Barriers to Access the Pap Smear Test for Cervical Cancer Screening in Rural Riverside Populations Covered by a Fluvial Primary Healthcare Team in the Amazon
Abstract
1. Introduction
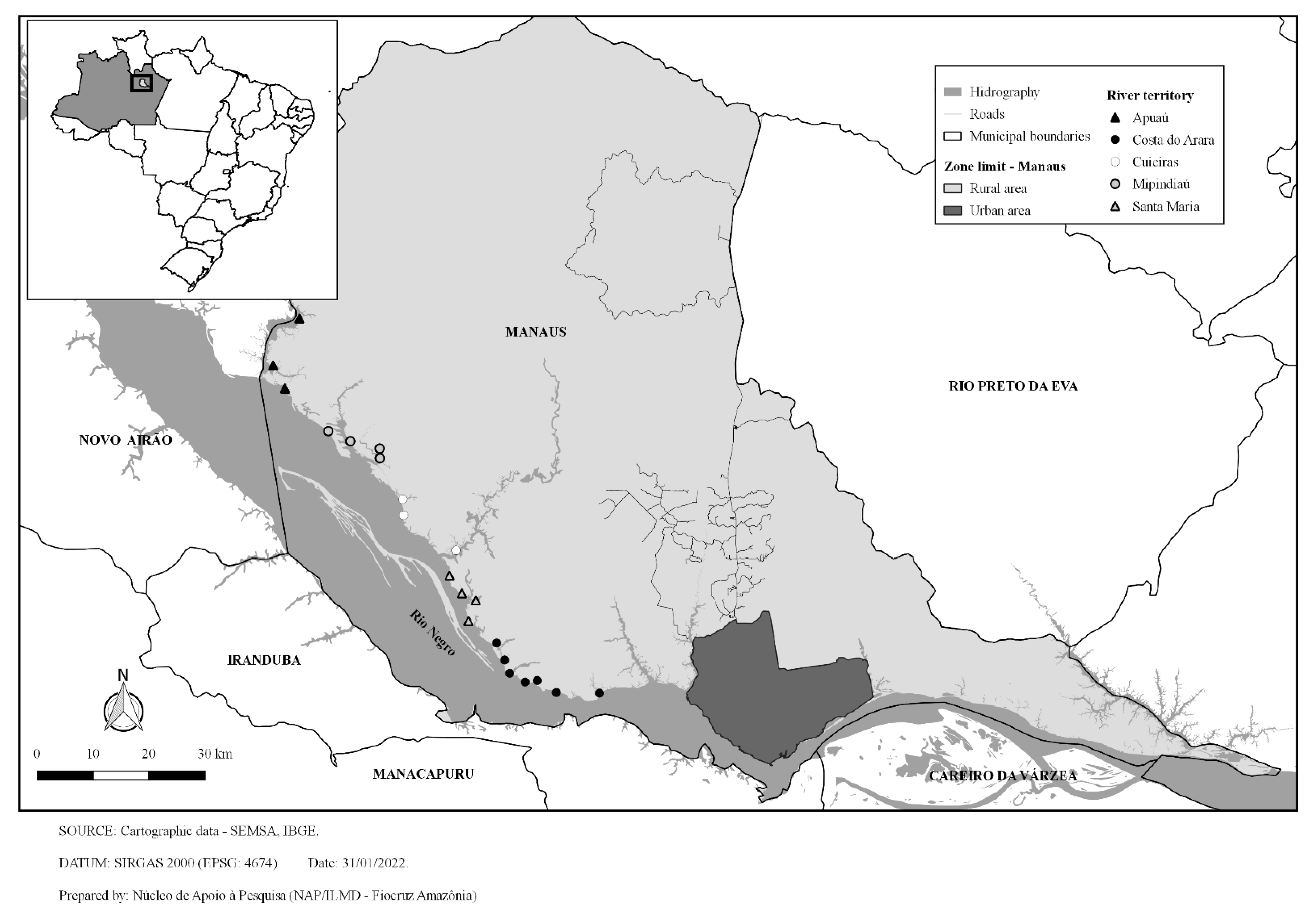
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, V.A.S.; Ribeiro, J.M. Cervical cancer control limiting factors and facilitators: A literature review. Cienc. Saude Coletiva 2019, 24, 3431–3442. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Strategy to Accelerate the Elimination of Cervical Cancer As a Public Health Problem; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394424. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde. Instituto Nacional do Câncer José de Alencar Gomes da Silva (INCA). Estimativa 2020: Incidência de câncer no Brasil. Rio de Janeiro: Ministério da Saúde. 2019. Available online: https://www.inca.gov.br/publicacoes/livros/estimativa-2020-incidencia-de-cancer-no-brasil (accessed on 20 October 2021).
