The In Vivo Toxicity Assessments of Water-Dispersed Fluorescent Silicon Nanoparticles in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Fluorescent SiNPs
2.2. C. elegans Strains and Administration
2.3. Nanoparticle Uptake Assays
2.4. RT-PCR Analysis
2.5. Statistical Analysis
3. Results
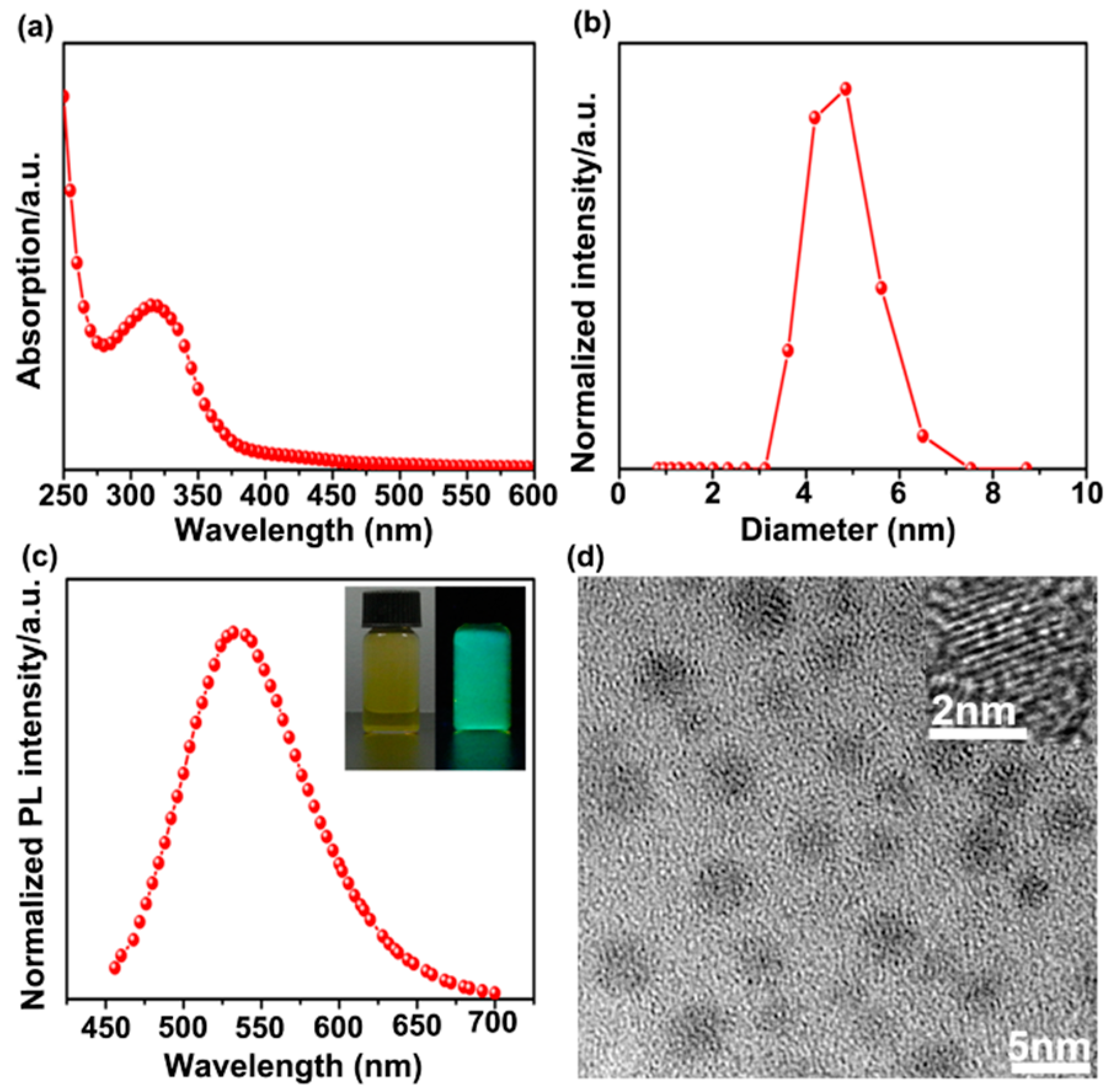
3.1. Characterization of SiNPs
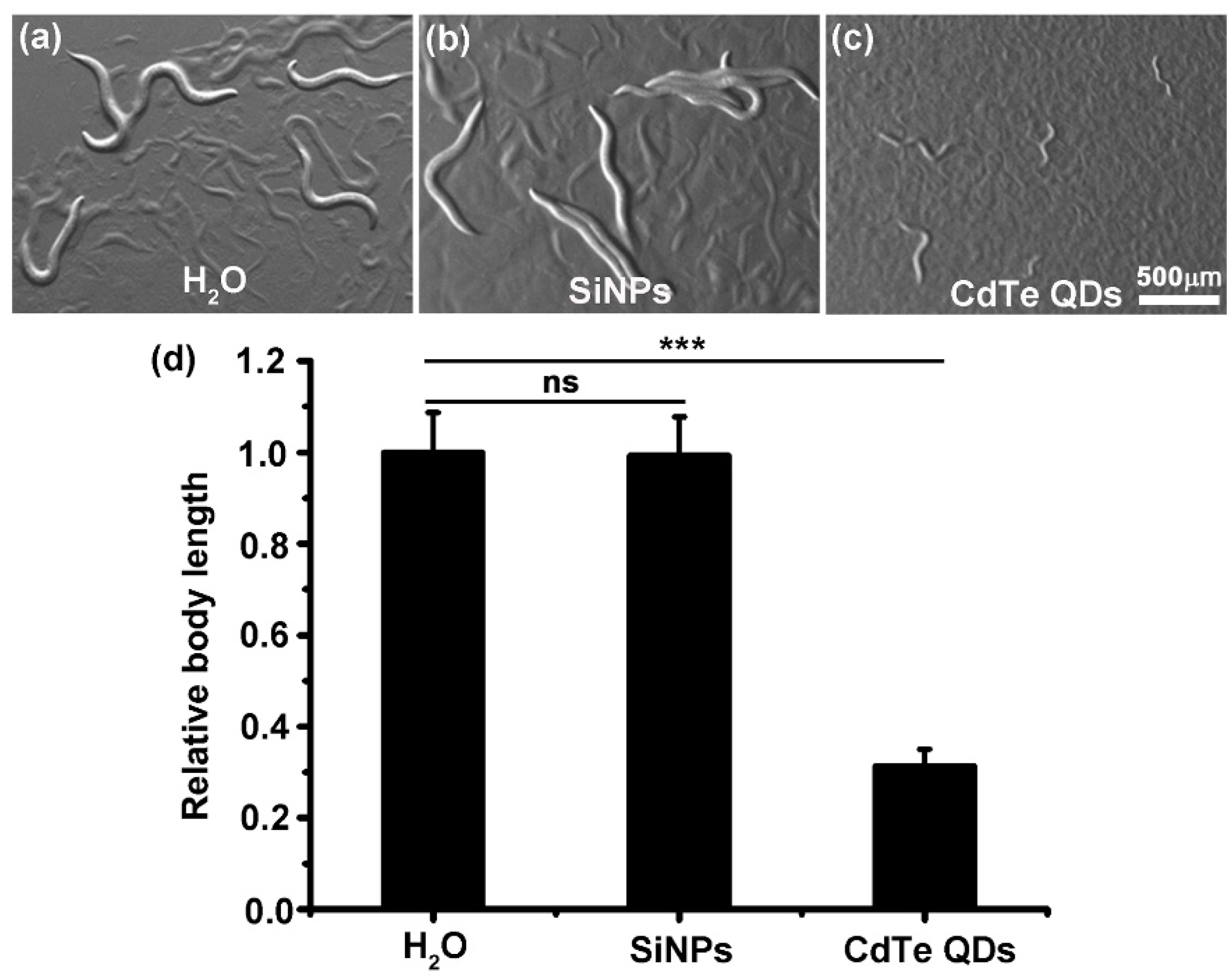
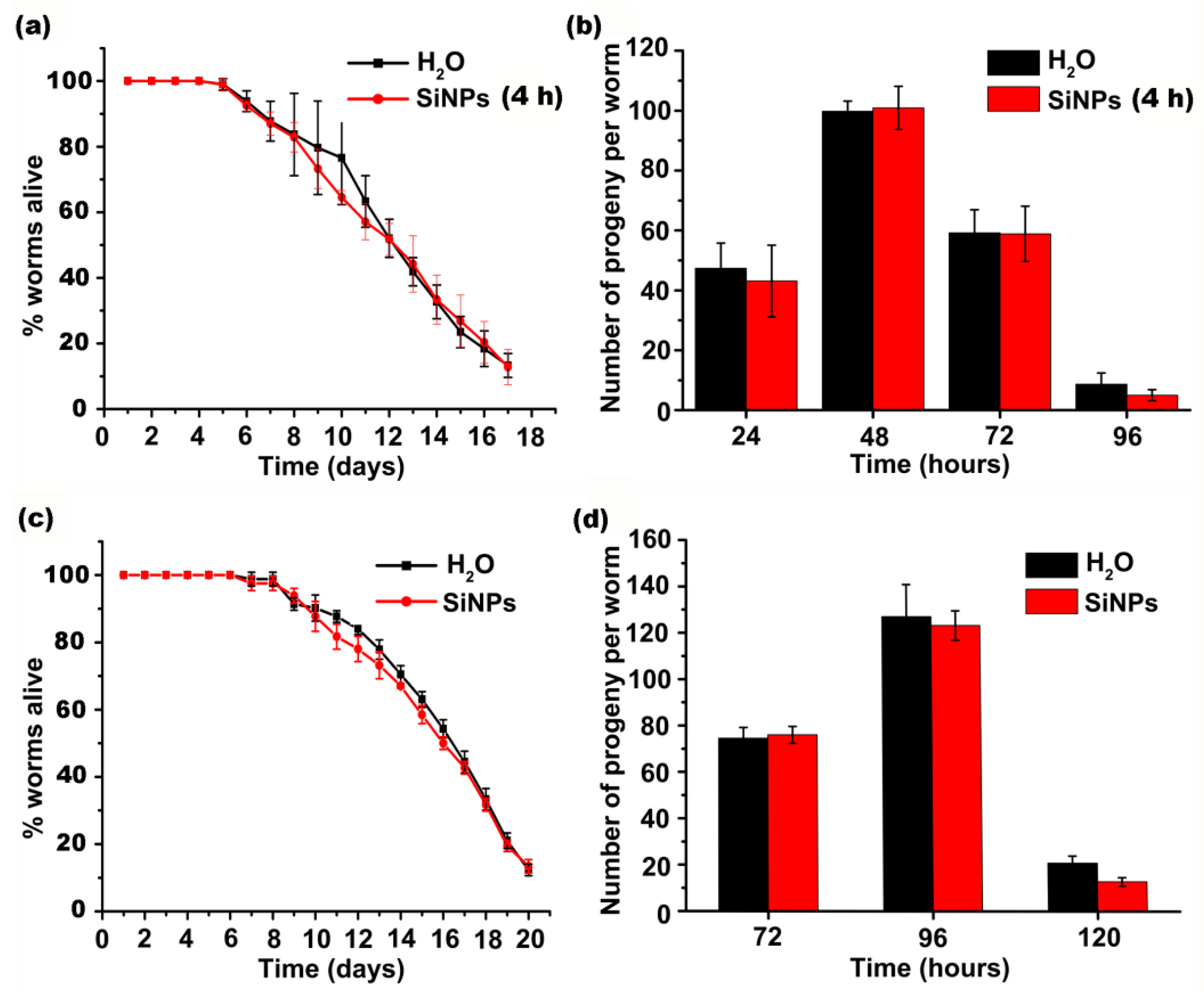
3.2. Overall Effects of SiNPs on C. elegans Growth, Longevity, and Reproductivity
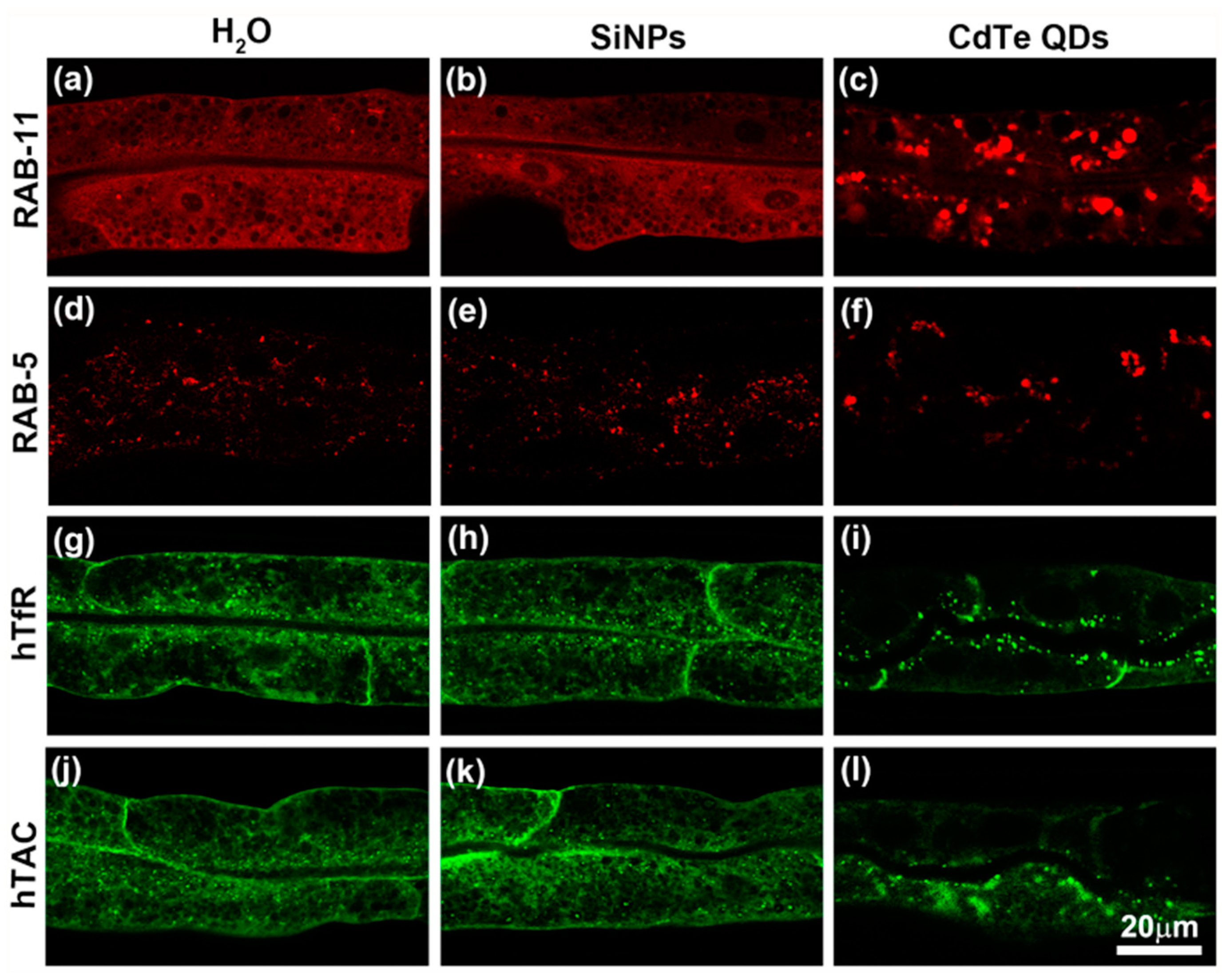
3.3. Subcellular Effects of SiNPs on C. elegans
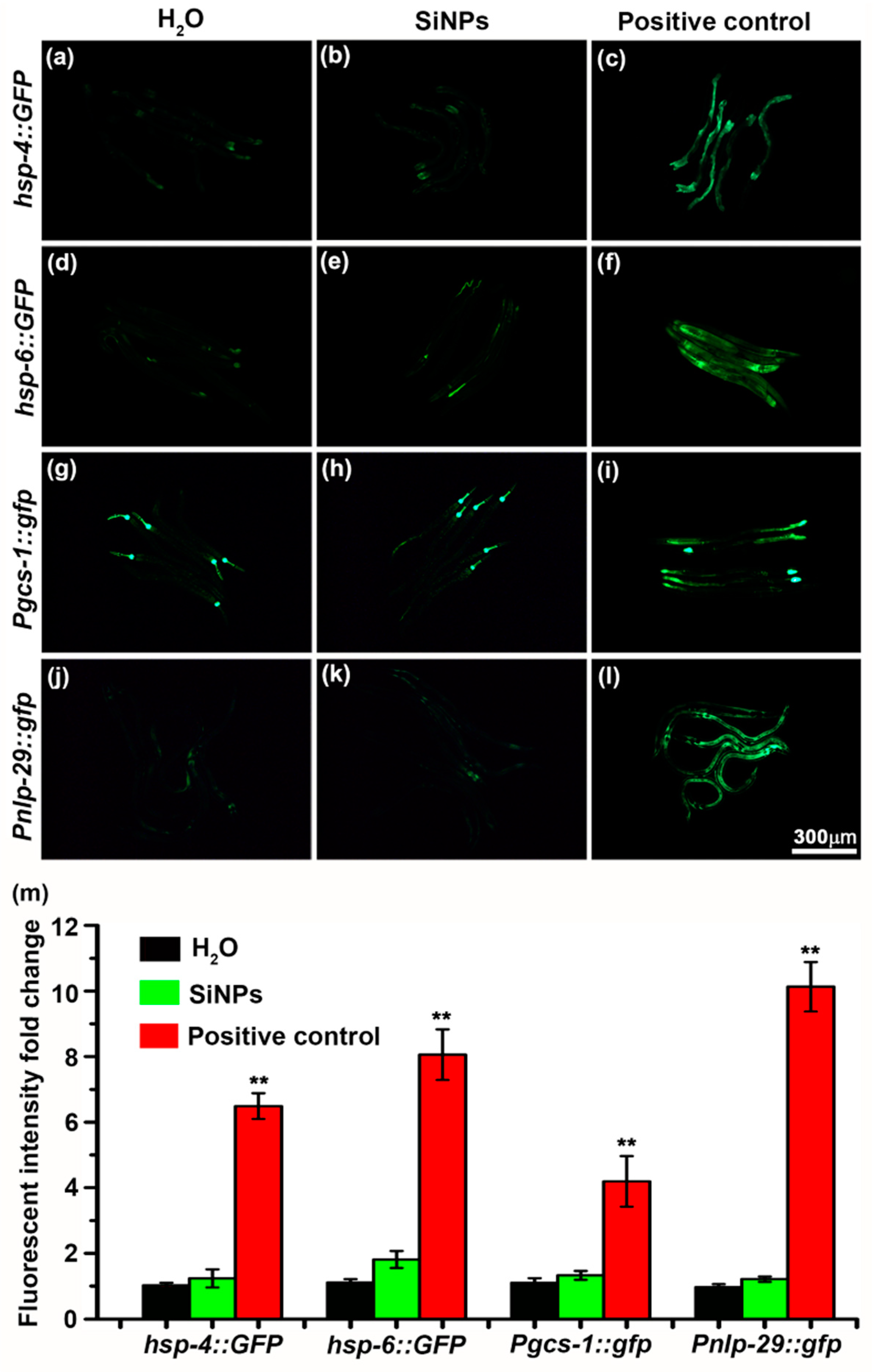
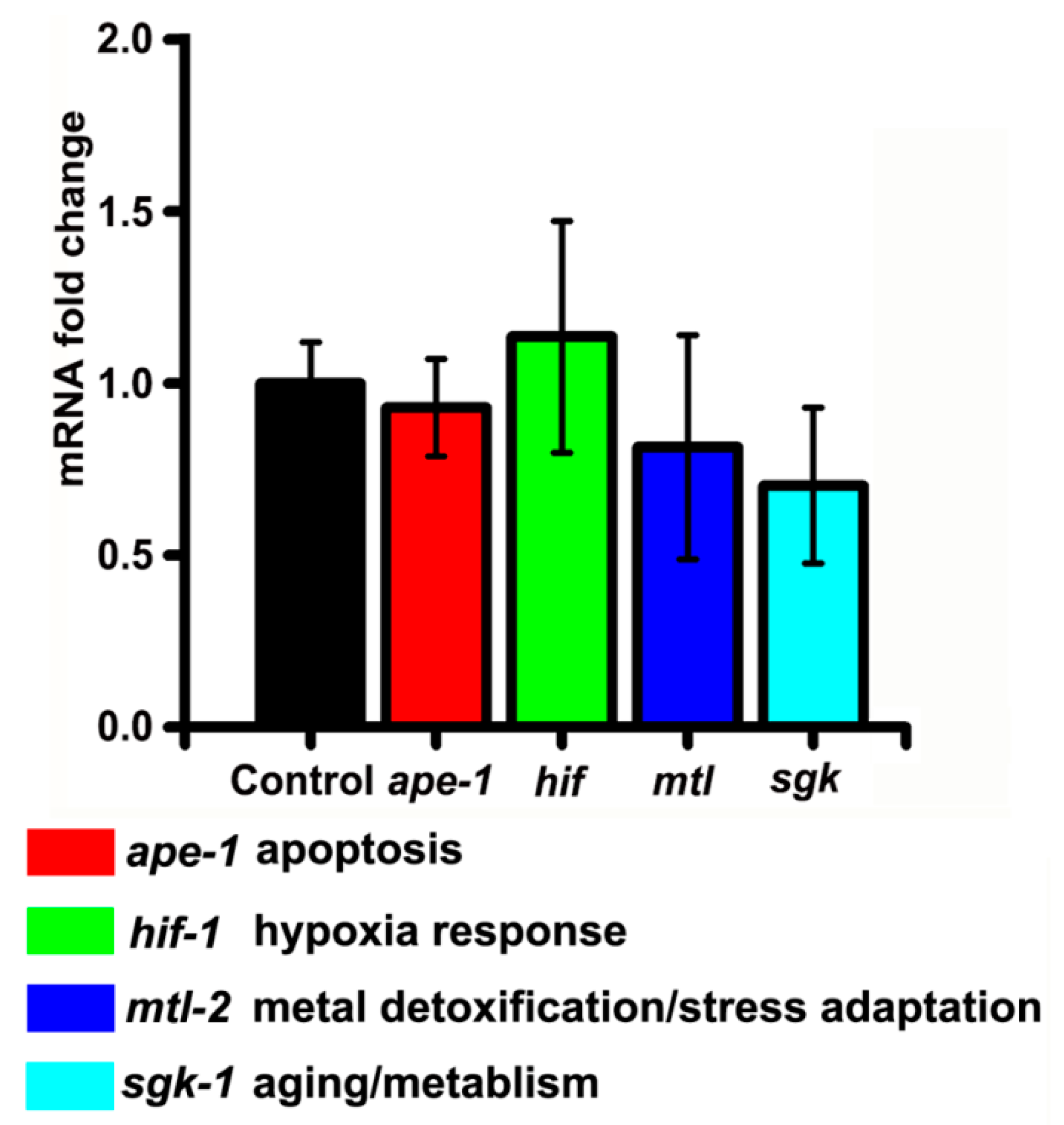
3.4. Potential of SiNPs to Trigger Self-Protective Pathways in C. elegans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, Y.I.; Lee, K.T.; Suh, Y.D.; Hyeon, T. Upconverting nanoparticles: A versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging. Chem. Soc. Rev. 2015, 44, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, X.G. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.D.; Hildebrandt, N. Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef]
- Xia, T.; Li, N.; Fang, X. Single-molecule fluorescence imaging in living cells. Annu. Rev. Phys. Chem. 2013, 64, 459–480. [Google Scholar] [CrossRef]
- Na, N.; Liu, L.; Taes, Y.E.; Zhang, C.; Huang, B.; Liu, Y.; Ouyang, J. Direct CdTe quantum-dot-based fluorescence imaging of human serum proteins. Small 2010, 6, 1589–1592. [Google Scholar] [CrossRef]
- Wang, W.; Hao, Q.; Wang, W.; Bao, L.; Lei, J.; Wang, Q.; Ju, H. Quantum dot-functionalized porous ZnO nanosheets as a visible light induced photoelectrochemical platform for DNA detection. Nanoscale 2014, 6, 2710–2717. [Google Scholar] [CrossRef]
- Xu, H.; Li, Q.; Wang, L.; He, Y.; Shi, J.; Tang, B.; Fan, C. Nanoscale optical probes for cellular imaging. Chem. Soc. Rev. 2014, 43, 2650–2661. [Google Scholar] [CrossRef]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining carbon dots: Synthesis and biomedical and optoelectronic applications. Nano Today 2016, 11, 565–586. [Google Scholar] [CrossRef]
- Montalti, M.; Prodi, L.; Rampazzo, E.; Zaccheroni, N. Dye-doped silica nanoparticles as luminescent organized systems for nanomedicine. Chem. Soc. Rev. 2014, 43, 4243–4268. [Google Scholar] [CrossRef]
