Cystic Fibrosis-Related Diabetes in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Population
2.3. Statistical Analysis
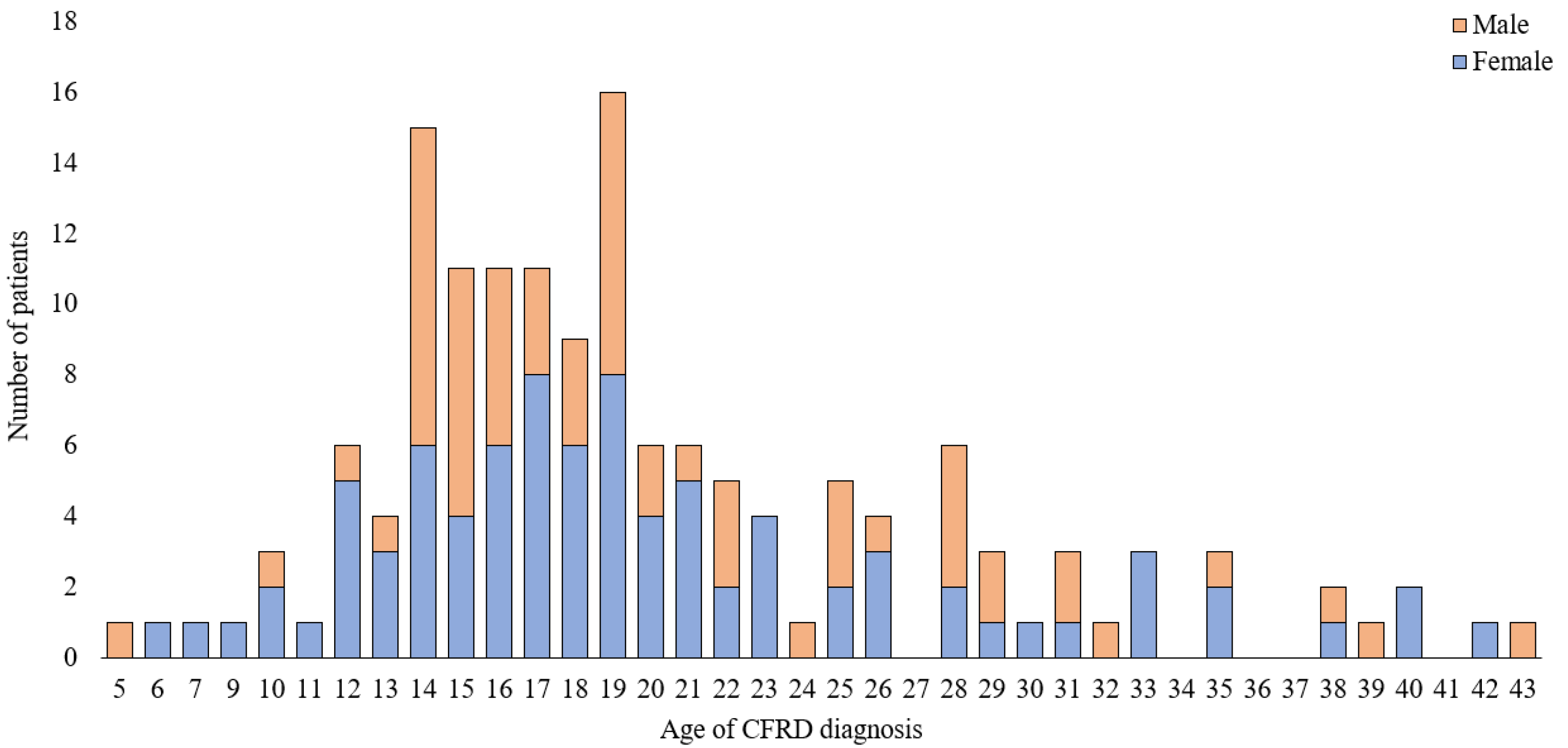
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scotet, V.; Gutierrez, H.; Farrell, P.M. Newborn screening for CF across the globe—Where is it worthwhile? Int. J. Neonatal Screen. 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Keogh, R.H.; Tanner, K.; Simmonds, N.J.; Bilton, D. The changing demography of the cystic fibrosis population: Forecasting future numbers of adults in the UK. Sci. Rep. 2020, 10, 10660. [Google Scholar] [CrossRef] [PubMed]
- Rachel, M.; Topolewicz, S.; Śliwczyński, A.; Galiniak, S. Managing Cystic Fibrosis in Polish Healthcare. Int. J. Environ. Res. Public Health 2020, 17, 7630. [Google Scholar] [CrossRef]
- Jackson, A.D.; Goss, C.H. Epidemiology of CF: How registries can be used to advance our understanding of the CF population. J. Cyst. Fibros. 2018, 17, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, N.J.; Cullinan, P.; Hodson, M.E. Growing old with cystic fibrosis—The characteristics of long-term survivors of cystic fibrosis. Respir. Med. 2009, 103, 629–635. [Google Scholar] [CrossRef][Green Version]
- Simmonds, N.J. Ageing in cystic fibrosis and long-term survival. Paediatr. Respir. Rev. 2013, 14 (Suppl. S1), 6–9. [Google Scholar] [CrossRef]
- Scotet, V.; L’Hostis, C.; Férec, C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef]
- Olesen, H.V.; Drevinek, P.; Gulmans, V.A.; Hatziagorou, E.; Jung, A.; Mei-Zahav, M.; Stojnic, N.; Thomas, M.; Zolin, A.; ECFSPR Steering Group. Cystic fibrosis related diabetes in Europe: Prevalence, risk factors and outcome; Olesen et al. J. Cyst. Fibros. 2020, 19, 321–327. [Google Scholar] [CrossRef]
- Alves, C.; Della-Manna, T.; Albuquerque, C.T.M. Cystic fibrosis-related diabetes: An update on pathophysiology, diagnosis, and treatment. J. Pediatr. Endocrinol. Metab. 2020, 33, 835–843. [Google Scholar] [CrossRef]
- Pozo, L.; Bello, F.; Mendez, Y.; Surani, S. Cystic fibrosis-related diabetes: The unmet need. World J. Diabetes 2020, 11, 213–217. [Google Scholar] [CrossRef]