- Organização Pan-Americana da Saúde (OPAS). Nota de Orientação da OPAS/OMS: Prevenção e Controle de Amplo Alcance do Câncer do Colo do Útero: Um Futuro Mais Saudável Para Meninas e Mulheres; OPAS: Washington, DC, USA, 2013. [Google Scholar]
- Instituto Nacional do Câncer José de Alencar Gomes da Silva (INCA). Diretrizes Brasileiras Para o Rastreamento do Câncer do Colo do Útero; INCA: Rio de Janeiro, Brazil, 2016.
- Carvalho, L.R.; Jurado, S.R. Motivos que influenciam a não realização do exame de Papanicolaou. Rev. Cient. Enferm. 2018, 8, 39–46. [Google Scholar] [CrossRef]
- Garnelo, L.; Lima, J.G.; Rocha, E.S.C.; Herkrath, F.J. Access and coverage of Primary Health Care for rural and urban populations in the northern region of Brazil. Saude Debate 2018, 42, 81–99. [Google Scholar] [CrossRef]
- Garnelo, L.; Parente, R.C.P.; Puchiarelli, M.L.R.; Correia, P.C.; Torres, M.V.; Herkrath, F.J. Barriers to access and organization of primary health care services for rural riverside populations in the Amazon. Int. J. Equity Health 2020, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Aquino, E.M.L.; do Carmo Leal, M.; Monteiro, C.A.; Barros, F.C.; Szwarcwald, C.L. Maternal and child health in Brazil: Progress and challenges. Lancet 2011, 377, 1863–1876. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Portaria GM no 2.488, de 21 de Setembro de 2017: Aprova a Política Nacional de Atenção Básica, Estabelecendo a Revisão de Diretrizes Para a Organização da Atenção Básica, no Âmbito do Sistema Único de Saúde (SUS). Brasília, DF: Diário Oficial da República Federativa do Brasil. 2011. Available online: http://bvsms.saude.gov.br/bvs/saudelegis/gm/2017/prt2436_22_09_2017.html (accessed on 17 June 2020).
- Kadri, M.R.; Santos, B.S.; Lima, R.T.S.; Schweickardt, J.C.; Martins, F.M. Unidade Básica de Saúde Fluvial: Um novo modelo da Atenção Básica para a Amazônia, Brasil. Interface 2019, 23, e180613. [Google Scholar] [CrossRef]
- Tiensoli, S.D.; Felisbino-Mendes, M.S.; Velasquez-Melendez, G. Evaluation of non-attendance for Pap test through the Surveillance System by telephone survey. Rev. Esc. Enferm. USP 2018, 52, e03390. [Google Scholar]
- World Health Organization (WHO). A Conceptual Framework for Action on the Social Determinants of Health; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Brito-Silva, K.; Bezerra, A.F.B.; Chaves, L.D.P.; Tanaka, O.U. Integrality in cervical cancer care: Evaluation of access. Rev. Saude Publica 2014, 48, 240–248. [Google Scholar] [CrossRef]
- Noé, B.R.; Trindade, F.R.; Dexheimer, G.M. Análise da periodicidade e da idade na realização do exame citopatológico cervicovaginal no Rio Grande do Sul. Rev. Saúde Desenvolv. 2018, 12, 104–120. [Google Scholar]