- Caltagirone, C.; Bettoschi, A.; Garau, A.; Montis, R. Silica-based nanoparticles: A versatile tool for the development of efficient imaging agents. Chem. Soc. Rev. 2015, 44, 4645–4671. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Tay, C.Y.; Docter, D.; Stauber, R.H.; Leong, D.T. Understanding and exploiting nanoparticles’ intimacy with the blood vessel and blood. Chem. Soc. Rev. 2015, 44, 8174–8199. [Google Scholar] [CrossRef] [PubMed]
- Krug, H.F. Nanosafety research—Are we on the right track. Angew. Chem. Int. Ed. 2014, 53, 12304–12319. [Google Scholar] [CrossRef] [PubMed]
- Tay, C.Y.; Cai, P.Q.; Setyawati, M.I.; Fang, W.R.; Tan, L.P.; Hong, C.H.L.; Chen, X.D.; Leong, D.T. Nanoparticles strengthen intracellular tension and retard cellular migration. Nano Lett. 2014, 14, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Matos, B.; Martins, M.; Samamed, A.C.; Sousa, D.; Ferreira, I.; Diniz, M.S. Toxicity evaluation of quantum dots (ZnS and CdS) singly and combined in Zebrafish (Danio rerio). Int. J. Environ. Res. Public Health 2020, 17, 232. [Google Scholar] [CrossRef]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.; Durazzo, A.; Souto, E.B. Nanotoxicology and nanosafety: Safety-by-design and testing at a glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Garriga, R.; Herrero-Continente, T.; Palos, M.; Cebolla, V.L.; Osada, J.; Munoz, E.; Rodriguez-Yoldi, M.J. Toxicity of Carbon Nanomaterials and Their Potential Application as Drug Delivery Systems: In Vitro Studies in Caco-2 and MCF-7 Cell Lines. Nanomaterials 2020, 10, 1617. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, Z.; Zhu, X. Toxic effects of TiO2 NPs on Zebrafish. Int. J. Environ. Res. Public Health 2019, 16, 523. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Tu, L.; Chen, D.; Luo, Z.; Liu, D.; Miao, Z.; Feng, G.; Qing, L.; Wang, S. Sub-Acute Toxicity Study of Graphene Oxide in the Sprague-Dawley Rat. Int. J. Environ. Res. Public Health 2016, 13, 1149. [Google Scholar] [CrossRef]
