The New Quadrivalent Adjuvanted Influenza Vaccine for the Italian Elderly: A Health Technology Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Domains
2.2. Non-Clinical Domains
3. Results
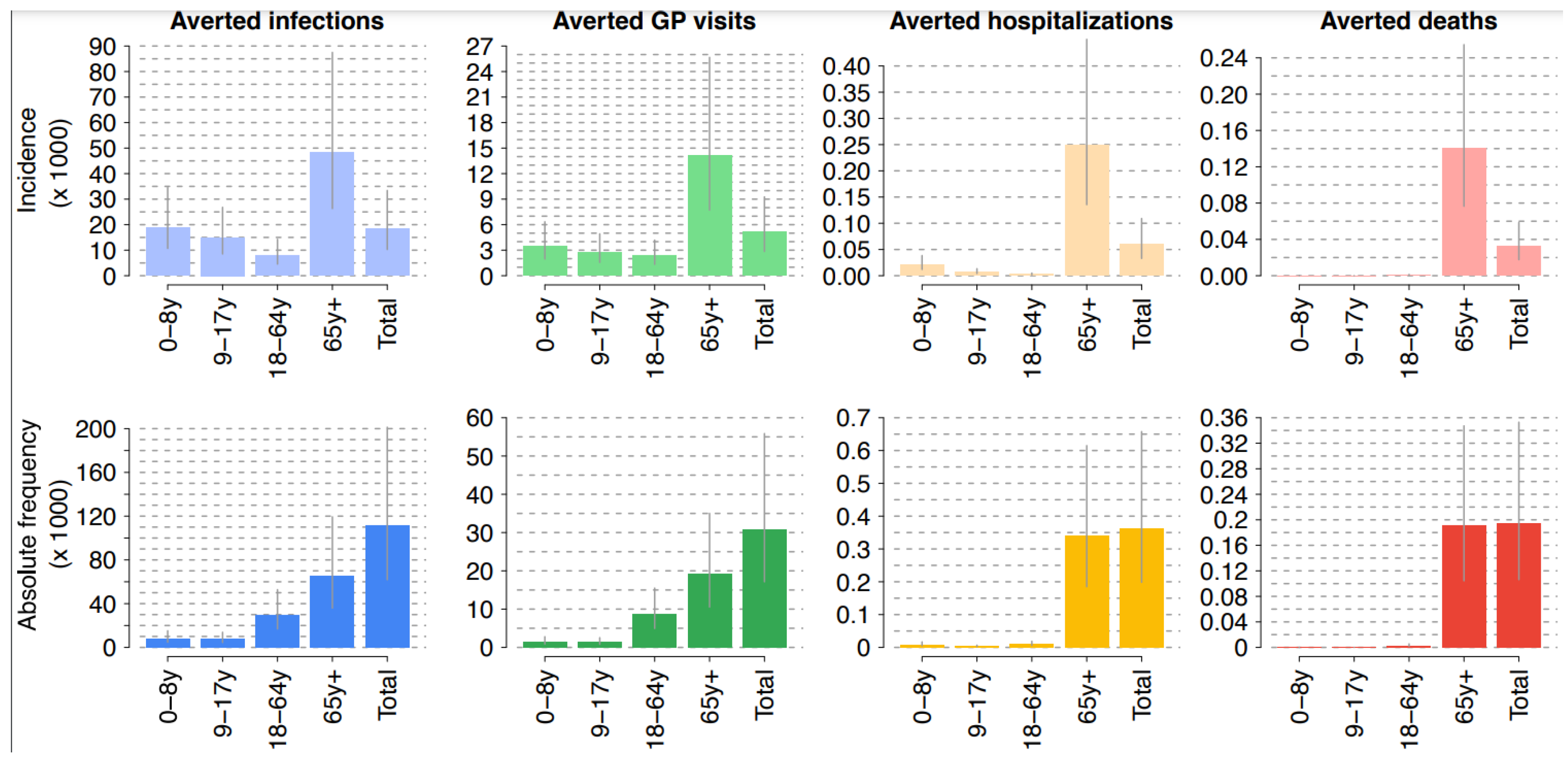
3.1. Clinical Domains
3.2. Non-Clinical Domains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Federici, C.; Cavazza, M.; Costa, F.; Jommi, C. Health care costs of influenza-related episodes in high income countries: A systematic review. PLoS ONE 2018, 13, e0202787. [Google Scholar] [CrossRef] [PubMed]
- WHO. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 29 December 2021).