- Tomasi, E.; Oliveira, T.F.; Fernandes, P.A.A.; Thumé, E.; Silveira, D.S.; Siqueira, F.V.; Duro, S.M.S.; Saes, M.O.; Nunes, B.P.; Fassa, A.G.; et al. Estrutura e processo de trabalho na prevenção do câncer de colo de útero na Atenção Básica à Saúde no Brasil: Programa de Melhoria do Acesso e da Qualidade—PMAQ. Rev. Bras. Saúde Matern. Infant. 2015, 15, 171–180. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Andrade, S.S.C.A.; Oliveira, P.P.V.; Silva, G.A.; Silva, M.M.A.; Malta, D.C. Pap-test coverage in women aged 25 to 64 years old, according to the National Health Survey and the Surveillance System for Risk and Protective Factors for Chronic Diseases by Telephone Survey, 2013. Rev. Bras Epidemiol. 2018, 21, e180014. [Google Scholar] [PubMed]
- Borges, M.F.S.O.; Dotto, L.M.G.; Koifman, R.J.; Cunha, M.A.; Muniz, P.T. Prevalência do exame preventivo de câncer do colo do útero em Rio Branco, Acre, Brasil, e fatores associados à não-realização do exame. Cad. Saude Publica 2012, 28, 1156–1166. [Google Scholar] [CrossRef]
- Navarro, C.; Fonseca, A.J.; Sibajev, A.; Souza, C.I.A.; Araújo, D.S.; Teles, D.A.F.; Carvalho, S.G.L.; Cavalcante, K.W.M.; Rabelo, W.L. Cervical cancer screening coverage in a high-incidence region. Rev. Saude Publica 2015, 49, 17. [Google Scholar] [CrossRef][Green Version]
- Duarte, D.V.; Vieira, R.C.; Brito, E.B.; Pinheiro, M.C.N.; Monteiro, J.S.V.; Valente, M.D.R.; Ishikawa, E.A.Y.; Fuzii, H.T.; Sousa, M.S. Prevalence of Human Papillomavirus Infection and Cervical Cancer Screening among Riverside Women of the Brazilian Amazon. Rev. Bras. Ginecol. Obstet. 2017, 39, 350–357. [Google Scholar] [CrossRef]
- Barbosa, I.R.; Souza, D.L.B.; Bernal, M.M.; Costa, I.C.C. Desigualdades regionais na mortalidade por câncer de colo de útero no Brasil: Tendências e projeções até o ano 2030. Cien. Saude Colet. 2016, 21, 253–262. [Google Scholar] [CrossRef][Green Version]
- Lobo, L.M.G.A.; Almeida, M.M.; Oliveira, F.B.M. Uterine column cancer, HPV and Papanicolaou experiment: A reflection on women’s knowledge. ReonFacema 2018, 4, 889–895. [Google Scholar]
- Melo, E.M.F.; Linhares, F.M.P.; Silva, T.M.; Pontes, C.M.; Santos, A.H.S.; Oliveira, S.C. Cervical cancer: Knowledge, attitude and practice on the prevention examination. Rev. Bras. Enferm. 2019, 72, 25–31. [Google Scholar] [CrossRef]
- Abiodun, O.A.; Olu-Abiodun, O.O.; Sotunsa, J.O.; Oluwole, F.A. Impact of health education intervention on knowledge and perception of cervical cancer and cervical screening uptake among adult women in rural communities in Nigeria. BMC Public Health 2014, 14, 814. [Google Scholar] [CrossRef]
- Silva, M.A.S.; Teixeira, E.M.B.; Ferrari, R.A.P.; Cestari, M.E.W.; Cardelli, A.A.M. Factors related to non-adherence to the realization of the Papanicolaou test. Rev. Rene 2015, 16, 532–539. [Google Scholar] [CrossRef]
- Iglesias, G.A.; Larrubia, L.G.; Campos Neto, A.S.; Pacca, F.C.; Iembo, T. Conhecimento e adesão ao Papanicolau de mulheres de uma rede de Atenção Primária à Saúde. Rev. Cienc. Med. 2019, 28, 21–30. [Google Scholar] [CrossRef]
