Abstract
The physiological changes during pregnancy may increase the risk of complications in pregnant women with coronavirus disease 2019 (COVID-19). Vitamin D is a fat-soluble secosteroid hormone and its role in immunity is appears to be of particular importance in this recent pandemic. Nevertheless, there is little research about the role of vitamin D levels regarding COVID-19 in pregnant women to date. This study aimed to establish a relationship between serum 25-hydroxyvitamin D (25(OH)D) levels in pregnant women and COVID-19. A comparative case-control study was performed with a study population of 256 pregnant women (82 pregnant women with infection and 174 women in control group). Serum 25(OH)D levels were significantly lower in pregnant women with COVID-19 infection than in those without infection. In addition, 89% of COVID-19-positive pregnant women had 25(OH)D deficiency, while in the control group the percentage was 75.30%, finding statistically significant differences (ORa = 2.68; 95% CI 1.19–6.06; p = 0.01). Our results find a relationship between vitamin D deficiency in pregnant women and COVID-19 infection. This finding could be relevant for actual clinical practice. Thus, more research is needed in this field.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China, and it spread rapidly throughout the world. The World Health Organization (WHO) declared a global health emergency on 31 January 2020, and subsequently, a pandemic on 11 March 2020. There are more than 400 million confirmed cases of people infected with this coronavirus, including nearly 6 million deaths worldwide at the time of writing [1]. Symptoms of SARS-CoV-2 infection in pregnant women are similar to those in the general population. However, pregnant women, when they present clinical symptoms, are more likely to require treatment in the intensive care unit (ICU) when compared to non-pregnant ones [2]. Some authors state that this is due to the different physiological changes during gestation, as pregnant women may have an increased risk of complications [3,4,5].
Vitamin D is a fat-soluble secosteroid hormone [6]. The classic functions that have been attributed to vitamin D are related to calcium and phosphate homeostasis, acting in the intestines, kidneys, and bones [7]. However, in recent years, other functions of vitamin D have been described, for example, its important immune function [8,9]. In this sense, vitamin D deficiency has been associated with an increased risk of developing autoimmune diseases [10] and respiratory infections [11]. Furthermore, vitamin D deficiency has been linked with adverse effects on pregnancy and offspring such as placental insufficiency [12,13], preterm delivery [14], gestational diabetes [15], low birth weight, and being small for gestational age [16].
As the outbreak of coronavirus disease 2019 (COVID-19) develops, the prevention and management of COVID-19 among pregnant women and possible maternal complications induced by the infection have become a major concern. Nevertheless, there is little research about the role of vitamin D levels regarding the COVID-19 in pregnant women. Therefore, our objective was to determine the relationship between serum 25-hydroxyvitamin D (25(OH)D) levels, specifically decreased levels, in pregnant women with COVID-19 infection and the association with the severity of the disease.
2. Materials and Methods
2.1. Study Design
An observational comparative case-control study was performed between singleton gestations with COVID-19 infection versus uninfected pregnant women who completed delivery in the same period.
This study was carried out in the Obstetrics and Gyneacology Department of Torrecardenas Hospital in the period between November 2020 and September 2021, with a subsequent 4-week follow-up of the patients included in the study. Torrecardenas Hospital is the reference hospital of the province of Almeria, Spain, with 727,945 inhabitants, and attends about 3000 deliveries per year.
2.2. Study Population
The study population consisted of singleton pregnant women who were admitted for ongoing labour or any other reason but who terminated their pregnancy by delivery or caesarean section during their admission.
