Is Altered Oculomotor Control during Smooth Pursuit Neck Torsion Test Related to Subjective Visual Complaints in Patients with Neck Pain Disorders?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
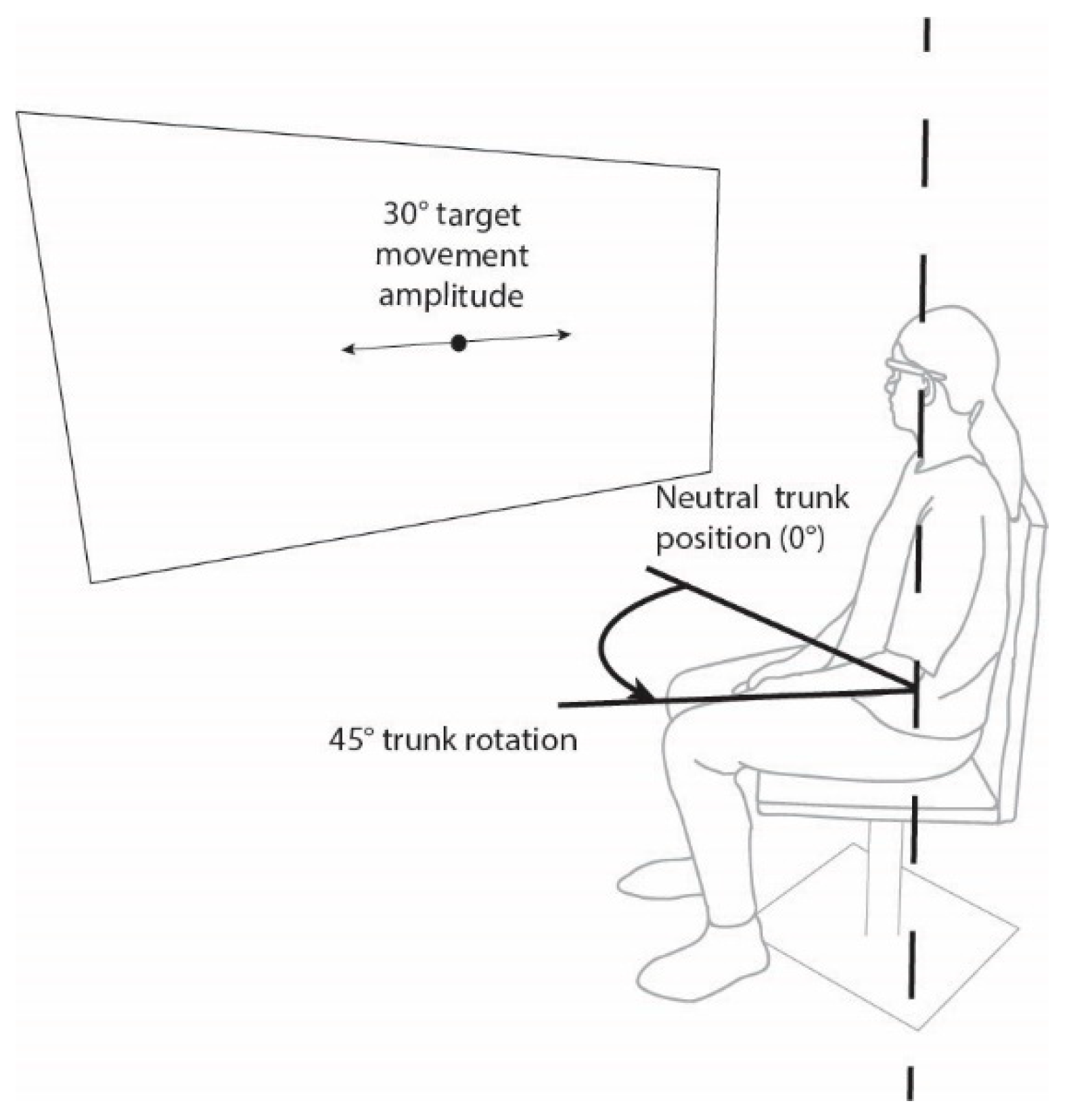
2.2. Assessment
2.3. Equipment
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Classification Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalland Knapstad, M.; Goplen, F.; Skouen, J.S.; Ask, T.; Nordahl, S.H.G. Symptom Severity and Quality of Life in Patients with Concurrent Neck Pain and Dizziness. Disabil. Rehabil. 2020, 42, 2743–2746. [Google Scholar] [CrossRef] [PubMed]
- Kristjansson, E.; Treleaven, J. Sensorimotor Function and Dizziness in Neck Pain: Implications for Assessment and Management. J. Orthop. Sports Phys. Ther. 2009, 39, 364–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogduk, N.; Govind, J. Cervicogenic Headache: An Assessment of the Evidence on Clinical Diagnosis, Invasive Tests, and Treatment. Lancet Neurol. 2009, 8, 959–968. [Google Scholar] [CrossRef]
- Thoomes, E.J.; van Geest, S.; van der Windt, D.A.; Falla, D.; Verhagen, A.P.; Koes, B.W.; Thoomes-de Graaf, M.; Kuijper, B.; Scholten-Peeters, W.G.M.; Vleggeert-Lankamp, C.L. Value of Physical Tests in Diagnosing Cervical Radiculopathy: A Systematic Review. Spine J. 2018, 18, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treleaven, J.; Jull, G.; LowChoy, N. Smooth Pursuit Neck Torsion Test in Whiplash-Associated Disorders: Relationship to Self-Reports of Neck Pain and Disability, Dizziness and Anxiety. J. Rehabil. Med. 2005, 37, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Daly, L.; Giffard, P.; Thomas, L.; Treleaven, J. Validity of Clinical Measures of Smooth Pursuit Eye Movement Control in Patients with Idiopathic Neck Pain. Musculoskelet. Sci. Pract. 2018, 33, 18–23. [Google Scholar] [CrossRef]
- Treleaven, J. Dizziness, Unsteadiness, Visual Disturbances, and Sensorimotor Control in Traumatic Neck Pain. J. Orthop. Sports Phys. Ther. 2017, 47, 492–502. [Google Scholar] [CrossRef]
- Treleaven, J.; Takasaki, H. Characteristics of Visual Disturbances Reported by Subjects with Neck Pain. Man. Ther. 2014, 19, 203–207. [Google Scholar] [CrossRef]
- Hülse, M.; Hölzl, M. Vestibulospinal reactions in cervicogenic disequilibrium. Cervicogenic imbalance. HNO 2000, 48, 295–301. [Google Scholar] [CrossRef]
- Jaiswal, S.; Asper, L.; Long, J.; Lee, A.; Harrison, K.; Golebiowski, B. Ocular and Visual Discomfort Associated with Smartphones, Tablets and Computers: What We Do and Do Not Know. Clin. Exp. Optom. 2019, 102, 463–477. [Google Scholar] [CrossRef] [Green Version]