- Giacchetta, I.; Primieri, C.; Cavalieri, R.; Domnich, A.; de Waure, C. The burden of seasonal influenza in Italy: A systematic review of influenza-related complications, hospitalizations, and mortality. Influenza Other Respir. Viruses 2021, 16, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Bartoszko, J.; Loeb, M. The burden of infuenza in older adults: Meeting the challenge. Aging Clin. Exp. Res. 2021, 33, 711–717. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Seasonal Influenza Vaccination Strategies. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/vaccination-strategies (accessed on 6 January 2022).
- Andrew, M.K.; Bowles, S.K.; Pawelec, G.; Haynes, L.; Kuchel, G.A.; McNeil, S.A.; McElhaney, J.E. Infuenza vaccination in older adults: Recent innovations and practical applications. Drugs Aging 2019, 36, 29–37. [Google Scholar] [CrossRef]
- Gellin, B.G.; Shen, A.K.; Fish, R.; Zettle, M.A.; Uscher-Pines, L.; Ringel, J.S. The National Adult Immunization Plan: Strengthening Adult Immunization Through Coordinated Action. Am. J. Prev. Med. 2016, 51, 1079–1083. [Google Scholar] [CrossRef]
- O’Rourke, B.; Oortwijn, W.; Schuller, T. The new definition of health technology assessment: A milestone in international collaboration. Int. J. Technol. Assess. Health Care 2020, 36, 187–190. [Google Scholar] [CrossRef]
- EUnetHTA. HTA Core Model. Available online: https://www.eunethta.eu/hta-core-model/ (accessed on 6 January 2022).
- Calabrò, G.E.; Boccalini, S.; Bonanni, P.; Bechini, A.; Panatto, D.; Lai, P.L.; Amicizia, D.; Rizzo, C.; Ajelli, M.; Trentini, F.; et al. Valutazione di Health Technology Assessment (HTA) del Vaccino Antinfluenzale Quadrivalente Adiuvato: Fluad Tetra. QIJPH 2021, 10, 1–168. Available online: https://www.ijph.it/hta-vaccino-antinfluenzale-quadrivalente-adiuvato-fluad-tetra (accessed on 6 January 2022).
- Riassunto delle Caratteristiche del Prodotto (RCP). Fluad Tetra. Available online: https://www.ema.europa.eu/en/documents/product-information/fluad-tetra-epar-product-information_it.pdf (accessed on 6 January 2022).
- Di Pietro, M.L.; Poscia, A.; Specchia, M.L.; de Waure, C.; Zace, D.; Gasparini, R.; Amicizia, D.; Lai, P.L.; Panatto, D.; Arata, L.; et al. Valutazione di Health Technology Assessment (HTA) del vaccino antinfluenzale adiuvato nella popolazione anziana italiana. QIJPH 2017, 6, 1–104. Available online: https://www.ijph.it/hta-vaccino-antinfluenzale-adiuvato (accessed on 6 January 2022).
- Trentini, F.; Pariani, E.; Bella, A.; Diurno, G.; Crottogini, L.; Rizzo, C.; Merler, S.; Ajelli, M. Characterizing the transmission patterns of seasonal influenza in Italy: Lessons from the last decade. BMC Public Health 2022, 22, 19. [Google Scholar] [CrossRef]
- Merler, S.; Ajelli, M.; Camilloni, B.; Puzzelli, S.; Bella, A.; Rota, M.C.; Tozzi, A.E.; Muraca, M.; Meledandri, M.; Iorio, A.M.; et al. Pandemic influenza A/H1N1pdm in Italy: Age, risk and population susceptibility. PLoS ONE 2013, 8, e74785. [Google Scholar] [CrossRef][Green Version]
- Rizzo, C.; Rota, M.C.; Bella, A.; Alfonsi, V.; Declich, S.; Caporali, M.G.; Ranghiasci, A.; Lapini, G.; Piccirella, S.; Salmaso, S.; et al. Cross-reactive antibody responses to the 2009 A/H1N1v influenza virus in the Italian population in the pre-pandemic period. Vaccine 2010, 28, 3558–3562. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità (ISS). InfluNet. Available online: https://www.epicentro.iss.it/influenza/influnet (accessed on 29 January 2021).