- Fede, C.; Millino, C.; Pacchioni, B.; Celegato, B.; Compagnin, C.; Martini, P.; Selvestrel, F.; Mancin, F.; Celotti, L.; Lanfranchi, G.; et al. Altered Gene Transcription in Human Cells Treated with Ludox® Silica Nanoparticles. Int. J. Environ. Res. Public Health 2014, 11, 8867–8890. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. Dysfunction of various organelles provokes multiple cell death after quantum dot exposure. Int. J. Nanomed. 2018, 13, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, Y.J.; Liu, L.; Xu, Y.C.; Liu, X.; Lin, J.; Zhou, W.; Wei, P.F.; Jin, P.P.; Wen, L.P. Inhibition of lanthanide nanocrystal-induced inflammasome activation in macrophages by a surface coating peptide through abrogation of ROS production and TRPM2-mediated Ca2+ influx. Biomaterials 2016, 108, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Yang, S.W.; Deng, Y.; Chai, P.W.; Yang, Y.C.; He, X.Y.; Xie, X.M.; Kang, Z.H.; Ding, G.Q.; Zhou, H.F.; et al. Emancipating target—Functionalized carbon dots from autophagy vesicles for a novel visualized tumor therapy. Adv. Funct. Mater. 2018, 28, 1800881. [Google Scholar] [CrossRef]
- Lin, W.S.; Huang, Y.W.; Zhou, X.D.; Ma, Y.F. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol. Appl. Pharm. 2006, 217, 252–259. [Google Scholar] [CrossRef]
- Erogbogbo, F.; Yong, K.T.; Roy, I.; Hu, R.; Law, W.C.; Zhao, W.; Ding, H.; Wu, F.; Kumar, R.; Swihart, M.T.; et al. In vivo targeted cancer imaging, sentinel lymph node mapping and multi-channel imaging with biocompatible silicon nanocrystals. ACS Nano 2011, 5, 413–423. [Google Scholar] [CrossRef]
- Joo, J.; Liu, X.; Kotamraju, V.R.; Ruoslahti, E.; Nam, Y.; Sailor, M.J. Gated Luminescence Imaging of Silicon Nanoparticles. ACS Nano 2015, 9, 6233–6241. [Google Scholar] [CrossRef]
- Tang, J.; Chu, B.; Wang, J.; Song, B.; Su, Y.; Wang, H.; He, Y. Multifunctional nanoagents for ultrasensitive imaging and photoactive killing of Gram-negative and Gram-positive bacteria. Nat. Commun. 2019, 10, 4057. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, C.; Ma, W.; Jun Li, J.; Xiao, X.; Zhao, D. One-step hydrothermal synthesis of yellow and green emitting silicon quantum dots with synergistic effect. Nanomaterials 2019, 9, 466. [Google Scholar] [CrossRef]
- Dou, Y.; Shang, Y.; He, X.; Li, W.; Li, Y.; Zhang, Y. Preparation of a ruthenium-complex-functionalized two-photon-excited red fluorescence silicon nanoparticle composite for targeted fluorescence imaging and photodynamic therapy in vitro. ACS Appl. Mater. Interfaces 2019, 11, 13954–13963. [Google Scholar] [CrossRef]