- Marshall, B.C.; Butler, S.M.; Stoddard, M.; Moran, A.M.; Liou, T.G.; Morgan, W.J. Epidemiology of cystic fibrosis-related diabetes. J. Pediatr. 2005, 146, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Minicucci, L.; Lorini, R.; Giannattasio, A.; Colombo, C.; Iapichino, L.; Reali, M.F.; Padoan, R.; Calevo, M.G.; Casciaro, R.; De Alessandri, A.; et al. Liver disease as risk factor for cystic fibrosis-related diabetes development. Acta Paediatr. 2007, 96, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Desimone, M.; Kasim, N.; Chan, C.L. Cystic fibrosis-related diabetes: Prevalence, screening, and diagnosis. J. Clin. Transl. Endocrinol. 2021, 27, 100290. [Google Scholar] [CrossRef]
- Hjelm, M.; Tumin, D.; Nemastil, C.J.; Salvator, A.E.; Hayes, D., Jr. Influence of Cystic Fibrosis-Related Diabetes on Mental Health in Adults: A Single-Center Study. Lung 2020, 198, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Okoniewski, W.; Hughan, K.S.; Weiner, G.A.; Weiner, D.J.; Forno, E. Glycemic control and FEV1 recovery during pulmonary exacerbations in pediatric cystic fibrosis-related diabetes. J. Cyst. Fibros. 2020, 19, 460–465. [Google Scholar] [CrossRef]
- Moran, A.; Brunzell, C.; Cohen, R.C.; Katz, M.; Marshall, B.C.; Onady, G.; Robinson, K.A.; Sabadosa, K.A.; Stecenko, A.; Slovis, B.; et al. Clinical care guidelines for cystic fibrosis-related diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010, 33, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.; Pillay, K.; Becker, D.; Granados, A.; Hameed, S.; Acerini, C.L. ISPAD Clinical Practice Consensus Guidelines 2018: Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. S27), 64–74. [Google Scholar] [CrossRef]
- Chung, W.K.; Erion, K.; Florez, J.C.; Hattersley, A.T.; Hivert, M.F.; Lee, C.G.; McCarthy, M.I.; Nolan, J.J.; Norris, J.M.; Pearson, E.R.; et al. Precision Medicine in Diabetes: A Consensus Report From the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 1617–1635. [Google Scholar] [CrossRef]
- Zolin, A.; Orenti, A.; Naehrlich, L.; van Rens, J. ECFSPR Annual Report 2017; European Cystic Fibrosis Society: Karup, Denmark, 2017; Available online: https://www.ecfs.eu/sites/default/files/general-content-images/working-groups/ecfs-patient-registry/ECFSPR_Report2017_v1.3.pdf (accessed on 5 June 2021).