- Ministero della Salute. Circolare “Prevenzione e Controllo Dell’Influenza: Raccomandazioni per la Stagione 2020–2021”. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2020&codLeg=74451&parte=1%20&serie=null (accessed on 6 January 2022).
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Chu, S.; Wernecke, M.; et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among the U.S. elderly, 2017–2018. J. Infect. Dis. 2019, 220, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Wernecke, M.; MaCurdy, T.; et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018–2019. J. Infect. Dis. 2020, 222, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Van Aalst, R.; Gravenstein, S.; Mor, V.; Mahmud, S.M.; Wilschut, J.; Postma, M.; Chit, A. Comparative effectiveness of high dose versus adjuvanted influenza vaccine: A retrospective cohort study. Vaccine 2020, 38, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Pelton, S.I.; Divino, V.; Shah, D.; Mould-Quevedo, J.; DeKoven, M.; Krishnarajah, G.; Postma, M.J. Evaluating the relative vaccine effectiveness of adjuvanted trivalent influenza vaccine compared to high-dose trivalent and other egg-based influenza vaccines among older adults in the US during the 2017–2018 influenza season. Vaccines 2020, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Lu, M.; Kelman, J.; Lu, Y.; Lindaas, A.; Loc, J.; Pratt, D.; Wei, Y.; Chillarige, Y.; Wernecke, M.; et al. Comparative effectiveness of influenza vaccines among U.S. Medicare beneficiaries ages 65 years and older during the 2019–2020 season. Clin. Infect. Dis. 2020, 73, e4251–e4259. [Google Scholar] [CrossRef]
- Ministero della Salute. Influenza, Coperture Vaccinali Stagione 2019–2020. Available online: http://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=5048 (accessed on 29 January 2021).
- Istituto Superiore di Sanità (ISS). La Sorveglianza “Passi”: Vaccinazione Antinfluenzale. Available online: https://www.epicentro.iss.it/passi/dati/ (accessed on 29 January 2021).
- Istituto Superiore di Sanità (ISS). La Sorveglianza “Passi d’Argento”: Vaccinazione Antinfluenzale. Available online: https://www.epicentro.iss.it/passi-argento/dati/VaccinazioneAntinfluenzale (accessed on 29 January 2021).
- Perrotta, D.; Bella, A.; Rizzo, C.; Paolotti, D. Participatory Online Surveillance as a Supplementary Tool to Sentinel Doctors for Influenza-Like Illness Surveillance in Italy. PLoS ONE 2017, 12, e0169801. [Google Scholar] [CrossRef]
- Esposito, S.; Cantarutti, L.; Molteni, C.G.; Daleno, C.; Scala, A.; Tagliabue, C.; Pelucchi, C.; Giaquinto, C.; Principi, N. Clinical manifestations and socio-economic impact of influenza among healthy children in the community. J. Infect. 2011, 62, 379–387. [Google Scholar] [CrossRef]
- Sessa, A.; Costa, B.; Bamfi, F.; Bettoncelli, G.; D’Ambrosio, G. The incidence, natural history and associated outcomes of influenza-like illness and clinical influenza in Italy. Fam. Pract. 2001, 18, 629–634. [Google Scholar] [CrossRef]
- Rosano, A.; Bella, A.; Gesualdo, F.; Acampora, A.; Pezzotti, P.; Marchetti, S.; Ricciardi, W.; Rizzo, C. Investigating the impact of influenza on excess mortality in all ages in Italy during recent seasons (2013/14–2016/17 seasons). Int. J. Infect. Dis. 2019, 88, 127–134. [Google Scholar] [CrossRef]
- Calabrò, G.E.; Boccalini, S.; Del Riccio, M.; Ninci, A.; Manzi, F.; Bechini, A.; Bonanni, P.; Panatto, D.; Lai, P.L.; Amicizia, D.; et al. Valutazione di Health Technology Assessment (HTA) del vaccino antinfluenzale quadrivalente da coltura cellulare: Flucelvax Tetra. QIJPH 2019, 8, 1–170. Available online: https://www.ijph.it/hta-vaccino-antinfluenzale-quadrivalente-flucelvax-tetraC (accessed on 6 January 2022).
- World Health Organization (WHO). Guidance on the Economic Evaluation of Influenza Vaccination. Available online: https://apps.who.int/iris/bitstream/handle/10665/250086/WHO-IVB16.05-eng.pdf?sequence=1 (accessed on 6 August 2021).