- Kim, G.H.; Lee, G.; Kang, M.H.; Kim, M.; Jin, Y.; Beck, S.; Cheon, J.; Sung, J.; Joo, J. Luminescent silicon nanoparticles for distinctive tracking of cellular targeting and trafficking. Faraday Discuss. 2020, 222, 304–317. [Google Scholar] [CrossRef]
- Zhong, Y.; Sun, X.; Wang, S.; Peng, F.; Bao, F.; Su, Y.; Li, Y.; Lee, S.T.; He, Y. Facile, Large-Quantity Synthesis of Stable, Tunable-Color Silicon Nanoparticles and Their Application for Long-Term Cellular Imaging. ACS Nano 2015, 9, 5958–5967. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Peng, F.; Hu, Z.; Chu, B.; Zhong, Y.; Su, Y.; He, S.; He, Y. In vitro cellular behaviors and toxicity assays of small-sized fluorescent silicon nanoparticles. Nanoscale 2017, 9, 7602–7611. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Erogbogbo, F.; Yong, K.T.; Ye, L.; Hu, R.; Chen, H.; Hu, Y.; Yang, Y.; Yang, J.; Roy, I.; et al. Assessing clinical prospects of silicon quantum dots: Studies in mice and monkeys. ACS Nano 2013, 7, 7303–7310. [Google Scholar] [CrossRef] [PubMed]
- Waterston, R. Genome sequence of the nematode C. elegans: A platform for investigating biology. Science 1998, 282, 2012–2018. [Google Scholar]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef]
- Lu, J.H.; Hou, W.C.; Tsai, M.H.; Chang, Y.T.; Chao, H.R. The impact of background-level carboxylated single-walled carbon nanotubes (SWCNTs−COOH) on induced toxicity in Caenorhabditis elegans and human cells. Int. J. Environ. Res. Public Health 2022, 19, 1218. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, Y.; Fang, J.; Wang, D. Immune response is required for the control of in vivo translocation and chronic toxicity of graphene oxide. Nanoscale 2014, 6, 5894–5906. [Google Scholar] [CrossRef]
- Zanni, E.; De Bellis, G.; Bracciale, M.P.; Broggi, A.; Santarelli, M.L.; Sarto, M.S.; Palleschi, C.; Uccelletti, D. Graphite nanoplatelets and Caenorhabditis elegans: Insights from an in vivo model. Nano Lett. 2012, 12, 2740–2744. [Google Scholar] [CrossRef]