- Waugh, N.; Royle, P.; Craigie, I.; Ho, V.; Pandit, L.; Ewings, P.; Adler, A.; Helms, P.; Sheldon, C. Screening for cystic fibrosis-related diabetes: A systematic review. Health Technol. Assess. 2012, 16, 1–179. [Google Scholar] [CrossRef]
- Frost, F.; Dyce, P.; Ochota, A.; Pandya, S.; Clarke, T.; Walshaw, M.J.; Nazareth, D. Cystic fibrosis-related diabetes: Optimizing care with a multidisciplinary approach. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 545–552. [Google Scholar] [CrossRef]
- Gomes, A.; Patusco, R.; Chung, M.; Dreker, M.R.; Byham-Gray, L.; Lapin, C.; Ziegler, J. The associations between pediatric weight status and cystic fibrosis-related diabetes status and health-related quality of life among children and young adults with cystic fibrosis: A systematic review. Pediatr. Pulmonol. 2021, 56, 2413–2425. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, H.K.; Abbott, J.; Hurley, M.A.; Dye, L.; Lawton, C.L.; Mansfield, M.W.; Peckham, D. Cystic fibrosis-related diabetes (CFRD) and cognitive function in adults with cystic fibrosis. J. Cyst. Fibros. 2021, S1569–1993, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Keenan, K.; Gong, J.; Panjwani, N.; Avolio, J.; Lin, F.; Adam, D.; Barrett, P.; Bégin, S.; Berthiaume, Y.; et al. Cystic fibrosis-related diabetes onset can be predicted using biomarkers measured at birth. Genet. Med. 2021, 23, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Moheet, A.; Moran, A. New concepts in the pathogenesis of cystic fibrosis-related diabetes. J. Clin. Endocrinol. Metab. 2022, 2, dgac020. [Google Scholar] [CrossRef] [PubMed]
- de Souza Dias Lopes, P.; Machado, S.H.; Lucena, I.R.S.; Marostica, P.J.C. Ultrasound findings of pubertal development in girls with cystic fibrosis and their association with clinical outcomes and Tanner staging. Arch. Endocrinol. Metab. 2021, 65, 632–639. [Google Scholar] [PubMed]
- Fendler, W.; Borowiec, M.; Baranowska-Jazwiecka, A.; Szadkowska, A.; Skala-Zamorowska, E.; Deja, G.; Jarosz-Chobot, P.; Techmanska, I.; Bautembach-Minkowska, J.; Mysliwiec, M.; et al. Prevalence of monogenic diabetes amongst Polish children after a nationwide genetic screening campaign. Diabetologia 2012, 55, 2631–2635. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation Patient Registry 2013 Annual Data Report to the Center Directors Bethesda, Maryland. Available online: https://www.cff.org/2013_cff_annual_data_report_to_the_center_directors.pdf (accessed on 12 June 2021).
