Parenting and the Serotonin Transporter Gene (5HTTLPR), Is There an Association? A Systematic Review of the Literature
Abstract
1. Introduction
- Has a relationship between 5HTTLPR and parenting ever been established in humans?
- Are there other variables involved in this relationship?
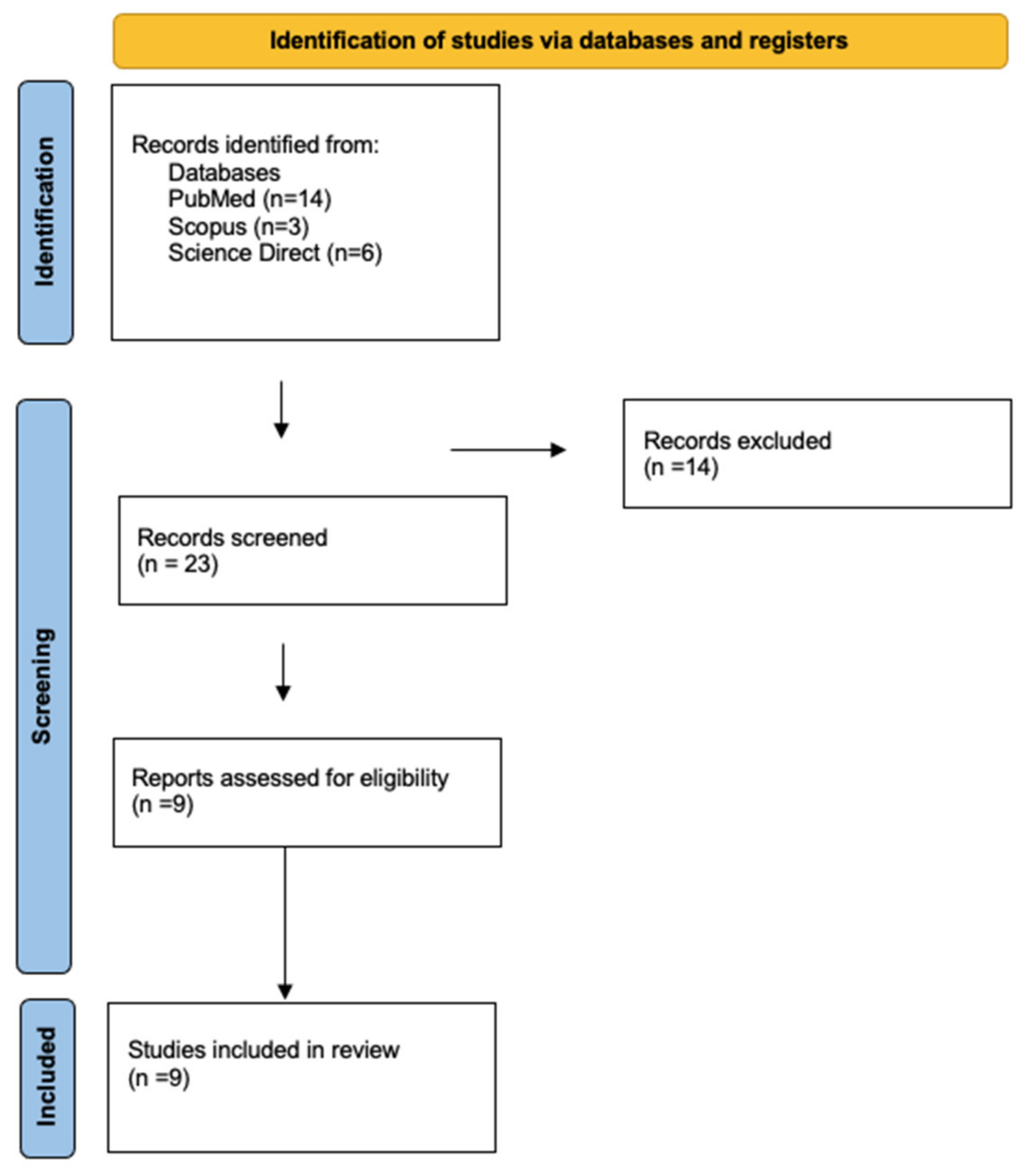
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cents, R.A.; Kok, R.; Tiemeier, H.; Lucassen, N.; Székely, E.; Bakermans-Kranenburg, M.J.; Hofman, A.; Jaddoe, V.W.; van IJzendoorn, M.H.; Verhulst, F.C.; et al. Variations in maternal 5-HTTLPR affect observed sensitive parenting. J. Child Psychol. Psychiatry 2014, 55, 1025–1032. [Google Scholar] [CrossRef]
- Morgan, J.E.; Hammen, C.; Lee, S.S. Parental Serotonin Transporter Polymorphism (5-HTTLPR) Moderates Associations of Stress and Child Behavior with Parenting Behavior. J. Clin. Child Psychol. 2016, 47, 76–87. [Google Scholar] [CrossRef]
- Groh, A.M.; Roisman, G.I.; van Ijzendoorn, M.H.; Bakermans-Kranenburg, M.J.; Fearon, R.P. The significance of insecure and disorganized attachment for children’s internalizing symptoms: A meta-analytic study. Child Dev. 2012, 83, 591–610. [Google Scholar] [CrossRef]
- Ainsworth, M.D.S.; Blehar, M.C.; Waters, E.; Wall, S. Patterns of Attachment: A Psychological Study of the Strange Situation; Psychology Press: Hillsdale, NJ, USA, 1978. [Google Scholar]
- Belsky, J. The determinants of parenting: A process model. Child Dev. 1978, 55, 83–96. [Google Scholar] [CrossRef]
- Rosenblatt, J.S. Nonhormonal basis of maternal behavior in the rat. Science 1967, 156, 1512–1514. [Google Scholar] [CrossRef]
- Kuroda, K.O.; Tachikawa, K.; Yoshida, S.; Tsuneoka, Y.; Numan, M. Neuromolecular basis of parental behavior in laboratory mice and rats: With special emphasis on technical issues of using mouse genetics. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1205–1231. [Google Scholar] [CrossRef]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- Kenna, G.A.; Roder-Hanna, N.; Leggio, L.; Zywiak, W.H.; Clifford, J.; Edwards, S.; Kenna, J.A.; Shoaff, J.; Swift, R.M. Association of the 5-HTT gene-linked promoter region (5-HTTLPR) polymorphism with psychiatric disorders: Review of psychopathology and pharmacotherapy. Pharm. Pers. Med. 2012, 5, 19–35. [Google Scholar] [CrossRef]
- Caspi, A.; Hariri, A.R.; Holmes, A.; Uher, R.; Moffitt, T.E. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am. J. Psychiatry 2010, 167, 509–527. [Google Scholar] [CrossRef]
- Lesch, K.P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Müller, C.R.; Hamer, D.H.; Murphy, D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef]
- Baião, R.; Fearon, P.; Belsky, J.; Teixeira, P.; Soares, I.; Mesquita, A. Does 5-HTTLPR moderate the effect of the quality of the environmental context on maternal sensitivity? Testing the maternal susceptibility hypothesis. Psychiatr. Genet. 2020, 30, 49–56. [Google Scholar] [CrossRef]
- Mileva-Seitz, V.R.; Kennedy, J.; Atkinson, L.; Steiner, M.; Levitan, R.; Matthews, S.G.; Meaney, M.J.; Sokolowski, M.B.; Fleming, A.S. Serotonin transporter allelic variation in mothers predicts maternal sensitivity, behavior and attitudes toward 6-month-old infants. Genes Brain Behav. 2011, 10, 325–333. [Google Scholar] [CrossRef]