- Fernandes, N.F.S.; Galvão, J.R.; Assis, M.M.A.; Almeida, P.F.; Santos, A.M. Acesso ao exame citológico do colo do útero em região de saúde: Mulheres invisíveis e corpos vulneráveis. Cad. Saude Publica 2019, 35, e00234618. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, L.C.; Sousa, A.A.S.; Sousa, C.M.S.; Brito, E.A.S.; Leite, R.S.S.; Santana, W.J.; Vieira, P.D. Percepção de mulheres sobre o exame de prevenção de colo de útero Papanicolau: Uma revisão integrativa da literatura. Rev. Mult. Psic. 2019, 13, 434–445. [Google Scholar]
- Onofre, M.F.; Vieira, R.D.; Bueno, G.H. Principais fatores que dificultam a adesão ao exame de citologia oncótica: Uma revisão de literatura. Enferm. Rev. 2019, 22, 231–242. [Google Scholar]
- Silva, M.C.M.; Brito, I.S.; Bernardo, B.C.; Rocha, E.N.S.G.N.; Pascoal, A.M.J.G.J.; Candeia, J.A.V. Cervical Cancer Prevention: Empowerment of women from a community of Luanda. Omnia 2016, 5, 75–91. [Google Scholar] [CrossRef]
- Rico, A.M.; Iriart, J.A.B. “Tem mulher, tem preventivo”: Sentidos das práticas preventivas do câncer do colo do útero entre mulheres de Salvador, Bahia, Brasil. Cad. Saude Publica 2013, 29, 1763–1773. [Google Scholar] [CrossRef]
- Jia, Y.; Li, S.; Yang, R.; Zhou, H.; Xiang, O.; Hu, T.; Zhang, Q.; Chen, Z.; Ma, D.; Feng, L. Knowledge about cervical cancer and barriers of screening program among women in Wufeng County, a high-incidence region of cervical cancer in China. PLoS ONE 2013, 8, e67005. [Google Scholar] [CrossRef]
- Andrade, M.S.; Almeida, M.M.G.; Araújo, T.M.; Santos, K.O.B. Fatores associados à não adesão ao Papanicolau entre mulheres atendidas pela Estratégia Saúde da Família em Feira de Santana, Bahia, 2010. Epidemiol. Serv. Saude 2014, 23, 111–120. [Google Scholar] [CrossRef]
- Santos, A.C.S.; Varela, C.D.S. Prevenção do câncer de colo uterino: Motivos que influenciam a não realização do exame de papanicolaou. Rev. Enferm. Contemp. 2016, 4, 179–188. [Google Scholar] [CrossRef][Green Version]
- Costa, R.S.L.; Silva, M.V.R.E.; Souza, T.N. Fatores que levam a não adesão ao exame preventivo do câncer do colo uterino em uma unidade de saúde do Acre em 2014. DêCiência Foco 2018, 2, 5–18. [Google Scholar]
- Oliveira, B.S.; Oliveira, S.S.; Santos, T.H.A.; Andrade, T.R.S.F.; Cavalcante, A.B.; Ferrari, Y.A.C. Fatores associados à não adesão ao exame citopatológico do colo uterino: Uma revisão integrativa. Rev. Saúde Desenvolv. 2020, 14, 131–141. [Google Scholar]
- Pitilin, E.B.; Lentsck, M.H. Primary Health Care from the perception of women living in a rural area. Rev. Esc. Enferm. USP 2015, 49, 726–732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aguilar, R.P.; Soares, D.A. Barreiras à realização do exame Papanicolau: Perspectivas de usuárias e profissionais da Estratégia de Saúde da Família da cidade de Vitória da Conquista-BA. Physis 2015, 25, 359–379. [Google Scholar] [CrossRef]
- Acosta, D.F.; Dantas, T.S.; Cazeiro, C.C.; Acosta, D.F.; Gomes, V.L.O. Vivenciando o exame papanicolau: Entre o (não) querer e o fazer. Rev. Enferm UFPE Line 2017, 11, 3031–3038. [Google Scholar]