- Cong, W.; Wang, P.; Qu, Y.; Tang, J.; Bai, R.; Zhao, Y.; Chen, C.; Bi, X. Evaluation of the influence of fullerenol on aging and stress resistance using Caenorhabditis elegans. Biomaterials 2015, 42, 78–86. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Q.; Wang, D. An epigenetic signal encoded protection mechanism is activated by graphene oxide to inhibit its induced reproductive toxicity in Caenorhabditis elegans. Biomaterials 2016, 79, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Donner, J.S.; Thompson, S.A.; Alonso-Ortega, C.; Morales, J.; Rico, L.G.; Santos, S.I.; Quidant, R. Imaging of plasmonic heating in a living organism. ACS Nano 2013, 7, 8666–8672. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kwak, J.I.; An, Y.J. Multigenerational study of gold nanoparticles in Caenorhabditis elegans: Transgenerational effect of maternal exposure. Environ. Sci. Technol. 2013, 47, 5393–5399. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Sim, S.J.; Yi, J.; Park, K.; Chung, K.H.; Ryu, D.; Choi, J. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ. Sci. Technol. 2009, 43, 3933–3940. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xu, S.; Yang, Y.; Li, L.; Chen, S.; Xu, A.; Wu, L. Insights into the Ecotoxicity of Silver Nanoparticles Transferred from Escherichia coli to Caenorhabditis elegans. Sci. Rep. 2016, 6, 36465. [Google Scholar] [CrossRef] [PubMed]
- Scharf, A.; Piechulek, A.; von Mikecz, A. Effect of nanoparticles on the biochemical and behavioral aging phenotype of the nematode Caenorhabditis elegans. ACS Nano 2013, 7, 10695–10703. [Google Scholar] [CrossRef]
- von Mikecz, A.; Schikowski, T. Effects of Airborne Nanoparticles on the Nervous System: Amyloid Protein Aggregation, Neurodegeneration and Neurodegenerative Diseases. Nanomaterials 2020, 10, 1349. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Song, B.; Wu, S.; Su, Y.; Zhang, H.; He, Y. A real-time documentation and mechanistic investigation of quantum dots-induced autophagy in live Caenorhabditis elegans. Biomaterials 2015, 72, 38–48. [Google Scholar] [CrossRef]
- Qu, Y.; Li, W.; Zhou, Y.; Liu, X.; Zhang, L.; Wang, L.; Li, Y.F.; Iida, A.; Tang, Z.; Zhao, Y.; et al. Full assessment of fate and physiological behavior of quantum dots utilizing Caenorhabditis elegans as a model organism. Nano Lett. 2011, 11, 3174–3183. [Google Scholar] [CrossRef]