- Bakermans-Kranenburg, M.J.; van Ijzendoorn, M.H. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc. Cogn. Affec. Neurosci. 2008, 3, 128–134. [Google Scholar] [CrossRef]
- Mileva-Seitz, V.R.; Bakermans-Kranenburg, M.J.; van IJzendoorn, M.H. Genetic mechanisms of parenting. Horm. Behav. 2008, 77, 211–223. [Google Scholar] [CrossRef]
- Sawano, E.; Doi, H.; Nagai, T.; Ikeda, S.; Shinohara, K. Interactive effects of 5-HTTLPR genotype and rearing environment on affective attitude towards own infant in Japanese mothers. Behav. Brain Res. 2008, 325, 173–180. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, 264–269. [Google Scholar] [CrossRef]
- Aromataris, E.; Pearson, A. The systematic review: An overview. Am. J. Nurs. 2014, 114, 53–58. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Sohani, Z.N.; Sarma, S.; Alyass, A.; De Souza, R.J.; Robiou-Du-Pont, S.; Li, A.; Mayhew, A.; Yazdi, F.; Reddon, H.; Lamri, A.; et al. Empirical evaluation of the Q-Genie tool: A protocol for assessment of effectiveness. BMJ Open 2016, 6, e010403. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Belsky, J.; Newman, D.; Widaman, K.; Rodkin, P.; Pluess, M.; Fraley, R.; Berry, D.; Helm, J.L.; Roisman, G.I. Differential susceptibility to effects of maternal sensitivity? A study of candidate plasticity genes. Dev. Psychopathol. 2015, 27, 725–746. [Google Scholar] [CrossRef]
- Kopala-Sibley, D.C.; Hayden, E.P.; Singh, S.M.; Sheikh, H.I.; Kryski, K.R.; Klein, D.N. Gene-environment correlations in the cross-generational transmission of parenting: Grandparenting moderates the effect of child 5-HTTLPR genotype on mothers’ parenting. Soc. Dev. 2017, 26, 724–739. [Google Scholar] [CrossRef]
- Sturge-Apple, M.L.; Davies, P.T.; Martin, M.J.; Cicchetti, D.; Hentges, R.F. An examination of the impact of harsh parenting contexts on children’s adaptation within an evolutionary framework. Dev. Psychol. 2012, 48, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.; Snidman, N.; Kahn, V.; Towsley, S.; Steinberg, L.; Fox, N.A. The Preservation of Two Infant Temperaments into Adolescence. Monogr. Soc. Res. Child Dev. 2007, 72, 1–75. [Google Scholar] [PubMed]
- Bakermans-Kranenburg, M.J.; Van IJzendoorn, M.H. Handbook of Attachment Third Edition, Theory, Research, and Clinical Applications; Guilford: New York, NY, USA, 2016. [Google Scholar]
- Brigman, J.L.; Mathur, P.; Harvey-White, J.; Izquierdo, A.; Saksida, L.M.; Bussey, T.J.; Fox, S.; Deneris, E.; Murphy, D.L.; Holmes, A. Pharmacological or Genetic Inactivation of the Serotonin Transporter Improves Reversal Learning in Mice. Cereb. Cortex 2010, 20, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Homberg, J.R.; Lesch, P.K. Looking on the Bright Side of Serotonin Transporter Gene Variation. Biol. Psychiatry 2011, 69, 513–519. [Google Scholar] [CrossRef]
- Jedema, H.; Gianaros, P.; Greer, P.; Kerr, D.D.; Liu, S.; Higley, J.D.; Suomi, S.J.; Olsen, A.S.; Porter, J.N.; Lopresti, B.J.; et al. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol. Psychiatry 2010, 15, 512–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chronis-Tuscano, A.; Raggi, V.L.; Clarke, T.L.; Rooney, M.E.; Diaz, Y.; Pian, J. Associations between Maternal Attention-Deficit/Hyperactivity Disorder Symptoms and Parenting. J. Abnorm. Child Psychol. 2008, 36, 1237–1250. [Google Scholar] [CrossRef]