- Santos, R.C.A.; Miranda, F.A.N. Importância do vínculo entre profissional-usuário na Estratégia de Saúde da Família. Rev. Enferm. UFSM 2016, 6, 350–359. [Google Scholar] [CrossRef][Green Version]
- Souza, M.S.; Lima, I.A.R.; Souza, L.F.; Teixeira, N.A.; Barbosa, G.P.; Nascimento, A.P.O.; Teles, M.A.B.; Siqueira, L.G. Perfil das mulheres que se submetem ao exame Papanicolau na Estratégia Saúde da Família. Rev. Uningá 2020, 57, 51–60. [Google Scholar]
- Falcão, G.B.; Ibiapina, F.L.P.; Feitosa, H.N.; Feitosa, T.S.; Lacerda, P.D.; Braga, J.U.; Carvalho, F.H.C. Fatores associados à realização de citologia para prevenção de câncer do colo uterino em uma comunidade urbana de baixa renda. Cad. Saude Colet. 2014, 22, 165–172. [Google Scholar] [CrossRef]
- Ribeiro, L.; Bastos, R.R.; Vieira, M.T.; Ribeiro, L.C.; Teixeira, M.T.B.; Leite, I.C.G. Rastreamento oportunístico versus perdas de oportunidade: Não realização do exame de Papanicolaou entre mulheres que frequentaram o pré-natal. Cad. Saude Publica 2016, 32, e00001415. [Google Scholar] [CrossRef]
- Fonseca, A.J.; Ferreira, L.P.; Dalla-Benetta, A.C.; Roldan, C.N.; Ferreira, M.L.S. Epidemiologia e impacto econômico do câncer de colo de útero no Estado de Roraima: A perspectiva do SUS. Rev. Bras Ginecol. Obstet. 2010, 32, 386–392. [Google Scholar] [CrossRef][Green Version]
- Junior, J.B.; Freitas, K.M.; Silva, V.K.G.; Duarte, R.B.; Carvalho, E.M.R. O câncer do colo do útero: Um rastreio nos sistemas de informações. Rev. Interdiscip. Encontro Ciências 2018, 1, 108–122. [Google Scholar]
- Ministério da Saúde. Controle dos Cânceres do Colo do Útero e Da Mama; Ministério da Saúde: Rio de Janeiro, Brazil, 2013.
- Instituto Nacional do Câncer José Alencar Gomes da Silva (INCA). Ficha Técnica de Indicadores das Ações de Controle do Câncer do Colo do Útero; INCA: Rio de Janeiro, Brazil, 2014.
- Viana, J.N.; Moysés, R.P.C.; Espir, T.T.; Sousa, G.A.; Barcellos, J.F.M.; Alves, M.G.P. Determinantes sociais da saúde e prevenção secundária do câncer do colo do útero no Estado do Amazonas, Brasil. Medicina 2019, 52, 110–120. [Google Scholar] [CrossRef]
- Moura, L.L.; Codeço, C.T.; Luz, P.M. Human papillomavirus (HPV) vaccination coverage in Brazil: Spatial and age cohort heterogeneity. Rev. Bras. Epidemiol. 2020, 24, e210001. [Google Scholar] [CrossRef]
- Torres, K.L.; Mariño, J.M.; Pires Rocha, D.A.; de Mello, M.B.; de Melo Farah, H.H.; Reis, R.D.S.; Alves, V.D.C.R.; Gomes, E.; Martins, T.R.; Soares, A.C.; et al. Self-sampling coupled to the detection of HPV 16 and 18 E6 protein: A promising option for detection of cervical malignancies in remote areas. PLoS ONE 2018, 13, e0201262. [Google Scholar] [CrossRef] [PubMed]
- Inturrisi, F.; Aitken, C.A.; Melchers, W.J.G.; van den Brule, A.J.C.; Molijn, A.; Hinrichs, J.W.J.; Niesters, H.G.M.; Siebers, A.G.; Schuurman, R.; Heideman, D.A.M.; et al. Clinical performance of high-risk HPV testing on self-samples versus clinician samples in routine primary HPV screening in the Netherlands: An observational study. Lancet Reg. Health Eur. 2021, 11, 100235. [Google Scholar] [CrossRef]
