] [CrossRef] [PubMed]
- Czyż, S.H.; Starościak, W. Perceived physical activity during stay-at-home COVID-19 pandemic lockdown March-April 2020 in Polish adults. PeerJ 2022, 10, e12779. [Google Scholar] [CrossRef] [PubMed]
- Schoofs, M.C.A.; Bakker, E.A.; de Vries, F.; Hartman, Y.A.W.; Spoelder, M.; Thijssen, D.H.J.; Eijsvogels, T.M.H.; Buffart, L.M.; Hopman, M.T.E. Impact of Dutch COVID-19 restrictive policy measures on physical activity behavior and identification of correlates of physical activity changes: A cohort study. BMC Public Health 2022, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Terakawa, A.; Bouchi, R.; Kodani, N.; Hisatake, T.; Sugiyama, T.; Matsumoto, M.; Ihana-Sugiyama, N.; Ohsugi, M.; Ueki, K.; Kajio, H. Living and working environments are important determinants of glycemic control in patients with diabetes during the COVID-19 pandemic: A retrospective observational study. J. Diabetes Investig. 2022. [Google Scholar] [CrossRef]
- Aldukhayel, A. The COVID-19 lockdown does not necessarily worsen diabetes control, in spite of lower physical activity—A systematic review. Endokrynol. Pol. 2022, 73, 131–148. [Google Scholar] [CrossRef]
- Poon, E.T.C.; Wongpipit, W.; Ho, R.S.T.; Wong, S.H.S. Interval training versus moderate-intensity continuous training for cardiorespiratory fitness improvements in middle-aged and older adults: A systematic review and meta-analysis. J. Sports Sci. 2021, 39, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Jelleyman, C.; Yates, T.; O’Donovan, G.; Gray, L.J.; King, J.A.; Khunti, K.; Davies, M.J. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes. Rev. 2015, 16, 942–961. [Google Scholar] [CrossRef] [Green Version]
- Thum, J.S.; Parsons, G.; Whittle, T.; Astorino, T.A. High-intensity interval training elicits higher enjoyment than moderate intensity continuous exercise. PLoS ONE 2017, 12, e0166299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackwell, J.; Atherton, P.J.; Smith, K.; Doleman, B.; Williams, J.P.; Lund, J.N.; Phillips, B.E. The efficacy of unsupervised home-based exercise regimens in comparison to supervised laboratory-based exercise training upon cardio-respiratory health facets. Physiol. Rep. 2017, 5, e13390. [Google Scholar] [CrossRef] [PubMed]
- Schwendinger, F.; Pocecco, E. Counteracting physical inactivity during the COVID-19 pandemic: Evidence-based recommendations for home-based exercise. Int. J. Environ. Res. Public Health 2020, 17, 3909. [Google Scholar] [CrossRef] [PubMed]
- Cora, L.C.; Alison, L.M.; Michael, S.; Adrian, E.B.; Michael, L.B.; Barbara, E.A.; Michael, P.; Ulf, E.; Agneta, Y.; James, F.S.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar]
- Wolthuis, R.A.; Froelicher, V.F.; Fischer, J.; Noguera, I.; Davis, G.; Stewart, A.J.; Triebwasser, J.H. New practical treadmill protocol for clinical use. Am. J. Cardiol. 1977, 39, 697–700. [Google Scholar] [CrossRef]
- Kendzierski, D.; DeCarlo, K.J. Physical activity enjoyment scale: Two validation studies. J. Sport Exerc. Physiol. 1991, 13, 50–64. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.B.D. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef]
- Shambrook, P.; Kingsley, M.I.; Wundersitz, D.W.; Xanthos, P.D.; Wyckelsma, V.L.; Gordon, B.A. Glucose response to exercise in the post-prandial period is independent of exercise intensity. Scand. J. Med. Sci. Sports 2018, 28, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wongpipit, W.; Sun, F.H.; Sheridan, S.; Huang, W.Y.J.; Sit, C.H.P.; Wong, S.H.S. Walking initiated 20 minutes before the time of individual postprandial glucose peak reduces the glucose response in young men with overweight or obesity: A randomized crossover study. J. Nutr. 2021, 151, 866–875. [Google Scholar] [CrossRef]