- Murray, C.; Johnston, C. Parenting in mothers with and without attention-deficit/hyperactivity disorder. J. Abnorm. Psychol. 2006, 115, 52–61. [Google Scholar] [CrossRef]
- Deater-Deckard, K.; Sewell, M.D.; Petrill, S.A.; Thompson, L.A. Maternal working memory and reactive negativity in parenting. Psychol. Sci. 2010, 21, 75–79. [Google Scholar] [CrossRef]
- Praschak-Rieder, N.; Kennedy, J.; Wilson, A.A.; Hussey, D.; Boovariwala, A.; Willeit, M.; Ginovart, N.; Tharmalingam, S.; Masellis, M.; Houle, S.; et al. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: A [(11)C] DASB positron emission tomography study. Biol. Psychiatry 2007, 62, 327–331. [Google Scholar] [CrossRef]
- Johns, J.M.; Joyner, P.W.; McMurray, M.S.; Elliott, D.L.; Hofler, V.E.; Middleton, C.L.; Knupp, K.; Greenhill, K.W.; Lomas, L.M.; Walker, C.H. The effects of dopaminergic/serotonergic reuptake inhibition on maternal behavior, maternal aggression, and oxytocin in the rat. Pharmacol. Biochem. Behav. 2005, 81, 769–785. [Google Scholar] [CrossRef]
- Barofsky, A.L.; Taylor, J.; Tizabi, Y.; Kumar, R.; Jones-Quartey, K. Specific Neurotoxin Lesions of Median Raphe Serotonergic Neurons Disrupt Maternal Behavior in the Lactating. Rat. Endocrinol. 1983, 113, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Galbally, M.; Lewis, A.J.; van Ijzendoorn, M.; Permezel, M. The role of oxytocin in mother-infant relations: A systematic review of human studies. Harv. Rev. Psychiatry 2011, 19, 1–14. [Google Scholar] [CrossRef]
- Swain, J.E.; Lorberbaum, J.P.; Kose, S.; Strathearn, L. Brain basis of early parent-infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. J. Child Psychol. Psychiatry 2007, 48, 262–287. [Google Scholar] [CrossRef] [PubMed]
- Skuse, D.H.; Gallagher, L. Genetic influences on social cognition. Pediatr. Res. 2011, 69, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.; Riis, M.; Knigge, U.; Kjaer, A.; Warberg, J. Serotonin receptors involved in vasopressin and oxytocin secretion. J. Neuroendocrinol. 2003, 15, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Repetti, R.L.; Taylor, S.E.; Seeman, T.E. Risky families: Family social environments and the mental and physical health of offspring. Psychol. Bull. 2002, 128, 330–366. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Insel, T.R. Hormonal and Nonhormonal Basis of Maternal Behavior. The Neurobiology of Parental Behavior; Springer: New York, NY, USA, 2003. [Google Scholar]
- Belsky, J.; Jonassaint, C.; Pluess, M.; Stanton, M.; Brummett, B.; Williams, R. Vulnerability genes or plasticity genes? Mol. Psychiatry 2009, 14, 746–754. [Google Scholar] [CrossRef]
- Kinnally, E.L.; Tarara, E.R.; Mason, W.A.; Mendoza, S.P.; Abel, K.; Lyons, L.A.; Capitanio, J.P. Serotonin transporter expres- sion is predicted by early life stress and is associated with disinhibited behavior in infant rhesus macaques. Genes Brain Behav. 2010, 9, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.L.; Lesch, K.-P. Targetting the murine serotonin transporter: Insights into human neurobiology. Nat. Rev. Neurosci. 2008, 9, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.K.; Blakely, R.D. The functional impact of SLC6 transporter genetic variation. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 401–441. [Google Scholar] [CrossRef]