- Wu, T.; He, K.; Zhan, Q.; Ang, S.; Ying, J.; Zhang, S.; Zhang, T.; Xue, Y.; Tang, M. MPA-capped CdTe quantum dots exposure causes neurotoxic effects in nematode Caenorhabditis elegans by affecting the transporters and receptors of glutamate, serotonin and dopamine at the genetic level, or by increasing ROS, or both. Nanoscale 2015, 7, 20460–20473. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Y.; Song, B.; Zhong, Y.; Wu, S.; Cui, R.; Cong, H.; Su, Y.; Zhang, H.; He, Y. Linking Subcellular Disturbance to Physiological Behavior and Toxicity Induced by Quantum Dots in Caenorhabditis elegans. Small 2016, 12, 3143–3154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.C.; Yang, Z.L.; Dong, W.; Tang, R.J.; Sun, L.D.; Yan, C.H. Bioimaging and toxicity assessments of near-infrared upconversion luminescent NaYF4: Yb, Tm nanocrystals. Biomaterials 2011, 32, 9059–9067. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.F.; Riehn, R.; Ryu, W.S.; Khanarian, N.; Tung, C.K.; Tank, D.; Austin, R.H. In vivo and scanning electron microscopy imaging of up-converting nanophosphors in Caenorhabditis elegans. Nano Lett. 2006, 6, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Chen, C.S.; Hsieh, H.H.; Wu, Y.C.; Chang, H.C. In vivo imaging and toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegans. Nano Lett. 2010, 10, 3692–3699. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Larsen, P.L. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1993, 90, 8905–8909. [Google Scholar] [CrossRef]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef]
- Shen, X.H.; Ellis, R.E.; Lee, K.; Liu, C.Y.; Yang, K.; Solomon, A.; Yoshida, H.; Morimoto, R.; Kurnit, D.M.; Mori, K.; et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 2001, 107, 893–903. [Google Scholar] [CrossRef]
- Benedetti, C.; Haynes, C.M.; Yang, Y.; Harding, H.P.; Ron, D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics 2006, 174, 229–239. [Google Scholar] [CrossRef]
- Choi, C.H.J.; Zuckerman, J.E.; Webster, P.; Davis, M.E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl. Acad. Sci. USA 2011, 108, 6656–6661. [Google Scholar] [CrossRef]
- Zhang, X.D.; Luo, Z.T.; Chen, J.; Song, S.S.; Yuan, X.; Shen, X.; Wang, H.; Sun, Y.M.; Gao, K.; Zhang, L.F.; et al. Ultrasmall glutathione-protected gold nanoclusters as next generation radiotherapy sensitizers with high tumor uptake and high renal clearance. Sci. Rep. 2015, 5, 8669. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Chen, D.H. Magnetic nanoparticles grafted with cyclodextrin for hydrophobic drug delivery. Chem. Mater. 2007, 19, 6345–6349. [Google Scholar] [CrossRef]