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.; Mauskopf, J.; Loder, E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur. J. Health Econ. 2013, 14, 367–372. [Google Scholar] [CrossRef]
- ISTAT. Rapporto Annuale 2021. Available online: https://www.istat.it/it/archivio/259418 (accessed on 2 August 2021).
- Ministero della Salute. Piano Nazionale Prevenzione Vaccinale 2017–2019. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf (accessed on 29 January 2021).
- Ministero della Salute. Prevenzione e Controllo Dell’Influenza: Raccomandazioni per la Stagione 2018–2019. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2018&codLeg=64381&parte=1%20&serie=null (accessed on 29 January 2021).
- Ministero della Salute. Conferenza Permanente per i Rapporti tra lo Stato, le Regioni e le Province Autonome di Trento e Bolzano. Prevenzione e Controllo Dell’Influenza: Raccomandazioni per la Stagione 2019–2020. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2019&codLeg=70621&parte=1%20&serie=null (accessed on 29 January 2021).
- Ministero della Salute. Prevenzione e Controllo Dell’Influenza: Raccomandazioni per la Stagione 2021–2022. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=79647&parte=1%20&serie=null (accessed on 6 January 2022).
- European Commission. Expert Panel on Effective Ways of Investing in Health (EXPH). Opinion on Defining Value in “Value-Based Healthcare”. 2019. Available online: https://ec.europa.eu/health/sites/default/files/expert_panel/docs/024_defining-value-vbhc_en.pdf (accessed on 6 January 2022).
- European Commission; WHO. Ten Actions towards Vaccination for All. 2019. Available online: https://ec.europa.eu/health/sites/health/files/vaccination/docs/10actions_en.pdf (accessed on 6 January 2022).
- European Commission; Larson, H.; de Figueiredo, A.; Karafillakis, E.; Rawa, M. State of vaccine confidence in the EU 2018. 2018. Available online: https://ec.europa.eu/health/sites/health/files/vaccination/docs/2018_vaccine_confidence_en.pdf (accessed on 6 January 2022).
- European Commission. Directorate-General for Health and Food Safety and Co-ordinated by the Directorate-General for Communication. Special Eurobarometer 488. “Europeans’ Attitudes towards Vaccination” Report. 2019. Available online: https://ec.europa.eu/health/sites/default/files/vaccination/docs/20190426_special-eurobarometer-sp488_en.pdf (accessed on 6 January 2022).
- European Commission. Expert Panel on Effective Ways of Investing in Health (EXPH). Vaccination Programmes and Health Systems in the European Union. 2018. Available online: https://ec.europa.eu/health/sites/default/files/expert_panel/docs/020_vaccinationpgms_en.pdf (accessed on 6 January 2022).
- Censis. «Generazione di… influenzati?». 2019. Available online: https://www.censis.it/welfare-e-salute/sanit%C3%A0-l%E2%80%99influenza-tutti-la-conoscono-ma-pochi-la-temono (accessed on 29 January 2021).
- Cittadinanzattiva. La Nostra Indagine Civica Sull’Approvvigionamento delle dosi di Vaccine Antinfluenzale. Ottobre 2020. Available online: https://www.cittadinanzattiva.it/comunicati/salute/13598-indagine-civica-di-cittadinanzattiva-sull-approvvigionamento-da-parte-della-regione-delle-dosi-di-vaccino-antinfluenzale-solo-10-regioni-rispondono.html (accessed on 29 January 2021).
- Sacchini, D.; Virdis, A.; Refolo, P.; Pennacchini, M.; de Paula, I.C. Health technology assessment (HTA): Ethical aspects. Med. Health Care Philos. 2009, 12, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità (ISS). InfluNet. Sistema di Sorveglianza Integrata Dell’Influenza. Available online: https://w3.iss.it/site/RMI/influnet/pagine/rapportoInflunet.aspx (accessed on 29 January 2021).