- Champagne, F.A.; Weaver, I.C.; Diorio, J.; Dymov, S.; Szyf, M.; Meaney, M.J. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 2006, 147, 2909–2915. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.L.; Lubin, F.D.; Funk, A.J.; Sweatt, J.D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry 2009, 65, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.; Diorio, J.; Liu, D.; Meaney, M.J. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 1999, 286, 1155–1158. [Google Scholar] [CrossRef]
- McGowan, P.O.; Sasaki, A.; D’Alessio, A.C.; Dymov, S.; Labonte, B.; Szyf, M.; Turecki, G.; Meaney, M.J. Epigenetic regulation of hippocampal glucocorticoid receptor gene expression in human suicide victims. Nat. Neurosci. 2009, 12, 342–348. [Google Scholar] [CrossRef]
- Lohaus, A.; Keller, H.; Ball, J.; Voelker, S.; Elben, C. Maternal sensitivity in interactions with three- and 12-month-old infants: Stability, structural composition, and developmental consequences. Infant Child Dev. 2004, 13, 235–252. [Google Scholar] [CrossRef]
- Pauli-Pott, U. Mothers with depressive symptoms: Cross-situational consistency and temporal stability of their parenting behaviour. Infant Behav. Dev. 2008, 31, 679–687. [Google Scholar] [CrossRef]
- Gotlib, I.H.; Joormann, J.; Minor, K.L.; Hallmayer, J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol. Psychiatry 2008, 63, 847–851. [Google Scholar] [CrossRef]
- Belsky, J. Mother-infant interaction at home and in the laboratory: A comparative study. J. Genet. Psychol. 1980, 137, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.R.; Tessitore, A.; Mattay, V.S.; Fera, F.; Weinberger, D.R. The amygdala response to emotional stimuli: A comparison of faces and scenes. Neuroimage 2002, 17, 317–323. [Google Scholar] [CrossRef]
- Canli, T.; Lesch, K.P. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007, 10, 1103–1109. [Google Scholar] [CrossRef]
- Belsky, J.; Beaver, K.M. Cumulative-genetic plasticity, parenting and adolescent self-regulation. J. Child Psychol. Psychiatry 2011, 52, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.E.; Way, B.M.; Welch, W.T.; Hilmert, C.J.; Lehman, B.J.; Eisenberger, N.I. Early family environment, current adversity, the serotonin transporter polymorphism, and depressive symptomatology. Biol. Psychiatry 2006, 60, 671–676. [Google Scholar] [CrossRef] [PubMed]
| Baiao et al. 2020 [12] | Bakerman et al. 2008 [14] | Belsky et al. 2015 [22] | Cents et al. 2014 [1] | Kopala-Sibley et al. 2017 [23] | Mileva-Seitz et al. 2011 [11] | Morgan et al. 2016 [2] | Sawano et al. 2016 [16] | Sturge Apple et al. 2012 [24] | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Rationale for study: Was a scientific rationale for chosen genes presented to avoid selective reporting of positive results? | 6 | 6 | 7 | 6 | 6 | 6 | 6 | 5 | 6 |
| 2. Selection and definition of outcome of interest: Were the cases appropriately defined? Were participants appropriately sampled? Were the case/outcome assessors blinded to the genotype status? | 5 | 6 | 6 | 5 | 6 | 5 | 5 | 5 | 6 |