Two groups were established: case group or pregnant women with COVID-19 infection and control group or pregnant women without infection. Inclusion criteria for the case group were: pregnant women who tested positive for SARS-CoV-2 using real-time quantitative reverse transcription (qRT-PCR) from nasopharyngeal and oropharyngeal samples, with single pregnancy and who had pregnancies with date of last known menstrual period and normal prenatal controls normal up to the time of diagnosis. Inclusion criteria for the control group were: pregnant women with negative qRT-PCR who completed delivery in the same period as the case group, with single pregnancy, and who had pregnancies with a date of last known menstrual period, and normal prenatal controls up to the time of diagnosis. Two control subjects were matched prospectively to each case and deliveries were immediately after the delivery of pregnant women with COVID-19 infection. Exclusion criteria for both groups were: pregnant women under 18 years old, who did not give informed consent at the time of inclusion in the study, who had multiple gestation, and who took extra vitamin D supplements during pregnancy.
2.3. Sample Size
The 2:1 ratio was based on a priori power calculations. It was necessary to recruit a minimum of 31 patients in the case group and 62 in the control group to achieve a power of 80% to detect differences in the contrast of the null hypothesis (Ho), taking into account that the significance level was 5% and assuming that the proportion in the control group was 5% and that the expected loss percentage was 10%. The case group included 87 pregnant women, and the control group 174 pregnant women. However, after study exclusions (Scheme 1), the final case group included 82 pregnant women.
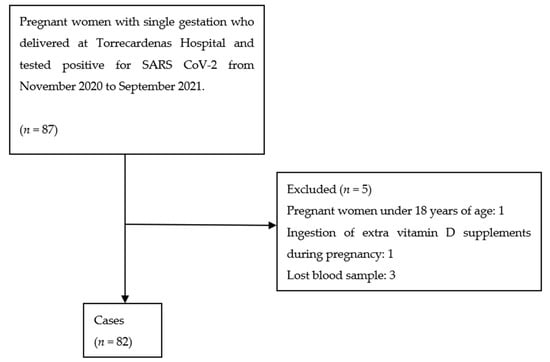
Scheme 1.
Case selection process.
2.4. Study Variables
The following variables were collected: maternal age; level of education; race (the race was included because skin colour influences vitamin D levels [17,18]); tobacco consumption; pre-pregnancy body mass index (BMI, calculated as weight in kilograms divided by the square of height in meters), pre-pregnancy obesity (pre-pregnancy BMI ≥ 30 kg/m2 was defined as obese); parity; chronic hypertension; pregestational diabetes; asthma; gestational hypertension; preeclampsia; gestational diabetes; gestational hypothyroidism; gestational week at the delivery; preterm birth; cause of preterm birth; labour beginning; labour ending; neonatal outcomes; and the following laboratory parameters at the time of delivery: 25(OH)D (ng/mL), haemoglobin, haematocrit, leukocytes, lymphocytes, platelets, prothrombin time, and fibrinogen. Variables of symptoms, type of symptoms, hospital admission criteria, admission criteria to the ICU, and severity were also collected by the researchers in women with COVID-19 infection. These pregnant women were classified as mild, moderate, severe, or critical according to the WHO Living guidance for clinical management of COVID-19 [19].
2.5. Data Collection
Pregnant women who met the study inclusion criteria, after obtaining informed consent, responded to an interview where some of the variables studied were collected. Subsequently, a blood sample was extracted at delivery, and medical records were reviewed. Serum 25(OH)D levels were measured on a Roche Modular E 170 analyser (Roche Diagnostics, West Sussex, England). Results are expressed in ng/mL (1 ng/mL = 2.496 nmol/L). This method has been validated against high-performance liquid chromatography (HPLC) and radioimmunoassay methodology and can detect 25(OH)D levels in the range of 4 to 96 ng/mL [20]. Levels of 25(OH)D were classified following the Spanish Society of Endocrinology and Nutrition (SEEN) as sufficient (≥30 ng/mL), insufficient (20–29 ng/mL), deficient (10–19 ng/mL), and a last classification was added called severe deficiency (<10 ng/mL). This variable was also grouped into a new variable called 25(OH)D deficiency, showed as a dichotomous qualitative variable: Yes/No. Vitamin D deficiency was defined when values were below 20 ng/mL, according to the SEEN criteria.