- Leung, M.C.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moragas, L.; Roig, A.; Laromaine, A. C. elegans as a tool for in vivo nanoparticle assessment. Adv. Colloid Interface Sci. 2015, 219, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Jiang, Y.; Zeng, S.; Yan, J.; Li, X.; Zhang, Y.; Zou, W.; Wang, X. Endocytic sorting and recycling require membrane phosphatidylserine asymmetry maintained by TAT-1/CHAT-1. PLoS Genet. 2010, 6, e1001235. [Google Scholar] [CrossRef] [PubMed]
- Leo, O.; Foo, M.; Sachs, D.H.; Samelson, L.E.; Bluestone, J.A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc. Natl. Acad. Sci. USA 1987, 84, 1374–1378. [Google Scholar] [CrossRef]
- Andon, F.T.; Fadeel, B. Programmed cell death: Molecular mechanisms and implications for safety assessment of nanomaterials. Acc. Chem. Res. 2013, 46, 733–742. [Google Scholar] [CrossRef]
- Peynshaert, K.; Manshian, B.B.; Joris, F.; Braeckmans, K.; De Smedt, S.C.; Demeester, J.; Soenen, S.J. Exploiting intrinsic nanoparticle toxicity: The pros and cons of nanoparticle-induced autophagy in biomedical research. Chem. Rev. 2014, 114, 7581–7609. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Haynes, C.M.; Petrova, K.; Benedetti, C.; Yang, Y.; Ron, D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev. Cell 2007, 13, 467–480. [Google Scholar] [CrossRef]
- An, J.H.; Blackwell, T.K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genet. Dev. 2003, 17, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, W.; Li, L.; Li, Y.; Fu, R.; Zhu, Y.; Li, J.; Zhou, Y.; Xiong, S.; Zhang, H. Structural damage in the C. elegans epidermis causes release of STA-2 and induction of an innate immune response. Immunity 2015, 42, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Zhong, Y.; Fu, R.; Wu, S.; Wang, Q.; Wang, H.; Su, Y.; Zhang, H.; He, Y. The in vivo targeted molecular imaging of fluorescent silicon nanoparticles in Caenorhabditis elegans. Nano Res. 2018, 11, 2336–2346. [Google Scholar] [CrossRef]