| 3. Selection and comparability of comparison groups Were the controls appropriately defined? Were the controls sampled in a way to minimize selection bias? Was a detailed description of selection procedure (i.e., eligibility criteria, sources and methods of ascertainment, methods of matching if applicable) outlined or referenced? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 4. Technical classification of the exposure Was the source (e.g., buffy coat) and method of storage for the DNA sample appropriate? Was agreement with the Hardy–Weinberg equilibrium tested in controls? | 5 | 6 | 6 | 5 | 6 | 5 | 6 | 5 | 6 |
| 5. Non-technical classification of the exposure Did a blinded assessor conduct the genotyping? Was genotyping conducted in all the participants from the study simultaneously or in smaller batches? | 5 | 6 | 6 | 5 | 6 | 5 | 6 | 5 | 6 |
| 6. Other sources of bias | N/A | 5 | 7 | 5 | 6 | N/A | N/A | N/A | N/A |
| 7. Sample size and power Was the sample size appropriate? Was an a priori power analysis conducted? | 5 | 6 | 6 | 5 | 6 | 5 | 6 | 5 | 6 |
| 8. A priori planning of analyses Was the analysis plan appropriate and sufficiently described? | 5 | 6 | 6 | 5 | 6 | 6 | 6 | 5 | 6 |
| 9. Statistical methods and control for confounding Were important confounders appropriately controlled? Were missing data for samples and genetic variants appropriately handled? >10% missing genotype data is often unacceptable. Were the results adjusted for multiple testing to avoid false positive results? | 6 | 6 | 6 | 5 | 6 | 6 | 6 | 5 | 6 |
| 10. Testing of assumptions and inferences for genetic analyses Were all assumptions concerning the genetic analysis tested? | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 5 | 6 |
| 11. Appropriateness of inferences drawn from results | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 5 | 6 |
| Total Score | 43 | 59 | 62 | 53 | 60 | 50 | 59 | 45 | 54 |
| First Author | Year | Participants | Ethnic Group | Diagnostic Instrument | Genotype | GxE | Findings | Effect Size for the Association between 5-HTTLPR and Maternal Sensitivity |
|---|---|---|---|---|---|---|---|---|
| Baião et al. [12] | 2020 | 210 mothers and their preschool children. Children’s ages ranged from 40 to 77 months (M = 58.26, SD = 7.63) | Caucasian | Maternal sensitivity was measured observationally. Mother–child interaction was videotaped in a quiet room (at the family home or at the preschool) across three 5-min episodes. The mother’s ability to accurately perceive the infant’s signals was assessed using Ainsworth et al.’s (1974) 9-level maternal sensitivity scale. | SS/LL/Ls | Yes Family context | The findings revealed a gene-X–environment interaction, with short allele homozygotes proving more sensitive to the family context than long allele carriers, depending on the environmental context. | - |