- Rizzo, C.; Viboud, C.; Montomoli, E.; Simonsen, L.; Miller, M.A. Influenza-related mortality in the Italian elderly: No decline associated with increasing vaccination coverage. Vaccine 2006, 24, 6468–6475. [Google Scholar] [CrossRef]
- Adlhoch, C.; Dias, J.G.; Bonmarin, I.; Hubert, B.; Larrauri, A.; Domínguez, J.A.O.; Sanz, M.C.D.; Brytting, M.; Carnahan, A.; Popovici, O.; et al. Determinants of Fatal Outcome in Patients Admitted to Intensive Care Units With Influenza, European Union 2009–2017. Open Forum Infect. Dis. 2019, 6, ofz462. [Google Scholar] [CrossRef] [PubMed]
- Essink, B.; Fierro, C.; Rosen, J.; Figueroa, A.L.; Zhang, B.; Verhoeven, C.; Edelman, J.; Smolenov, I. Immunogenicity and safety of MF59-adjuvanted quadrivalent influenza vaccine versus standard and alternate B strain MF59-adjuvanted trivalent influenza vaccines in older adults. Vaccine 2020, 38, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Beran, J.; Reynales, H.; Poder, A.; Yu, C.Y.; Pitisuttithum, P.; Yuan, L.L.; Vermeulen, W.; Verhoeven, C.; Leav, B.; Zhang, B.; et al. Prevention of influenza during mismatched seasons in older adults with an MF59-adjuvanted quadrivalent influenza vaccine: A randomised, controlled, multicentre, phase 3 efficacy study. Lancet Infect. Dis. 2021, 21, 1027–1037. [Google Scholar] [CrossRef]
- Moa, A.M.; Chughtai, A.A.; Muscatello, D.J.; Turner, R.M.; MacIntyre, C.R. Immunogenicity and safety of inactivated quadrivalent influenza vaccine in adults: A systematic review and meta-analysis of randomised controlled trials. Vaccine 2016, 34, 4092–4102. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, J.; Shi, J.; Zhao, W.; Huang, X.; Cheng, L.; Yang, X. Immunogenicity of influenza vaccine in elderly people: A systematic review and meta-analysis of randomized controlled trials, and its association with real-world effectiveness. Hum. Vaccines Immunother. 2020, 16, 2680–2689. [Google Scholar] [CrossRef]
- Rizzo, C.; Gesualdo, F.; Loconsole, D.; Pandolfi, E.; Bella, A.; Orsi, A.; Guarona, G.; Panatto, D.; Icardi, G.; Napoli, C.; et al. Moderate Vaccine Effectiveness against Severe Acute Respiratory Infection Caused by A(H1N1)pdm09 Influenza Virus and No Effectiveness against A(H3N2)Influenza Virus in the 2018/2019 Season in Italy. Vaccines 2020, 8, 427. [Google Scholar] [CrossRef]
- Bellino, S.; Bella, A.; Puzelli, S.; Di Martino, A.; Facchini, M.; Punzo, O.; Pezzotti, P.; Castrucci, M.R.; the InfluNet Study Group. Moderate influenza vaccine effectiveness against A(H1N1)pdm09 virus, and low effectiveness against A(H3N2) subtype, 2018/19 season in Italy. Expert Rev. Vaccines 2019, 18, 1201–1209. [Google Scholar] [CrossRef]
- Stuurman, A.L.; Bollaerts, K.; Alexandridou, M.; Biccler, J.; Díez Domingo, J.; Nohynek, H.; Rizzo, C.; Turunen, T.; Riera-Montes, M. Vaccine effectiveness against laboratory-confirmed influenza in Europe—Results from the DRIVE network during season 2018/19. Vaccine 2020, 38, 6455–6463. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, P.; Boccalini, S.; Zanobini, P.; Dakka, N.; Lorini, C.; Santomauro, F.; Bechini, A. The appropriateness of the use of influenza vaccines: Recommendations from the latest seasons in Italy. Hum. Vaccines Immunother. 2018, 14, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Baio, G.; Pammolli, F.; Baldo, V.; Trivello, R. Object–oriented influence diagram for cost-effectiveness analysis of influenza vaccination in the Italian elderly population. Expert Rev. Pharm. Outcomes Res. 2006, 6, 293–301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iannazzo, S. Pharmacoeconomic evaluation of the MF59-adjuvanted influenza vaccine in the elderly population in Italy. J. Prev. Med. Hyg. 2011, 52, 1–8. [Google Scholar] [PubMed]
- De Waure, C.; Boccalini, S.; Bonanni, P.; Amicizia, D.; Poscia, A.; Bechini, A.; Barbieri, M.; Capri, S.; Specchia, M.L.; Di Pietro, M.L.; et al. Adjuvanted influenza vaccine for the Italian elderly in the 2018/19 season: An updated health technology assessment. Eur. J. Public Health 2019, 29, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 Influenza Season. MMWR Recomm. Rep. 2021, 70, 1–28. Available online: https://www.cdc.gov/mmwr/volumes/70/rr/pdfs/rr7005a1-H.pdf (accessed on 6 January 2022). [CrossRef]
- Public Health Agency of Canada. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI). Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2021–2022. May 2021. Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/vaccines-immunization/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2021-2022/naci-2021-2022-statement.pdf (accessed on 6 January 2022).