- Enerly, E.; Bonde, J.; Schee, K.; Pedersen, H.; Lönnberg, S.; Nygård, M. Self-Sampling for Human Papillomavirus Testing among Non-Attenders Increases Attendance to the Norwegian Cervical Cancer Screening Programme. PLoS ONE 2016, 11, e0151978. [Google Scholar] [CrossRef]
- Rodrigues, L.L.; Pilotto, J.H.; Lima, L.R.; Gaydos, C.A.; Hardick, J.; Morgado, M.G.; Martinelli, K.G.; de Paula, V.S.; Nicol, A.F. Self-collected versus clinician-collected samples for HSV-2 and HSV-2/HPV screening in HIV-infected and -uninfected women in the Tapajós region, Amazon, Brazil. Int. J. STD AIDS 2019, 30, 1055–1062. [Google Scholar] [CrossRef]
| Variable | Total Sample n (%) | <25 Years n (%) | 25–59 Years n (%) |
|---|---|---|---|
| Last screening test | |||
| Never | 18 (8.1) | 13 (28.9) | 5 (2.9) |
| Less than 1 year ago | 150 (67.9) | 25 (55.5) | 125 (71.4) |
| 1 -| 2 years ago | 26 (11.8) | 6 (13.3) | 20 (11.4) |
| 2 -| 3 years ago | 10 (4.5) | 1 (2.2) | 9 (5.1) |
| More than 3 years ago | 17 (7.7) | 1 (2.2) | 16 (9.1) |
| Reasons for not performing screening test (n = 18) | |||
| Never had intercourse | 1 (5.6) | 1 (7.7) | - |
| Did not deem necessary | 5 (27.8) | 1 (7.7) | 4 (80.0) |
| Felt ashamed | 3 (16.7) | 2 (15.4) | 1 (20.0) |
| Had never been instructed on undergoing the test | 1 (5.6) | 1 (7.7) | - |
| Had trouble scheduling the test | 2 (11.1) | 2 (15.4) | - |
| Because of age | 3 (16.7) | 3 (23.1) | - |
| Fear | 2 (11.1) | 2 (15.4) | - |
| Could not inform | 1 (5.6) | 1 (7.7) | - |
| Result waiting time (n = 203) | |||
| Less than a month | 15 (7.4) | 2 (6.2) | 13 (7.6) |
| 1 -| 3 months | 113 (55.7) | 20 (62.5) | 92 (54.1) |
| 3 -| 6 months | 38 (18.7) | 4 (12.5) | 34 (20.0) |
| Six months or more | 1 (0.5) | - | 1 (0.6) |
| Has not received the result yet | 32 (15.8) | - | 26 (15.3) |
| Never received the result | 3 (1.5) | 6 (18.8) | 3 (1.8) |
| Never went to get the result | 1 (0.5) | - | 1 (0.6) |
| Location of screening test (n = 203) | |||
| Public service | 194 (95.6) | 31 (96.9) | 162 (95.3) |
| Private service | 8 (3.9) | 1 (3.1) | 7 (4.1) |
| Does not know/did not answer | 1 (0.5) | - | 1 (0.6) |
| Variable | Mean (±SD)/ n (%) | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Structural determinants | ||||
| Age | 35 (±11.04) | 0.94 | 0.89–0.99 | 0.010 * |
| Years of education | 1.13 | 0.99–1.30 | 0.061 a | |
| Never went to school | 4 (1.8%) | |||
| 1–9 years of education | 136 (61.5%) | |||
| 10–12 years of education | 70 (31.7%) | |||
| 13 years of education of more | 9 (4.1%) | |||
| No information | 3 (1.4%) | |||
| Family income (R$) | 908.6 (±769.0) | 1.00 | 1.00–1.00 | 0.452 |
| Occupation | ||||
| Others | 119 (53.8%) | ref | ||
| Housewife | 102 (46.2%) | 0.36 | 0.14–0.93 | 0.034 * |
| Access to care | ||||
| Easy | ref | |||
| Difficult | 1.15 | 0.36–3.76 | 0.812 | |