- Kaminski, B.A.; Goldsweig, B.K.; Sidhaye, A.; Blackman, S.M.; Schindler, T.; Moran, A. Cystic fibrosis related diabetes: Nutrition and growth considerations. J. Cyst. Fibros. 2019, 18 (Suppl. S2), S32–S37. [Google Scholar] [CrossRef]
- Mozzillo, E.; Raia, V.; Fattorusso, V.; Falco, M.; Sepe, A.; De Gregorio, F.; Nugnes, R.; Valerio, G.; Franzese, A. Glucose derangements in very young children with cystic fibrosis and pancreatic insufficiency. Diabetes Care 2012, 35, e78. [Google Scholar] [CrossRef][Green Version]
- Koch, C.; Cuppens, H.; Rainisio, M.; Madessani, U.; Harms, H.; Hodson, M.; Mastella, G.; Navarro, J.; Strandvik, B.; McKenzie, S.; et al. European Epidemiologic Registry of Cystic Fibrosis (ERCF): Comparison of major disease manifestations between patients with different classes of mutations. Pediatr. Pulmonol. 2001, 31, 1–12. [Google Scholar] [CrossRef]
- Soave, D.; Miller, M.R.; Keenan, K.; Li, W.; Gong, J.; Ip, W.; Accurso, F.; Sun, L.; Rommens, J.M.; Sontag, M.; et al. Evidence for a causal relationship between early exocrine pancreatic disease and cystic fibrosis-related diabetes: A Mendelian randomization study. Diabetes 2014, 63, 2114–2119. [Google Scholar] [CrossRef]
- Kayani, K.; Mohammed, R.; Mohiaddin, H. Cystic Fibrosis-Related Diabetes. Front. Endocrinol. 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Blackman, S.M.; Nelson, A.; Oberdorfer, E.; Wells, D.; Dunitz, J.; Thomas, W.; Moran, A. Diabetes-related mortality in adults with cystic fibrosis. Role of genotype and sex. Am. J. Respir. Crit. Care Med. 2015, 191, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Terliesner, N.; Vogel, M.; Steighardt, A.; Gausche, R.; Henn, C.; Hentschel, J.; Kapellen, T.; Klamt, S.; Gebhardt, J.; Kiess, W.; et al. Cystic-fibrosis related-diabetes (CFRD) is preceded by and associated with growth failure and deteriorating lung function. J. Pediatr. Endocrinol. Metab. 2017, 30, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Cano Megías, M.; Guisado Vasco, P.; González Albarrán, O.; Lamas Ferreiro, A.; Máiz Carro, L. Association of the relative change in weight and body mass index with lung function in teenagers and adults with cystic fibrosis: Influence of gender and diabetes. Endocrinol. Nutr. 2015, 62, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Ararat, E.; Sonawalla, A.; Berlinski, A.; Tas, E. Nutritional status between 5-10 years is associated with cystic fibrosis-related diabetes in adolescence. Pediatr. Pulmonol. 2021, 56, 3217–3222. [Google Scholar] [CrossRef]