2.6. Ethical Implications
This study was approved by the Ethics Committee of the Torrecárdenas University Hospital on 27 January 2021, with protocol number 2607-143/2020. Ethical principles were taken into account and the confidentiality of the participants was respected, in compliance with the Declaration of Helsinki of the World Medical Association (WMA), which sets out the ethical principles for medical research on human beings.
Pregnant women who gave birth were informed about the observational nature of the study and asked to participate after giving written consent. The information was treated confidentially and anonymously.
2.7. Statistical Analysis
Statistical analysis was performed with SPSS version 25.0 software package (SPSS, Chicago, IL, USA). A descriptive analysis of the variables was carried out, calculating frequencies and percentages for those variables that were qualitative and measures of central tendency and dispersion for the quantitative variables. Prior to hypothesis contrast between quantitative and qualitative variables, a Kolmogorov–Smirnov test was performed to verify if they followed a normal distribution. According to this, nonparametric data were analysed with the Mann–Whitney U test (2 independent samples). The Pearson-X2 test was used to compare percentages, with Yates’ correction factor and Fisher’s exact test if necessary.
Finally, a regression study was carried out to complete the results. A binary logistic regression analysis to adjust for possible confounding factors was performed. The dependent variable used was COVID-19 infection and independent variables considered for the analysis were vitamin D deficiency, maternal age, education, and race. We divided the race into Caucasian and Arab women since they accounted for the majority of the sample, discarding the rest to avoid possible biases.
A value of p < 0.05 was considered statistically significant in all the hypothesis contrast tests.
3. Results
A total of 256 women participated in this study. Case group consisted of 82 (32%) women, while 174 (68%) women had no positive qRT-PCR for SARS-CoV-2.
Bivariant analyses showed that statistically significant differences were observed when comparing both groups regarding their level of education. Sixty-five (37.60%) pregnant women in the control group had a university education, compared to seven (8.60%) in the COVID-19 group (p < 0.001). The rest of the educational levels were higher in pregnant women with COVID-19 infection. The percentage of Caucasian women was significantly higher in the control group versus the COVID-19 group. However, the percentage of Arab, Hispanic, and Black races was higher in women with COVID-19 infection compared to women in the control group (p = 0.001). Preterm deliveries were more frequent in the case group women (19.50%) compared to the control group (5.20%), this difference being statistically significant (p < 0.001). The newborns of the case group´s mothers had a significantly lower weight than of those in the control group (p < 0.001), and they had significantly more foetal growth restriction (p = 0.02). In addition, there were significantly more newborns with Apgar score < 7 at 5 min in mothers with COVID-19 infection (4.9%) than in the control group (0.00%) (p = 0.002).
We found no statistically significant differences in the rest of the characteristics of patients and delivery (Table 1) between both groups, nor in the laboratory parameters: haemoglobin, haematocrit, leukocytes, lymphocytes, platelets, prothrombin time, and fibrinogen.

Table 1.
Characteristics of patients and delivery.
Serum 25(OH)D levels of the participant of the study are shown in Table 2. We found low serum 25(OH)D levels in both groups, but they were significantly higher in the control group, with a median of 13.80 [11.45] ng/mL, compared to the COVID-19 group, with a median of 10.15 [9.45] ng/mL (p = 0.005). Furthermore, statistically significant differences were found in vitamin D deficiency status. Vitamin D sufficiency, insufficiency, and deficiency were more frequent in the control group women, while severe deficiency was higher in women with COVID-19 infection (p = 0.004). Seventy-three (89%) pregnant women in the COVID-19 group had vitamin D deficiency (severe or not), while 131 (75.30%) women in the control group were deficient (p = 0.01, ORc = 2.66, 95% CI: 1.22–5.77). After adjusting for confounding factors, vitamin D deficiency was still statistically significantly different between both groups (p = 0.01, ORa = 2.68, 95% CI: 1.19–6.06) (Table 3). A binary logistic regression analysis, using COVID-19 as the dependent variable, and age, obesity, pre-pregnancy weight, weight in the third trimester of pregnancy, and type of delivery as independent variables, was performed. The analysis showed that the independent variables were not influential.