- Parker, L.; Shaw, C.S.; Banting, L.; Levinger, I.; Hill, K.M.; McAinch, A.J.; Stepto, N.K. Acute low-volume high-intensity interval exercise and continuous moderate-intensity exercise elicit a similar improvement in 24-h glycemic control in overweight and obese adults. Front. Physiol. 2017, 7, 661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellini, A.; Nicolò, A.; Bulzomì, R.; Bazzucchi, I.; Sacchetti, M. The effect of different postprandial exercise types on glucose response to breakfast in individuals with type 2 diabetes. Nutrients 2021, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Deemer, S.E.; Castleberry, T.J.; Irvine, C.; Newmire, D.E.; Oldham, M.; King, G.A.; Ben-Ezra, V.; Irving, B.A.; Biggerstaff, K.D. Pilot study: An acute bout of high intensity interval exercise increases 12.5 h GH secretion. Physiol. Rep. 2018, 6, e13563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.E.; Bourne, J.E.; Little, J.P. Where does HIT fit? An examination of the affective response to high-intensity intervals in comparison to continuous moderate-and continuous vigorous-intensity exercise in the exercise intensity-affect continuum. PLoS ONE 2014, 9, e114541. [Google Scholar] [CrossRef] [Green Version]
- Kriel, Y.; Askew, C.D.; Solomon, C. The effect of running versus cycling high-intensity intermittent exercise on local tissue oxygenation and perceived enjoyment in 18–30-year-old sedentary men. PeerJ 2018, 6, e5026. [Google Scholar] [CrossRef]
- Herregods, T.V.K.; Van Hoeij, F.B.; Oors, J.M.; Bredenoord, A.J.; Smout, A.J.P.M. Effect of running on gastroesophageal reflux and reflux mechanisms. Off. J. Am. Coll. Gastroenterol. 2016, 111, 940–946. [Google Scholar] [CrossRef]
- Kondo, T.; Nakae, Y.; Mitsui, T.; Kagaya, M.; Matsutani, Y.; Horibe, H.; Read, N.W. Exercise-induced nausea is exaggerated by eating. Appetite 2001, 36, 119–125. [Google Scholar] [CrossRef]
- Reid, K.; Grundy, D.; Khan, M.I.; Read, N.W. Gastric emptying and the symptoms of vection-induced nausea. Eur. J. Gastroenterol. Hepatol. 1995, 7, 103–108. [Google Scholar]
- Zhang, X.Y.; Sun, F.H.; Wongpipit, W.; Huang, W.Y.J.; Wong, S.H.S. Accuracy of flash glucose monitoring during postprandial rest and different walking conditions in overweight or obese young adults. Front. Physiol. 2021, 12, 732751. [Google Scholar] [CrossRef]

| Variable | Values |
|---|---|
| Age (years) | 24.3 ± 2.3 |
| Height (cm) | 173.3 ± 5.8 |
| Mass (kg) | 68.0 ± 10.4 |
| BMI (kg/m2) | 22.6 ± 2.3 |
| Fasting glucose (mg/dL) | 93.4 ± 6.7 |
| Peak glucose (mg/dL) | 155.6 ± 12.1 |
| VO2max (mL/kg/min) | 42.9 ± 3.8 |
| MICE velocity (km/h) | 5.8 ± 0.5 |
| Variable | Home-Based HIIE | MICE | p-Value |
|---|---|---|---|
| Total energy consumption (kcal) | 99.5 ± 52.1 | 210 ± 43.3 | <0.001 |
| Mean %VO2max (%) | 64.4 ± 7.4 | 49.3 ± 3.8 | <0.001 |
| Peak %VO2max (%) | 81.1 ± 9.3 | - | - |
| Mean %HRmax (%) | 79.4 ± 4.9 | 66.2 ± 5.0 | <0.001 |
| Peak %HRmax (%) | 88.9 ± 3.3 | - | - |
| Rating of Perceived Exertion (RPE) | 17 ± 1.8 | 11 ± 1.9 | <0.001 |
| Variable | H | M | C |
|---|---|---|---|
| Dinner on the day before each trial (g) | 106.8 ± 27.1 | 95.9 ± 26.3 | 102.2 ± 25.7 |
| Lunch on the day of each trial (g) | 92.0 ± 37.1 | 86.8 ± 34.4 | 89.2 ± 35.9 |
| Dinner on the day of each trial (g) | 98.2 ± 20.3 | 96.9 ± 20.5 | 98.6 ± 20.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, Y.; Ono, K.; Okagawa, J.; Urabe, J.; Yamau, R.; Ishikawa, A. Home-Based High-Intensity Interval Exercise Improves the Postprandial Glucose Response in Young Adults with Postprandial Hyperglycemia. Int. J. Environ. Res. Public Health 2022, 19, 4227. https://doi.org/10.3390/ijerph19074227
Nakayama Y, Ono K, Okagawa J, Urabe J, Yamau R, Ishikawa A. Home-Based High-Intensity Interval Exercise Improves the Postprandial Glucose Response in Young Adults with Postprandial Hyperglycemia. International Journal of Environmental Research and Public Health. 2022; 19(7):4227. https://doi.org/10.3390/ijerph19074227
Chicago/Turabian StyleNakayama, Yuto, Kumiko Ono, Junya Okagawa, Junji Urabe, Ryoga Yamau, and Akira Ishikawa. 2022. "Home-Based High-Intensity Interval Exercise Improves the Postprandial Glucose Response in Young Adults with Postprandial Hyperglycemia" International Journal of Environmental Research and Public Health 19, no. 7: 4227. https://doi.org/10.3390/ijerph19074227
APA StyleNakayama, Y., Ono, K., Okagawa, J., Urabe, J., Yamau, R., & Ishikawa, A. (2022). Home-Based High-Intensity Interval Exercise Improves the Postprandial Glucose Response in Young Adults with Postprandial Hyperglycemia. International Journal of Environmental Research and Public Health, 19(7), 4227. https://doi.org/10.3390/ijerph19074227