| Bakermans-Kranenburg & van Ijzendoorn [14] | 2008 | 159 mothers with their 2-year-old toddlers | Caucasian | During a series of problem-solving tasks, mothers’ sensitive interactions were observed. Mothers’ supportive presence, intrusiveness, and clarity of instruction were rated on 7-point scales. The Dutch Family Problems Questionnaire for marital discord. Young Adult Self-Report for maternal depression. | SS/LL/Ls | Yes Age of child, maternal education, level, depression, maternal sensitivity, or marital discord | The 5-HTTLPR SCL6A4 and OXTR rs53576 genes were found to have independent genetic effects on maternal sensitivity. Parents with the possibly less efficient variants of the serotonergic (5-HTT ss) and oxytonergic (AA/AG) system genes showed lower levels of sensitive responsiveness to their toddlers after controlling for differences in maternal education, depression, and marital discord. | partial η2 = 0.03 |
| Belsky et al. [22] | 2014 | 112 mothers and children | - | Child Behaviour Checklist and Teacher Report versions for total problem symptomatology. Social Skills Rating System for social competence. Mother–child interactions were videotaped during 15-min semi-structured tasks at 6, 15, 24, and 36 months. | SS/LL/Ls | Yes | There were few main effects in candidate genes, and they did not seem to interact with maternal sensitivity/insensitivity. | Cohen’s d = 0.11 |
| Cents et al. [1] | 2014 | 767 mother–child dyads. Children were assessed at 14, 36, and 48 months | Caucasian | Maternal sensitivity was repeatedly observed at the child’s age of 14 months, 36 months, and 48 months. Sensitivity was coded using the Ainsworth’s rating scales for sensitivity and cooperation and the revised Erickson rating scales for Supportive presence and Intrusiveness. Child social fearfulness was observed using the Stranger Approach episode of the Laboratory Temperament Assessment Battery at 36 months. | SS/LL/Ls | Yes Maternal age, educational level, marital status, and parity | Repeated measurement analyses revealed that maternal 5-HTTLPR has a consistent main effect on sensitivity; mothers with the S allele were more sensitive toward their children (p = 0.005). The 5-HTTLPR genotype of the child had no bearing on this effect. We found no evidence that the effect of 5-HTTLPR on sensitivity was moderated by child social fearfulness. | r = 0.17 |
| Kopala-Sibley et al. [23] | 2017 | Sample 1: participants were recruited from a community sample of 405 children (208 girls) and their primary caregivers as part of a study of child temperament. At baseline, children were between 36 and 47 months of age (M = 40.72, SD = 3.51). Sample 2: participants were 476 children (251 males) and their mothers from a larger longitudinal study of 569 three-year-old children (for details, see Olino et al., 2010). The mean age of the children was 43.5 months (SD = 2.8) | European American and non-Hispanic | Sample 1: Three-bag task from which maternal support and hostility were coded. The Measure of Parenting Styles (MOPS; Parker et al., 1997) as a measure of mothers’ parenting experiences as children. Sample 2: The Teaching Tasks battery (Egeland et al., 1995), from which maternal support and hostility were rated. The Parental Bonding Inventory (PBI), a self-report measure of mothers bonding with their mothers. | SS/LL/Ls | Yes | A child with a short allele on the 5-HTTLPR gene was linked to more maternal hostility and less maternal support, but only when the mother reported poor grandmother’s parenting. | r = −0.01 |