- Consortium of the Cystic Fibrosis Genetic Analysis Consortium. Available online: http://www.genet.sickkids.on.ca/cftr (accessed on 20 May 2021).
- Laubner, K.; Scheuing, N.; Bauer, M.; Konrad, K.; Lilienthal, E.; Lorenzen, N.; Poeplau, T.; Teeken, A.; Thon, A.; Seufert, J.; et al. Association of CFTR mutations with Cystic Fibrosis related Diabetes (CFRD) in Germany and Austria: A multicentre analysis from the DPV registry. Diabetol. Stoffwechs. 2015, 10, P75. [Google Scholar] [CrossRef]
- Street, M.E.; Spaggiari, C.; Ziveri, M.A.; Rossi, M.; Volta, C.; Viani, I.; Grzincich, G.L.; Sartori, C.; Zanzucchi, M.; Raia, V.; et al. Insulin Production and Resistance in Cystic Fibrosis: Effect of Age, Disease Activity, and Genotype. J. Endocrinol. Investig. 2012, 35, 246–253. [Google Scholar]
- Iafusco, F.; Maione, G.; Rosanio, F.M.; Mozzillo, E.; Franzese, A.; Tinto, N. Cystic Fibrosis-Related Diabetes (CFRD): Overview of Associated Genetic Factors. Diagnostics 2021, 1, 572. [Google Scholar] [CrossRef]
- Cawood, T.J.; McKenna, M.J.; Gallagher, C.G.; Smith, D.; Chung, W.Y.; Gibney, J.; O’Shea, D. Cystic fibrosis-related diabetes in adults. Ir. Med. J. 2006, 99, 83–86. [Google Scholar]
- van den Berg, J.M.; Morton, A.M.; Kok, S.W.; Pijl, H.; Conway, S.P.; Heijerman, H.G. Microvascular complications in patients with cystic fibrosis-related diabetes (CFRD). J. Cyst. Fibros. 2008, 7, 515–519. [Google Scholar] [CrossRef]
- Kempegowda, P.; Sunsoa, H.; Chandan, J.S.; Quinn, L.M.; Amrelia, P.M.; Atta, S.N.; Amir, S.; The, Y.S.; Chaudhry, S.; de Bray, A.; et al. Retinopathy and microalbuminuria are common microvascular complications in cystic fibrosis-related diabetes. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820966428. [Google Scholar] [CrossRef] [PubMed]
- Franzese, A.; Mozilo, E.; Fattorusso, V.; Raia, V.; Valerio, G. Screening of glucose metabolism derangements in pediatric cystic fibrosis patients: How, when, why. Acta Diabetol. 2015, 52, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, V.; Casale, A.; Raia, V.; Mozzillo, E.; Franzese, A. Long-Term Follow-Up in a Girl with Cystic Fibrosis and Diabetes Since the First Year of Life. Diabetes Ther. 2017, 8, 1187–1190. [Google Scholar] [CrossRef] [PubMed][Green Version]

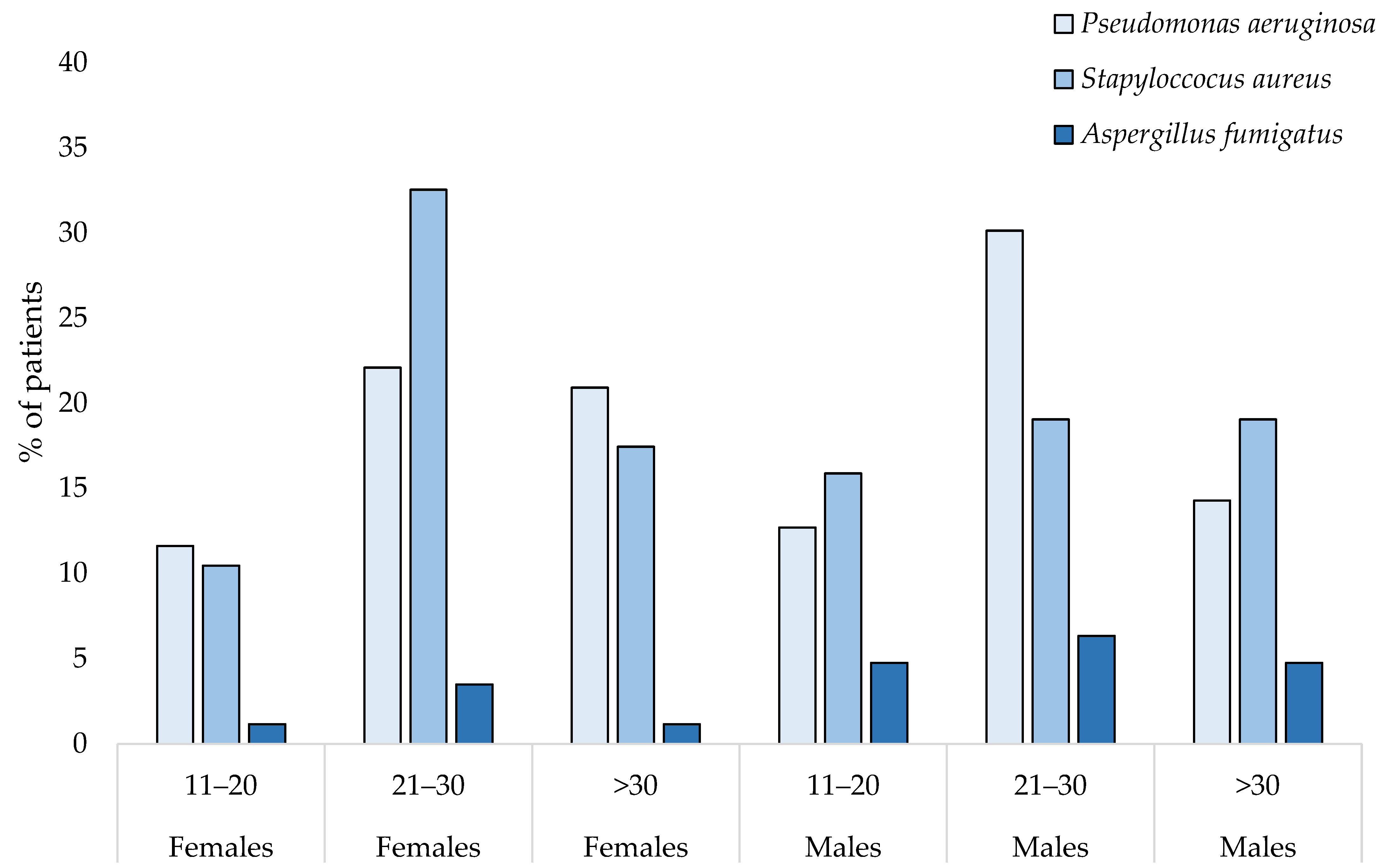
| Male | Female | p | |
|---|---|---|---|
| Number of patients | 63 | 86 | |
| Age | 26.5 ± 8.3 (12–49) | 27.8 ± 8.5 (11–51) | 0.382 |