- Joint Committee on Vaccination and Immunisation. Advice on influenza vaccines for 2021/22. November 2020. Available online: https://app.box.com/s/t5ockz9bb6xw6t2mrrzb144njplimfo0/file/737845224649 (accessed on 6 January 2022).
- Australian Technical Advisory Group on Immunisation (ATAGI) Clinical Advice. Statement on the Administration of Seasonal Influenza Vaccines in 2021—Updated December 2021. Available online: https://www.health.gov.au/sites/default/files/documents/2021/12/atagi-advice-on-seasonal-influenza-vaccines-in-2021-december-2021-update.pdf (accessed on 6 January 2022).
- CDC. Summary: ‘Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2021–2022′. Available online: https://www.cdc.gov/flu/professionals/acip/summary/summary-recommendations.htm (accessed on 6 January 2022).
- Coleman, B.L.; Sanderson, R.; Haag, M.D.M.; McGovern, I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir. Viruses 2021, 15, 813–823. [Google Scholar] [CrossRef]
- GeoDemo. Demografia in Cifre. Popolazione Residente al 1° Gennaio 2021 per età, Sesso e Stato Civile (Dati Provvisori) Italia. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCIS_POPRES1 (accessed on 6 January 2022).
- GeoDemo. Demografia in Cifre. Previsioni Della Popolazione 2018–2065. Available online: http://demo.istat.it/ (accessed on 6 January 2022).
- Istituto Superiore di Sanità (ISS). Epicentro. La Sorveglianza Passi d’Argento. Patologie Croniche. Available online: https://www.epicentro.iss.it/passi-argento/dati/croniche#dati (accessed on 29 December 2021).
- Bertram, M.; Dhaene, G.; Tan-Torres Edejer, T. (Eds.) Institutionalizing Health Technology Assessment Mechanisms: A How to Guide; World Health Organization: Geneva, Switzerland, 2021; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Laigle, V.; Postma, M.J.; Pavlovic, M.; Cadeddu, C.; Beck, E.; Kapusniak, A.; Toumi, M. Vaccine market access pathways in the EU27 and the United Kingdom—Analysis and recommendations for improvements. Vaccine 2021, 39, 5706–5718. [Google Scholar] [CrossRef]
- Stuurman, A.L.; Rizzo, C.; Haag, M. Investigating the procurement system for understanding seasonal influenza vaccine brand availability in Europe. PLoS ONE 2021, 16, e0248943. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. RegulatioN (EU) 2021/2282 on health technology assessment and amending Directive 2011/24/EU. Off. J. Eur. Union 22.12.2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R2282&from=EN (accessed on 6 January 2022).
- Eisenberg, J.M. Globalize the evidence, localize the decision: Evidence-based medicine and international diversity. Health Aff. 2002, 21, 166–168. [Google Scholar] [CrossRef][Green Version]
- Vaccines Europe. Joint Clinical Health Technology Assessment (HTA) for Vaccines in Europe. Available online: https://www.vaccineseurope.eu/news/position-papers/joint-clinical-health-technology-assessment-hta-for-vaccines-in-europe (accessed on 29 December 2021).