Table 2.
Vitamin D in the study population.

Table 3.
Binary Logistic Regression Analysis.
In the case group, 7 (8.50%) women had a severe, moderate, or critical infection, while 75 (91.50%) women had a mild infection. Four (4.90%) patients required ICU admission and 25 (30.50%) women had symptoms. Symptoms were sore throat (12%), fever (40%), anosmia (20%), ageusia (8%), cough (64%), respiratory distress (28%), and headache (28%). Thirty-six percent of women with COVID-19 infection also had pneumonia. The main reason for hospital admission was labour or obstetric reasons in 76 (92.7%) of the women, while only 6 (7.3%) of the pregnant women were admitted due to COVID-19 infection. Nasopharyngeal qRT-PCR of the newborns was positive in 2 cases.
Regarding vitamin D deficiency, an analysis of pregnant women with COVID-19 infection who had a mild infection versus moderate, severe, or critical infection was performed. Although serum 25(OH)D levels were higher in pregnant women with a mild infection, no statistically significant differences were found (p = 0.25). All patients with moderate, severe, or critical infection had vitamin D deficiency (p = 0.19) (Table 4). There were also no statistically significant differences in vitamin D deficiency in women who were admitted to the ICU versus those who were not (Table 5).

Table 4.
Vitamin D in patients with COVID-19 according to severity.

Table 5.
Vitamin D in patients with COVID-19 according to intensive to ICU admission.
4. Discussion
The main aim of this research was to analyse the relationship between decreased serum 25(OH)D levels in pregnant women with COVID-19 infection. We also sought to assess its association with the severity of infection.
The results of our study show a relationship between vitamin D levels and COVID-19 infection in pregnant women. Although levels were low in both groups, vitamin D levels were significantly lower in those women who had the infection. In addition, 89% of infected pregnant women had 25(OH)D deficiency (<20 ng/mL) while 75.30% of negative pregnant women had 25(OH)D deficiency, which was statistically significant. Adjusting for possible confounding factors, we found that pregnant women with 25(OH)D deficiency were 2.68 times more likely to have COVID-19 infection.
Investigations such as this one assessing 25(OH)D levels in pregnant women with COVID-19 infection are scarce to date. Vitamin D levels were also significantly lower in COVID-19-positive compared to -negative pregnant women, in a case-control study conducted in Ankara. These vitamin D levels were also statistically significantly lower in those women with moderate or severe COVID-19 infection compared to those with milder infection [21]. However, another cohort study in pregnant women found no statistically significant differences in vitamin D deficiency between pregnant women with COVID-19 infection and control group, nor the severity of infection [22]. Mean 25(OH)D levels were significantly lower than the cut-off values (30 ng/mL was taken as the cut-off value) in a published study of 44 pregnant women with COVID-19 infection. However, this study has limitations as it does not compare the results with uninfected pregnant women (controls), so it is not possible to establish causality [23].
Concerning the non-pregnant population, some authors are trying to find a possible relationship between vitamin D and COVID-19 infection. A study in Israel involving 7807 individuals (10.02% positive and 89.98% negative qRT-PCR for SARS-CoV-2) found that serum 25(OH)D levels were lower in positive than negative individuals. A significant and independent association was demonstrated between decreased 25(OH)D levels and increased likelihood of COVID-19 infection. These 25(OH)D levels were also associated as a risk factor for SARS-CoV-2 hospitalization [24]. Another study in Florida also showed an association between vitamin D deficiency and the risk of developing COVID-19 infection, even after adjusting for confounding factors [25].