| Mileva-Seitz et al. [11] | 2011 | 204 mothers and their children assessed to 72 months | Caucasian (90%), with 3% (n = 6) mixed ethnicity, 2% (n = 4) African, 1.5% Hispanic (n = 3), and 1% East Indian (n = 2); the rest were unknown or unspecified | At 6 months postpartum, it was recorded 30 min of non-feeding mother–infant interaction at the mothers’ homes. Maternal sensitivity was assessed using the Ainsworth maternal sensitivity scales (Ainsworth et al. 1978). The Childbearing Attitudes Questionnaire (CAQ) was used to assess mothers’ feelings and attitudes about a range of issues related to mothering and the infant. The Childhood Trauma Questionnaire (CTQ; Bernstein et al. 2003), was used to assess five types of childhood trauma: physical, emotional and sexual abuse; and emotional and physical neglect. The Parental Bonding Instrument (PBI; Parker et al. 1979), was used to assess the quality of parenting experienced during the subjects’ first 16 years of life. | S, LA, and LG | Yes | The genotype can predict differences in maternal sensitivity at 6 months postpartum, even after controlling for maternal age and parity: mothers with a S (or the functionally similar LG) allele were more sensitive than mothers without the allele during a 30-min recorded mother–infant interaction. Furthermore, highly significant gene–environment interactions in relation to maternal behaviour were found, such as mothers who lacked the S or LG alleles orienting away from their babies more frequently if they also reported poor early care quality. | Cohen’s d = 0.405 |
| Morgan et al. [2] | 2016 | 162 parents and their 6- to 9-year-old offspring. Families were sampled to include children with (n = 76) and without (n = 86) ADHD. The primary caregiver (defined as the parent who spends the most time with the child) and their child attended the laboratory in person; only the primary caregiver provided parent data. Because there was no difference between mothers and fathers in terms of positive (Z = 0.66, p = 0.51) and negative (Z = 0.15, p = 0.88) parenting, parenting data were pooled across gender. | Caucasian 62.6%, African American 8.4%, Hispanic 14.8%, Asian 7.1%, mixed 7.1% | To assess positive and negative parenting behaviours, the Dyadic Parent Child Interaction Coding System (DPICS; Eyberg, Nelson, Duke, and Boggs, 2005) was used. The task, which took approximately 20 min, required parents to play with their child in an activity of their choice. Child-related stress with the UCLA Life Stress Interview (LSI). The Parenting Stress Index–Short Form (PSI) for parenting assessment. Children’s disruptive behaviour was estimated using the number of symptoms of attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) from the Computerised Diagnostic Interview Questionnaire for Children. IV parental depression was assessed with the Beck Depression Inventory-II. Parental ADHD was self-reported via the 18-item Adult ADHD Self-Report Scale. | SS/LL/Ls | Yes | The S allele was associated with significantly less observed positive parenting than the LL genotype. There were also significant gene–environment interactions: parental negativity was negatively associated with child-related stress in SS/SL genotype parents but not in LL genotype parents; next, observed disruptive child behaviour was positively associated with parental negativity in both genotypes, but the effect was strongest in SS/SL parents. | r = 0.13 |