- Bronstein, A. Visual Symptoms and Vertigo. Neurol. Clin. 2005, 23, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.C.; Rivett, D.A.; Attia, J.R.; Parsons, M.; Levi, C. Risk Factors and Clinical Features of Craniocervical Arterial Dissection. Man. Ther. 2011, 16, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.; Giffard, P.; Johnston, V.; Treleaven, J. Computer Vision Symptoms in People with and without Neck Pain. Appl. Ergon. 2019, 80, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Tjell, C.; Rosenhall, U. Smooth Pursuit Neck Torsion Test: A Specific Test for Cervical Dizziness. Am. J. Otol. 1998, 19, 76–81. [Google Scholar] [PubMed]
- Majcen Rosker, Z.; Vodicar, M.; Kristjansson, E. Oculomotor Performance in Patients with Neck Pain: Does It Matter Which Angle of Neck Torsion Is Used in Smooth Pursuit Eye Movement Test and Is the Agreement between Angles Dependent on Target Movement Amplitude and Velocity? Musculoskelet. Sci. Pract. 2022, 59, 102535. [Google Scholar] [CrossRef]
- Majcen Rosker, Z.; Vodicar, M.; Kristjansson, E. Inter-Visit Reliability of Smooth Pursuit Neck Torsion Test in Patients with Chronic Neck Pain and Healthy Individuals. Diagnostics 2021, 11, 752. [Google Scholar] [CrossRef]
- Majcen Rosker, Z.; Rosker, J.; Vodicar, M.; Kristjansson, E. The Influence of Neck Torsion and Sequence of Cycles on Intra-Trial Reliability of Smooth Pursuit Eye Movement Test in Patients with Neck Pain Disorders. Exp. Brain Res. 2022, 240, 763–771. [Google Scholar] [CrossRef]
- Tjell, C.; Artur, T. Sören Sandström Smooth Pursuit Neck Torsion Test-A Specific Test for Whiplash Associated Disorders? J. Whiplash Relat. Disord. 2002, 1, 9–24. [Google Scholar] [CrossRef]
- Leube, A.; Rifai, K.; Rifai, K. Sampling Rate Influences Saccade Detection in Mobile Eye Tracking of a Reading Task. J. Eye Mov. Res. 2017, 10. [Google Scholar] [CrossRef]
- Niehorster, D.C.; Santini, T.; Hessels, R.S.; Hooge, I.T.C.; Kasneci, E.; Nyström, M. The Impact of Slippage on the Data Quality of Head-Worn Eye Trackers. Behav. Res. Methods 2020, 52, 1140–1160. [Google Scholar] [CrossRef] [Green Version]
- Stuart, S.; Parrington, L.; Martini, D.; Popa, B.; Fino, P.C.; King, L.A. Validation of a Velocity-Based Algorithm to Quantify Saccades during Walking and Turning in Mild Traumatic Brain Injury and Healthy Controls. Physiol. Meas. 2019, 40, 044006. [Google Scholar] [CrossRef]