| Intermediary determinants | ||||
| Self-perception of general health | ||||
| Very good/good | 121 (54.8%) | 1.24 | 0.25–6.21 | 0.797 |
| Regular | 71 (32.1%) | 1.26 | 0.23–6.85 | 0.788 |
| Poor/very poor | 15 (6.8%) | ref | ||
| No information | 14 (6.3%) | |||
| Level of difficulty to schedule appointments | 1.35 | 0.82–2.25 | 0.234 | |
| Very easy/easy | 86 (49.1%) | 1.06 | 0.36–3.12 | 0.915 |
| Not easy nor difficult | 29 (16.6%) | 3.91 | 0.45–34.21 | 0.218 |
| Difficult/very difficult | 49 (28%) | ref | ||
| Does not know/did not answer | 11 (6.3%) | |||
| First place you go when you are sick or in need of health care | ||||
| Primary care | 162 (73.4%) | 1.01 | 0.32–3.24 | 0.981 |
| Another healthcare service | 35 (15.8%) | ref | ||
| None/another/no information | 24 (10.9%) | |||
| How do you travel to get to the service you usually look for? | ||||
| On foot | 76 (34.4%) | ref | ||
| Boat/canoe | 127 (57.5%) | 0.57 | 0.21–1.56 | 0.277 |
| Other/no information | 18 (8.2%) | |||
| Knowledge of the healthcare unit responsible for covering the community | ||||
| Community health clinic or boat | 170 (76.9%) | ref | ||
| Healthcare unit outside the community | 26 (11.8%) | 0.98 | 0.21–4.69 | 0.984 |
| There is not one/does not know | 25 (11.3%) | 0.26 | 0.06–1.10 | 0.067 a |
| Time it takes from home to the healthcare unit during floods | 15.44 (±15.44) | 1.00 | 0.97–1.03 | 0.861 |
| Time it takes from home to the healthcare unit during drought | 29.19 (±32.40) | 1.00 | 0.98–1.01 | 0.887 |
| Opinion about the distance from home to the health unit | ||||
| Close | 141 (63.8%) | ref | ||
| Far | 54 (24.4%) | 1.78 | 0.49–6.52 | 0.385 |
| No information | 26 (11.8%) | |||
| How did you schedule the last appointment at the healthcare unit? | ||||
| Scheduled or through the team | 140 (63.4%) | ref | ||
| On their own | 55 (24.9%) | 1.76 | 0.48–6.46 | 0.393 |
| No information | 26 (11.8%) | |||
| Waiting time on the last appointment | 85.25 (±81.3) | 1.00 | 1.00–1.00 | 0.765 |
| Distance from the house to the boat stop | 2.4 (±3.4) | 0.99 | 0.88–1.12 | 0.885 |
| Distance from home to the health unit | 6.8 (±6.4) | 0.98 | 0.92–1.05 | 0.607 |
| Multiparity | 3.85 (±2.68) | 0.80 | 0.69–0.94 | 0.005 ** |
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Structural determinants | ||||||
| Age | 0.93 (0.89–0.98) | 0.006 ** | 0.96 (0.90–1.02) | 0.159 | ||
| Years of education | 0.97 (0.92–1.02) | 0.187 | ||||
| Occupation (ref. others) | ||||||
| Housewife | 0.36 (0.14–0.94) | 0.037 * | 0.30 (0.10–0.88) | 0.028 * | 0.31 (0.11–0.89) | 0.029 * |
| Intermediary determinants | ||||||
| Knowledge about the healthcare unit responsible for covering the community (ref. in the community) | ||||||
| Healthcare unit outside the community | 0.84 (0.16–4.30) | 0.835 | 0.86 (0.17–4.37) | 0.857 | ||
| There is not/does not know | 0.20 (0.04–1.01) | 0.051 | 0.18 (0.04–0.97) | 0.045 * | ||