| Sawano et al. [16] | 2016 | 93 mothers and their 4-month-old children | Asian | The parental bonding instrument (PBI) was used to assess the perceived quality of parental care received during the first 16 years of life. The Mother to Infant Bonding Scale (MIBS) was used to assess mothers’ affective attitude towards their own infant. The Edinburgh Postnatal Depression Scale (EPDS) was used to assess maternal symptoms of depression. | S/S l-carriers | Yes | On maternal attitude, it was discovered an interaction between the rearing environment and the 5-HTTLPR genotype. In particular, in mothers with homozygous short allele genotype, a poor rearing environment (characterised by low maternal care and high paternal overprotection) reduced a positive attitude toward one’s own infant. In long allele carriers, on the other hand, this negative effect was almost completely eliminated. Overall, our findings suggest that the 5-HTTLPR gene moderates the impact of maternal and parental behaviour on the experienced rearing environment, which is consistent with the idea that the short 5-HTTLPR allele amplifies environmental influence. | r = −0.09 |
| Sturge Apple et al. [24] | 2012 | 201 mothers and their two-year-old children | - | Mother–Child Problem Solving Task videotaped. Mother–Child Free Play/Compliance Task videotaped. Revised Conflict Tactics Scale (CTS2). Conflict and Problem-Solving Scale (CPS). Iowa Family Interaction Rating Scales (IFIRS). Empathetic Awareness Toward Children’s Needs scales from the Adult Adolescent Parenting Inventory (AAPI). Nurturance scale of the Parenting Dimensions Inventory (PDI). Computerised Diagnostic Interview Schedule IV (C DIS IV). | S/LG/LA genotypes | Yes | Mothers with one or two copies of the 5-HTTLPR S allele had a higher risk of both sensitive and harsh/punitive caregiving behaviours. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landoni, M.; Dalla Muta, A.; Di Tella, S.; Ciuffo, G.; Di Blasio, P.; Ionio, C. Parenting and the Serotonin Transporter Gene (5HTTLPR), Is There an Association? A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2022, 19, 4052. https://doi.org/10.3390/ijerph19074052
Landoni M, Dalla Muta A, Di Tella S, Ciuffo G, Di Blasio P, Ionio C. Parenting and the Serotonin Transporter Gene (5HTTLPR), Is There an Association? A Systematic Review of the Literature. International Journal of Environmental Research and Public Health. 2022; 19(7):4052. https://doi.org/10.3390/ijerph19074052
Chicago/Turabian StyleLandoni, Marta, Alice Dalla Muta, Sonia Di Tella, Giulia Ciuffo, Paola Di Blasio, and Chiara Ionio. 2022. "Parenting and the Serotonin Transporter Gene (5HTTLPR), Is There an Association? A Systematic Review of the Literature" International Journal of Environmental Research and Public Health 19, no. 7: 4052. https://doi.org/10.3390/ijerph19074052
APA StyleLandoni, M., Dalla Muta, A., Di Tella, S., Ciuffo, G., Di Blasio, P., & Ionio, C. (2022). Parenting and the Serotonin Transporter Gene (5HTTLPR), Is There an Association? A Systematic Review of the Literature. International Journal of Environmental Research and Public Health, 19(7), 4052. https://doi.org/10.3390/ijerph19074052