- Deravet, N.; Blohm, G.; de Xivry, J.-J.O.; Lefèvre, P. Weighted Integration of Short-Term Memory and Sensory Signals in the Oculomotor System. J. Vis. 2018, 18, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudner, L. Accuracy of Bayes and Logistic Regression Subscale Probabilities for Educational and Certification Tests. Pract. Assess. Res. Eval. 2019, 21, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, Y.; Li, Q.; Ma, J.; Li, S.; Lv, X.; Lv, W. Empirical Study of Seven Data Mining Algorithms on Different Characteristics of Datasets for Biomedical Classification Applications. Biomed. Eng. Online 2017, 16, 125. [Google Scholar] [CrossRef] [Green Version]
- Majcen Rosker, Z.; Vodicar, M.; Kristjansson, E. Video-Oculographic Measures of Eye Movement Control in the Smooth Pursuit Neck Torsion Test Can Classify Idiopathic Neck Pain Patients from Healthy Individuals: A Datamining Based Diagnostic Accuracy Study. Musculoskelet. Sci. Pract. 2022; manuscript submitted for publication. [Google Scholar]
- Reddy, R.S.; Tedla, J.S.; Dixit, S.; Abohashrh, M. Cervical Proprioception and Its Relationship with Neck Pain Intensity in Subjects with Cervical Spondylosis. BMC Musculoskelet. Disord. 2019, 20, 447. [Google Scholar] [CrossRef] [Green Version]
- Devecchi, V.; Rushton, A.B.; Gallina, A.; Heneghan, N.R.; Falla, D. Are Neuromuscular Adaptations Present in People with Recurrent Spinal Pain during a Period of Remission? A Systematic Review. PLoS ONE 2021, 16, e0249220. [Google Scholar] [CrossRef] [PubMed]
- Majcen Rosker, Z.; Kristjansson, E.; Vodicar, M.; Rosker, J. Postural Balance and Oculomotor Control Are Influenced by Neck Kinaesthetic Functions in Elite Ice Hockey Players. Gait Posture 2021, 85, 145–150. [Google Scholar] [CrossRef]
- Kongsted, A.; Jørgensen, L.V.; Bendix, T.; Korsholm, L.; Leboeuf-Yde, C. Are Smooth Pursuit Eye Movements Altered in Chronic Whiplash-Associated Disorders? A Cross-Sectional Study. Clin. Rehabil. 2007, 21, 1038–1049. [Google Scholar] [CrossRef]
- Velay, J.L.; Roll, R.; Lennerstrand, G.; Roll, J.P. Eye Proprioception and Visual Localization in Humans: Influence of Ocular Dominance and Visual Context. Vis. Res. 1994, 34, 2169–2176. [Google Scholar] [CrossRef]
- Madelain, L.; Krauzlis, R.J. Effects of Learning on Smooth Pursuit during Transient Disappearance of a Visual Target. J. Neurophysiol. 2003, 90, 972–982. [Google Scholar] [CrossRef]
- Garg, K.; Aggarwal, A. Effect of Cervical Decompression on Atypical Symptoms Cervical Spondylosis-A Narrative Review and Meta-Analysis. World Neurosurg. 2022, 157, 207–217.e1. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Muheremu, A.; Tian, W. Atypical Symptoms in Patients with Cervical Spondylosis: Comparison of the Treatment Effect of Different Surgical Approaches. Medicine 2018, 97, e10731. [Google Scholar] [CrossRef] [PubMed]