- Calabrò, G.E.; Carini, E.; Tognetto, A.; Giacchetta, I.; Bonanno, E.; Mariani, M.; de Waure, C.R.W. The Value(s) of Vaccination: Building the Scientific Evidence According to a Value-Based Healthcare Approach. Front. Public Health 2022, 10, 786662. [Google Scholar] [CrossRef]
- De Waure, C.; Calabrò, G.E.; Ricciardi, W. Value(s) of Vaccination Project Steering Committee. Recommendations to drive a value-based decision-making on vaccination. Expert Rev. Vaccines 2022, 21, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, G.E.; Carini, E.; Tognetto, A.; Mancinelli, S.; Sarnari, L.; Colamesta, V.; Ricciardi, W.; de Waure, C.; The BRAVE Project Expert Panel. Developing an Evidence-Based Tool for Planning and Evaluating Vaccination Strategies Aimed at Improving Coverage in Elderly and At-Risk Adult Population. Front. Public Health 2021, 9, 658979. [Google Scholar] [CrossRef] [PubMed]
- Thindwa, D.; Quesada, M.G.; Liu, Y.; Bennett, J.; Cohen, C.; Knoll, M.D.; von Gottberg, A.; Hayford, K.; Flasche, S. Use of Seasonal Influenza and Pneumococcal Polysaccharide Vaccines in Older Adults to Reduce COVID-19 mortality. Vaccine 2020, 38, 5398–5401. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.A.; Maschio, M.; Mould-Quevedo, J.F.; Drummond, M.; Weinstein, M.C. The cost-effectiveness of an adjuvanted quadrivalent influenza vaccine in the United Kingdom. Hum. Vaccines Immunother. 2021, 17, 4603–4610. [Google Scholar] [CrossRef] [PubMed]
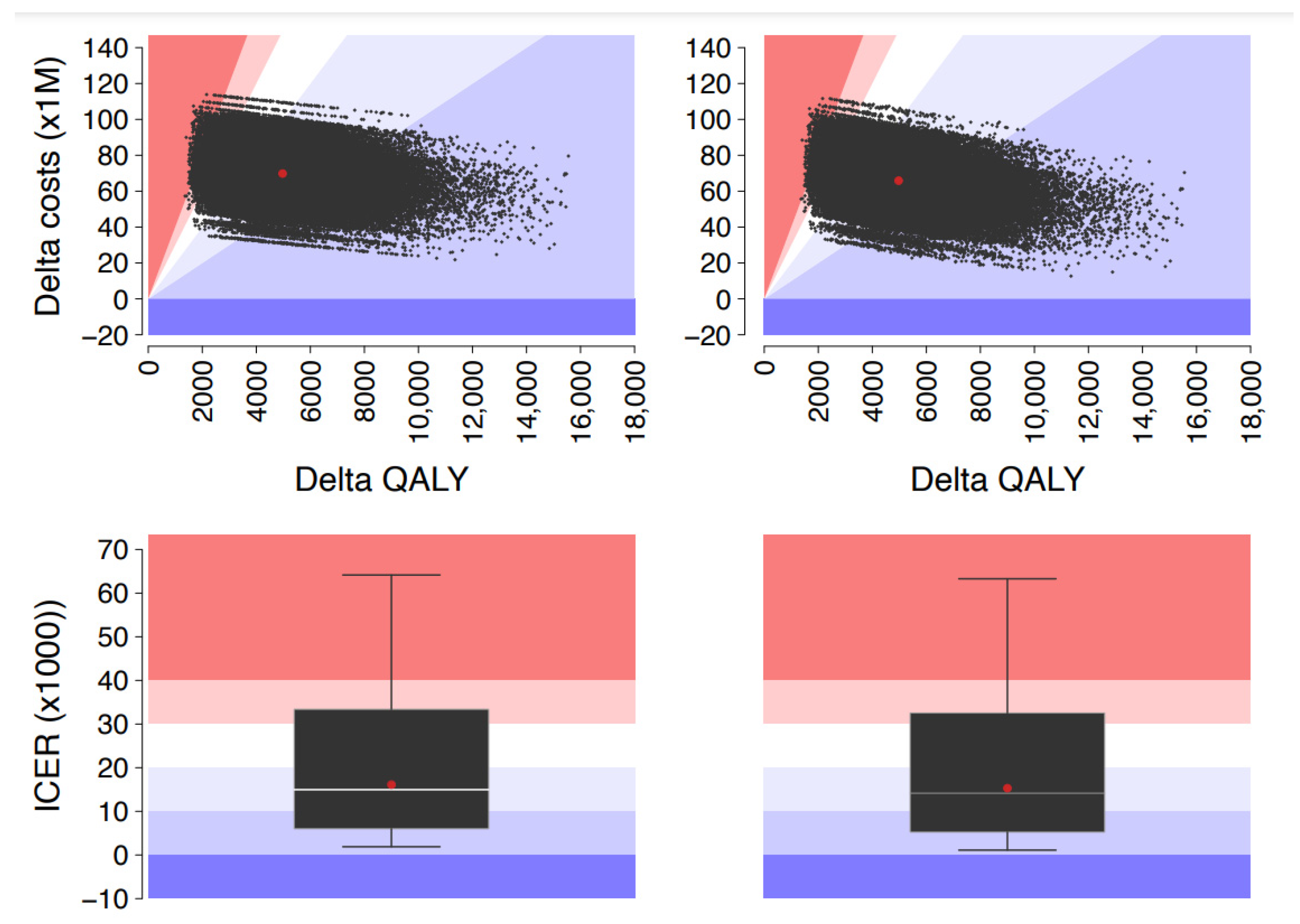
| Age Group | Total Costs, € | Incremental Cost, € | QALY | Incremental QALY, ΔQALY | ICER, €/QALY | ||
|---|---|---|---|---|---|---|---|
| Base Scenario | Alternative Scenario | Base Scenario | Alternative Scenario 1 | ||||
| National Health System (NHS) Perspective | |||||||
| 0.5–8 | 74,022,455 | 73,743,288 | −279,167 | 15,810 | 15,748 | 62 | Dominant |
| 9–17 | 45,665,939 | 45,473,834 | −192,105 | 11,414 | 11,365 | 49 | Dominant |
| 18–64 | 168,215,572 | 166,978,400 | −1,237,172 | 50,846 | 50,407 | 439 | Dominant |
| ≥65 | 147,357,045 | 213,374,855 | 66,017,809 | 41,046 | 36,474 | 4572 | 14,441 |
| Total | 435,261,011 | 499,570,376 | 64,309,365 | 119,116 | 113,994 | 5122 | 12,556 |