Moreover, decreased 25(OH)D levels have not only been associated with an increased likelihood of SARS-CoV-2 infection but have also been associated with disease severity and mortality in non-pregnant population [26,27,28]. Our study also assessed the vitamin D levels regarding the severity of the infection and the need for admission of the pregnant woman to the ICU. Although in the present study we found that pregnant women with moderate, severe, or critical infection had lower 25(OH)D levels compared to women with mild infection, this association was not significant. The same was true for women who required admission to the ICU. This may be because of the 82 women with COVID-19 infection, only seven women had moderate, severe, or critical infection, and four required ICU admission; these results may not be representative of trends in the general population.
Our results could be explained by the important role of vitamin D in the immune system. Its relationship with the prevention of respiratory infections and local inflammation at the pulmonary level has been established [11,29,30]. However, this alone does not explain its possible relationship to COVID-19 infection. SARS-CoV-2 decreases angiotensin 2-converting enzymes (ACE-2) and increases toxicity due to angiotensin II accumulation which, in turn, influences respiratory syndrome and myocarditis [31]. Vitamin D regulates the renin–angiotensin–aldosterone system (RAAS), decreasing renin and ACE expression and promoting ACE-2 expression, which may explain the possible protective role against acute lung injury [32,33]. Vitamin D may also influence the cytokine storm through its anti-inflammatory role in the immune system by decreasing the production of proinflammatory cytokines and increasing anti-inflammatory cytokines [31].
Furthermore, other studies show a relationship between vitamin D and some immune system factors. One study suggested that complement induces in T Helper 1 cells (Th1) the expression of vitamin D receptors and the CYP27B1 enzyme (which activates vitamin D), causing these cells to respond to vitamin D to suppress interferon-γ (IFN-γ) and increase interleukin-10 (IL-10). This could stop or modulate the hyperinflammatory response in patients with COVID-19 [34]. A link has also been described between vitamin D deficiency and decreased numbers of natural killer (NK) cells, preventing the cell barrier from forming during early viral infections [35].
Our research has some limitations. One of the limitations of this study is that only pregnant women with COVID-19 infection at the time of delivery were analysed and we did not analyse the possible impact of the infection at another time during pregnancy. COVID-19 vaccination was not yet recommended for pregnant women at the time of our study, so none of the women included in the study had been vaccinated. As the association between 25(OH)D levels and severity of infection could be affected by this intervening variable, this aspect could be considered for future research. In our study, no information about the diet and exposure to the sun was collected. As these variables could affect vitamin D status, they could be considered when performing similar research. Other limitations were the small sample and the low number of COVID-19-positive pregnant women with severe infection. However, despite the small sample, we completed the necessary number of cases required by the sample size to achieve a power of 80% to detect differences in contrast to the null hypothesis. Moreover, our study offers interesting results that may have relevance for clinical practice.
5. Conclusions
Our results show a relationship between vitamin D deficiency in pregnant women and COVID-19 infection. However, it is necessary to confirm with other studies whether decreased vitamin D levels may be a risk factor for developing COVID-19 infection upon coronavirus exposure. These findings can be relevant for actual clinical practice. Therefore, more research is needed in this important field.
Author Contributions
Conceptualization, N.F.-S., M.D.-G., V.M.-C., S.G.-S., B.J.N.-S., A.M.F.-A., and T.P.-C.; methodology, N.F.-S., M.D.-G., V.M.-C., S.G.-S., B.J.N.-S., A.M.F.-A., and T.P.-C.; formal analysis, N.F.-S., M.D.-G., V.M.-C., S.G.-S., B.J.N.-S., A.M.F.-A., and T.P.-C.; investigation, N.F.-S., M.D.-G., V.M.-C., S.G.-S., B.J.N.-S., A.M.F.-A., and T.P.-C.; data curation, N.F.-S., M.D.-G., V.M.-C., S.G.-S., B.J.N.-S., A.M.F.-A., and T.P.-C.; writing—original draft preparation, N.F.-S., M.D.-G., V.M.-C., S.G.-S., B.J.N.-S., A.M.F.-A., and T.P.-C.; writing—review and editing, N.F.-S., B.J.N.-S., A.M.F.-A., and T.P.-C.; supervision, N.F.-S., B.J.N.-S., A.M.F.-A., and T.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Torrecardenas University Hospital (protocol code 2607-143/2020 and date of approval: 27 January 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization (WHO). Coronavirus (COVID-19). 2021. Available online: https://who.sprinklr.com/ (accessed on 10 February 2022).