| Age at CF diagnosis (years) | 6.8 ± 7.4 (1–39) | 7.2 ± 7.5 (1–40) | 0.881 |
| Age at CFRD diagnosis (years) | 20.2 ± 7.5 (5–43) | 19.9 ± 7.6 (6–41) | 0.894 |
| CFRD duration (years) | 6.4 ± 4.5 (1–22) | 8.2 ± 5.5 (2–23) | 0.048 |
| BMI (Z-score), patients <20 years | −1.83 ± 1.39 (−4.91–0.48) | −1.53 ± 1.96 (−6.91–1.08) | 0.367 |
| BMI (kg/m2), patients >20 years | 19.5 ± 2.7 (14–28) | 20.1 ± 3 (13–31) | 0.38 |
| BMI at CFRD diagnosis (Z-score), patients <20 years | −1.75 ± 1.48 (−5.74–0.53) | −1.14 ± 1.47 (−4.45–1.29) | 0.143 |
| BMI at CFRD diagnosis (kg/m2), patients >20 years | 19.6 ± 3.1 (14–27) | 19.3 ± 3.7 (13–28) | 0.476 |
| Sweat chloride concentration (mmol/L) | 114.6 ± 18.9 (70–147) | 114.3 ± 19 (83–175) | 0.665 |
| Any use of pancreatic enzyme, n (%) | 56 (89%) | 80 (93%) | |
| Any hepatic dysfunction, n (%) | 14 (22%) | 17 (20%) | |
| Former organ transplantation, n (%) | 1 (1.6%) | 2 (2.3%) |
| ECFS Patient Registry | Own Research | Hazard Ratio | Comment | |
|---|---|---|---|---|
| CFTR mutation | ||||
| F508del/F508del | 0.686 | 0.67 | 1.17 | Similar chances |
| F508del/other | 0.739 | 0.689 | 0.93 | Similar chances |
| Other/other | 0.202 | 0.236 | 0.98 | Similar chances |
| BMI | ||||
| Underweight | 0.118 | 0.568 | 4.81 | Almost 5 times higher chance |
| Optimum | 6.061 | 1.614 | 0.27 | More than 4 times less chance |
| Overweight | 0.147 | 0.021 | 0.14 | More than 7 times less chance |
| Chronic infection | ||||
| P. aeruginosa | 0.2913 | 1.273 | 4.37 | More than 4 times higher chance |
| S. aureus | 1.3913 | 1.381 | 0.99 | Similar chances |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachel, M.; Biesiadecki, M.; Galiniak, S. Cystic Fibrosis-Related Diabetes in Poland. Int. J. Environ. Res. Public Health 2022, 19, 4069. https://doi.org/10.3390/ijerph19074069
Rachel M, Biesiadecki M, Galiniak S. Cystic Fibrosis-Related Diabetes in Poland. International Journal of Environmental Research and Public Health. 2022; 19(7):4069. https://doi.org/10.3390/ijerph19074069
Chicago/Turabian StyleRachel, Marta, Marek Biesiadecki, and Sabina Galiniak. 2022. "Cystic Fibrosis-Related Diabetes in Poland" International Journal of Environmental Research and Public Health 19, no. 7: 4069. https://doi.org/10.3390/ijerph19074069
APA StyleRachel, M., Biesiadecki, M., & Galiniak, S. (2022). Cystic Fibrosis-Related Diabetes in Poland. International Journal of Environmental Research and Public Health, 19(7), 4069. https://doi.org/10.3390/ijerph19074069