| Multiparity | 0.81 (0.67–0.96) | 0.018 * | 0.76 (0.64–0.90) | 0.002 ** | ||
| Variable | Total Sample 14–83 Years n (%) | <25 Years n (%) | 25–59 Years n (%) |
|---|---|---|---|
| Total of screening tests (n = 1097) | 1097 (100) | 229 (100) | 803 (100) |
| Inappropriately repeated | 35 (3.2) | 7 (3.1) | 26 (3.2) |
| Unnecessary performed | 105 (9.6) | 11 (4.8) | 87 (10.8) |
| Potentially delayed | 210 (19.1) | 45 (19.7) | 146 (18.2) |
| Types of epithelium cells (n = 1097) | |||
| No information | 102 (9.3) | 17 (7.4) | 85 (10.2) |
| Squamous epithelium | 257 (23.4) | 54 (23.6) | 193 (23.1) |
| Glandular epithelium | 1 (0.1) | 1 (0.4) | - |
| Squamous/glandular epithelium | 601 (54.8) | 137 (59.8) | 451 (53.9) |
| Squamous/metaplastic epithelium | 34 (3.1) | 4 (1.7) | 26 (3.1) |
| Squamous/glandular/metaplastic epithelium | 102 (9.3) | 16 (7.0) | 82 (9.8) |
| Tests with abnormal or unusual cells (n = 31) | |||
| Atypical squamous cells of undetermined significance not ruling out high-grade lesion–ASC-H | 7 (0.6) | 2 (0.9) | 5 (0.6) |
| Atypical, possibly non-neoplastic, squamous cells of undetermined significance–ASC-US | 14 (1.3) | 6 (2.6) | 8 (1.0) |
| High-grade intraepithelial lesion (cervical intraepithelial neoplasia grade II and III)–HSIL | 3 (3.3) | - | 3 (3.4) |
| Low-grade intraepithelial lesion (cytopathic effect linked to HPV and cervical intraepithelial neoplasia grade II and III)–LSIL-H | 2 (0.2) | 1 (0.4) | 1 (0.1) |
| Low-grade intraepithelial lesion (cytopathic effect linked to HPV and cervical intraepithelial neoplasia grade I)–LSIL | 5 (5.5) | 4 (1.7) | 1 (0.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, D.C.B.; Garnelo, L.; Herkrath, F.J. Barriers to Access the Pap Smear Test for Cervical Cancer Screening in Rural Riverside Populations Covered by a Fluvial Primary Healthcare Team in the Amazon. Int. J. Environ. Res. Public Health 2022, 19, 4193. https://doi.org/10.3390/ijerph19074193
da Silva DCB, Garnelo L, Herkrath FJ. Barriers to Access the Pap Smear Test for Cervical Cancer Screening in Rural Riverside Populations Covered by a Fluvial Primary Healthcare Team in the Amazon. International Journal of Environmental Research and Public Health. 2022; 19(7):4193. https://doi.org/10.3390/ijerph19074193
Chicago/Turabian Styleda Silva, Débora C. B., Luiza Garnelo, and Fernando J. Herkrath. 2022. "Barriers to Access the Pap Smear Test for Cervical Cancer Screening in Rural Riverside Populations Covered by a Fluvial Primary Healthcare Team in the Amazon" International Journal of Environmental Research and Public Health 19, no. 7: 4193. https://doi.org/10.3390/ijerph19074193
APA Styleda Silva, D. C. B., Garnelo, L., & Herkrath, F. J. (2022). Barriers to Access the Pap Smear Test for Cervical Cancer Screening in Rural Riverside Populations Covered by a Fluvial Primary Healthcare Team in the Amazon. International Journal of Environmental Research and Public Health, 19(7), 4193. https://doi.org/10.3390/ijerph19074193