- Madjunkov, M.; Dviri, M.; Librach, C. A comprehensive review of the impact of COVID-19 on human reproductive biology, assisted reproduction care and pregnancy: A Canadian perspective. J. Ovarian Res. 2020, 13, 140. [Google Scholar] [CrossRef]
- Wastnedge, E.A.N.; Reynolds, R.M.; Van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2020, 101, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of Coronavirus spectrum infections (SARS, MERS, COVID 1-19) during pregnancy: A systematic review and meta-analysis. AJOG MFM 2020, 2, 100107. [Google Scholar] [CrossRef] [PubMed]
- Narang, K.; Enninga, E.A.L.; Gunaratne, M.D.; Ibirogba, E.R.; Trad, A.T.A.; Elrefaei, A.; Theiler, R.N.; Ruano, R.; Szymanski, L.M.; Chakraborty, R.; et al. SARS-CoV-2 infection and COVID-19 during pregnancy: A multidisciplinary review. Mayo Clin. Proc. 2020, 95, 1750–1765. [Google Scholar] [CrossRef]
- Norman, A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am J. Clin. Nutr. 2008, 88, 491S–499S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, A.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and novel actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Extraskeletal actions of vitamin D. Ann. N. Y. Acad. Sci. 2016, 1376, 29–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of vitamin D beyond the skeletal function: A review of the molecular and clinical studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef] [Green Version]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin D: Not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat. Weight. Disord. 2017, 22, 27–41. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.A.; Kirkpatrick, D.R.; Kovilam, O.; Agrawal, D.K. Immunomodulatory role of vitamin D in the pathogenesis of preeclampsia. Expert Rev. Clin. Immunol. 2015, 11, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.V.; Cruze, L.; Wei, W.; Gehris, J.; Wagner, C.L. Maternal vitamin D sufficiency and reduced placental gene expression in angiogenic biomarkers related to comorbidities of pregnancy. J. Steroid Biochem. Mol. Biol. 2017, 173, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.L.; Lu, F.G.; Yang, S.H.; Xu, H.L.; Luo, B.A. Does maternal vitamin D deficiency increase the risk of preterm birth: A meta-analysis of observational studies. Nutrients 2016, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Song, Y.; Zhang, Z. Serum vitamin D deficiency and risk of gestational diabetes mellitus: A meta-analysis. Arch. Med. Sci. 2020, 16, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, C.; Bi, W.G.; Leduc, L.; Tabatabaei, N.; Jantchou, P.; Luo, Z.-C.; Audibert, F.; Nuyt, A.M.; Wei, S.Q. Prenatal vitamin D status and offspring’s growth, adiposity and metabolic health: A systematic review and meta-analysis. Br. J. Nutr. 2018, 119, 310–319. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Weishaar, T.; Rajan, S.; Keller, B. Probability of vitamin D deficiency by body weight and race/ethnicity. J. Am. Board Fam. Med. 2016, 29, 226–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Living Guidance for Clinical Management of COVID-19. 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (accessed on 14 January 2022).