- Bergamaschi, D.; Samuels, Y.; O’Neil, N.J.; Trigiante, G.; Crook, T.; Hsieh, J.K.; O’Connor, D.J.; Zhong, S.; Campargue, I.; Tomlinson, M.L. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat. Genet. 2003, 33, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.P.; Leibold, E.A. Mechanisms of iron metabolism in Caenorhabditis elegans. Front. Pharmacol. 2014, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Haas, K.L.; Freedman, J.H. Role of MTL-1, MTL-2, and CDR-1 in mediating cadmium sensitivity in Caenorhabditis elegans. Toxicol. Sci. 2012, 128, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Tullet, J.M.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef]
- Singh, M.P.; Atkins, T.M.; Muthuswamy, E.; Kamali, S.; Tu, C.; Louie, A.Y.; Kauzlarich, S.M. Development of iron-doped silicon nanoparticles as bimodal imaging agents. ACS Nano 2012, 6, 5596–5604. [Google Scholar] [CrossRef][Green Version]
| Genes | Accession Number | Primer Forward | Primer Reverse |
|---|---|---|---|
| hif-1 | 180,359 | TGTCTTTCCTGGTTCATTCAAA | ATCCGAAACGAAAGTGATGC |
| mtl-2 | 179,899 | TGCAACACCGGAACTAAAGA | TTAATGAGCAGCCTGAGCAC |
| sgk-1 | 181,697 | TTCTTCCTTCCGGTTGATTG | TCGTGATCTCGATGAGTGACA |
| ape-1 | 179,601 | ACCTCGTGCTTCTGTCGAAC | ATGGCTCCGTCGGTATTTTT |
| act-1 | 179,535 | TCCTTACCGAGCGTGGTTAC | GTTTCCGACGGTGATGACTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhu, Y.; Song, B.; Fu, R.; Zhou, Y. The In Vivo Toxicity Assessments of Water-Dispersed Fluorescent Silicon Nanoparticles in Caenorhabditis elegans. Int. J. Environ. Res. Public Health 2022, 19, 4101. https://doi.org/10.3390/ijerph19074101
Wang Q, Zhu Y, Song B, Fu R, Zhou Y. The In Vivo Toxicity Assessments of Water-Dispersed Fluorescent Silicon Nanoparticles in Caenorhabditis elegans. International Journal of Environmental Research and Public Health. 2022; 19(7):4101. https://doi.org/10.3390/ijerph19074101
Chicago/Turabian StyleWang, Qin, Yi Zhu, Bin Song, Rong Fu, and Yanfeng Zhou. 2022. "The In Vivo Toxicity Assessments of Water-Dispersed Fluorescent Silicon Nanoparticles in Caenorhabditis elegans" International Journal of Environmental Research and Public Health 19, no. 7: 4101. https://doi.org/10.3390/ijerph19074101
APA StyleWang, Q., Zhu, Y., Song, B., Fu, R., & Zhou, Y. (2022). The In Vivo Toxicity Assessments of Water-Dispersed Fluorescent Silicon Nanoparticles in Caenorhabditis elegans. International Journal of Environmental Research and Public Health, 19(7), 4101. https://doi.org/10.3390/ijerph19074101