| Societal Perspective | |||||||
| 0.5–8 | 104,431,875 | 104,033,764 | −398,112 | 15,810 | 15,748 | 62 | Dominant |
| 9–17 | 58,791,065 | 58,542,301 | −248,764 | 11,414 | 11,365 | 49 | Dominant |
| 18–64 | 626,765,954 | 621,568,549 | −5,197,404 | 50,846 | 50,407 | 439 | Dominant |
| ≥65 | 147,357,045 | 213,374,855 | 66,017,809 | 41,046 | 36,474 | 4572 | 14,441 |
| Total | 937,345,939 | 997,519,469 | 60,173,530 | 119,116 | 113,994 | 5122 | 11,748 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrò, G.E.; Boccalini, S.; Panatto, D.; Rizzo, C.; Di Pietro, M.L.; Abreha, F.M.; Ajelli, M.; Amicizia, D.; Bechini, A.; Giacchetta, I.; et al. The New Quadrivalent Adjuvanted Influenza Vaccine for the Italian Elderly: A Health Technology Assessment. Int. J. Environ. Res. Public Health 2022, 19, 4166. https://doi.org/10.3390/ijerph19074166
Calabrò GE, Boccalini S, Panatto D, Rizzo C, Di Pietro ML, Abreha FM, Ajelli M, Amicizia D, Bechini A, Giacchetta I, et al. The New Quadrivalent Adjuvanted Influenza Vaccine for the Italian Elderly: A Health Technology Assessment. International Journal of Environmental Research and Public Health. 2022; 19(7):4166. https://doi.org/10.3390/ijerph19074166
Chicago/Turabian StyleCalabrò, Giovanna Elisa, Sara Boccalini, Donatella Panatto, Caterina Rizzo, Maria Luisa Di Pietro, Fasika Molla Abreha, Marco Ajelli, Daniela Amicizia, Angela Bechini, Irene Giacchetta, and et al. 2022. "The New Quadrivalent Adjuvanted Influenza Vaccine for the Italian Elderly: A Health Technology Assessment" International Journal of Environmental Research and Public Health 19, no. 7: 4166. https://doi.org/10.3390/ijerph19074166
APA StyleCalabrò, G. E., Boccalini, S., Panatto, D., Rizzo, C., Di Pietro, M. L., Abreha, F. M., Ajelli, M., Amicizia, D., Bechini, A., Giacchetta, I., Lai, P. L., Merler, S., Primieri, C., Trentini, F., Violi, S., Bonanni, P., & de Waure, C. (2022). The New Quadrivalent Adjuvanted Influenza Vaccine for the Italian Elderly: A Health Technology Assessment. International Journal of Environmental Research and Public Health, 19(7), 4166. https://doi.org/10.3390/ijerph19074166