- Leino, A.; Turpeinen, U.; Koskinen, P. Automated measurement of 25-OH vitamin D3 on the Roche Modular E170 Analyzer. Clin. Chem. 2008, 54, 2059–2062. [Google Scholar] [CrossRef] [Green Version]
- Sinaci, S.; Ocal, D.F.; Yetiskin, D.F.Y.; Hendem, D.U.; Buyuk, G.N.; Ayhan, S.G.; Tanacan, A.; Ozgu-Erdinc, A.S.; Tekin, O.M.; Sahin, D. Impact of vitamin D on the course of COVID-19 during pregnancy: A case control study. J. Steroid Biochem. Mol. Biol. 2021, 213, 105964. [Google Scholar] [CrossRef]
- Tekin, A.B.; Yassa, M.; Birol, P.; Unlu, S.N.; Sahin, T.; Buran, A.M.; Ayanoglu, E.; Tug, N. Vitamin D status is not associated with clinical severity of COVID-19 in pregnant women. Eur. J. Nutr. 2021, 61, 1035–1041. [Google Scholar] [CrossRef]
- Bahat, P.Y.; Talma, M.A.; Bestel, A.; Selcuki, N.F.T.; Aydın, Z.; Polat, İ. Micronutrients in COVID-19 Positive Pregnancies. Cureus 2020, 12, e10609. [Google Scholar] [CrossRef]
- Merzon, E.; Tworowski, D.; Gorohovski, A.; Vinker, S.; Cohen, A.G.; Green, I.; Frenkel-Morgenstern, M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. FEBS J. 2020, 287, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Yue, S.; Xue, W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition 2021, 84, 111106. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Dantas Damascena, A.; Galvão Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and metaanalysis. Crit. Rev. Food Sci. Nutr. 2020, 62, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Liao, Q.; Shen, Y.; Li, H.; Cheng, L. Vitamin D deficiency is inversely associated with COVID-19 incidence and disease severity in Chinese people. J. Nutr. 2021, 151, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Angelidi, A.M.; Belanger, M.J.; Lorinsky, M.K.; Karamanis, D.; Chamorro-Pareja, N.; Ognibene, J.; Palaiodimos, L.; Mantzoros, C.S. Vitamin D status is associated with in-hospital mortality and mechanical ventilation: A cohort of COVID-19 hospitalized patients. Mayo Clin. Proc. 2021, 96, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Cates, C.J.; Urashima, M.; Jensen, M.; Griffiths, A.P.; Nurmatov, U.; Sheikh, A.; Griffiths, C.J. Vitamin D for the management to asthma. Cochrane Database Syst. Rev. 2016, 9, CD011511. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, P.E.; Lu, H.; Mann, E.H.; Chen, Y.-H.; Ho, T.-R.; Cousins, D.J.; Corrigan, C.; Kelly, F.J.; Mudway, I.S.; Hawrylowicz, C.M. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS ONE 2018, 13, e0200040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musavi, H.; Abazari, O.; Barartabar, Z.; Kalaki-Jouybari, F.; Hemmati-Dinarvand, M.; Esmaeili, P.; Mahjoub, S. The benefits of Vitamin D in the COVID-19 pandemic: Biochemical and immunological mechanisms. Arch. Physiol. Biochem. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.L.; Tajer, C.; Mariani, J.; Inserra, F.; Ferder, L.; Manucha, W. Vitamin D high doses supplementation could represent a promising alternative to prevent or treat COVID-19 infection. Clín. Investig. Arter. 2020, 32, 267–277. [Google Scholar] [CrossRef]
- Ferrari, D.; Locatelli, M.; Briguglio, M.; Lombardi, G. Is there a link between vitamin D status, SARS-CoV-2 infection risk and COVID-19 severity? Cell Biochem. Funct. 2021, 39, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, A.G.; Jahaj, E.; Pratikaki, M.; Keskinidou, C.; Detsika, M.; Grigoriou, E.; Psarra, K.; Orfanos, S.E.; Tsirogianni, A.; Dimopoulou, I.; et al. Vitamin D deficiency correlates with a reduced number of natural killer cells in intensive care unit (ICU) and non-ICU patients with COVID-19 pneumonia. Hell. J. Cardiol. C 2021, 62, 381–383. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).