- Gimse, R.; Tjell, C.; Bjørgen, I.A.; Saunte, C. Disturbed Eye Movements after Whiplash Due to Injuries to the Posture Control System. J. Clin. Exp. Neuropsychol. 1996, 18, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, M.C.; Gutiérrez-Sánchez, E.; Sánchez-González, J.-M.; Rebollo-Salas, M.; Ruiz-Molinero, C.; Jiménez-Rejano, J.J.; Pérez-Cabezas, V. Visual System Disorders and Musculoskeletal Neck Complaints: A Systematic Review and Meta-Analysis. Ann. N. Y. Acad. Sci. 2019, 1457, 26–40. [Google Scholar] [CrossRef]
- Richter, H.O.; Bänziger, T.; Abdi, S.; Forsman, M. Stabilization of Gaze: A Relationship between Ciliary Muscle Contraction and Trapezius Muscle Activity. Vis. Res. 2010, 50, 2559–2569. [Google Scholar] [CrossRef] [Green Version]
- Tilikete, C.; Vighetto, A. Oscillopsia: Causes and Management. Curr. Opin. Neurol. 2011, 24, 38–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, J.L.; Daye, P.M.; Thomson, G.T.D. Inaccurate Saccades and Enhanced Vestibulo-Ocular Reflex Suppression during Combined Eye-Head Movements in Patients with Chronic Neck Pain: Possible Implications for Cervical Vertigo. Front. Neurol. 2017, 8, 23. [Google Scholar] [CrossRef]
- Giffard, P.; Daly, L.; Treleaven, J. Influence of Neck Torsion on near Point Convergence in Subjects with Idiopathic Neck Pain. Musculoskelet. Sci. Pract. 2017, 32, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Kerry, R.; Taylor, A.J.; Mitchell, J.; McCarthy, C. Cervical Arterial Dysfunction and Manual Therapy: A Critical Literature Review to Inform Professional Practice. Man. Ther. 2008, 13, 278–288. [Google Scholar] [CrossRef]
- Kerry, R.; Taylor, A.J. Cervical Arterial Dysfunction: Knowledge and Reasoning for Manual Physical Therapists. J. Orthop. Sports Phys. Ther. 2009, 39, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Treleaven, J.; Clamaron-Cheers, C.; Jull, G. Does the Region of Pain Influence the Presence of Sensorimotor Disturbances in Neck Pain Disorders? Man. Ther. 2011, 16, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Ris, I.; Juul-Kristensen, B.; Boyle, E.; Kongsted, A.; Manniche, C.; Søgaard, K. Chronic Neck Pain Patients with Traumatic or Non-Traumatic Onset: Differences in Characteristics. A Cross-Sectional Study. Scand. J. Pain 2017, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, H.; Treleaven, J.; Johnston, V.; Jull, G. Contributions of Physical and Cognitive Impairments to Self-Reported Driving Difficulty in Chronic Whiplash-Associated Disorders. Spine 2013, 38, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
| Intensity 1 | Frequency 2 | ||||
|---|---|---|---|---|---|
| Visual Symptom | Median 3 | IQR 4 | Median 3 | IQR 4 | % of Patients 5 |
| Concentrating to read | 2 | 2 | 2 | 2 | 63.6% |
| Words moving on page | 0 | 2 | 0 | 3 | 45.5% |
| Blurred vision | 1 | 1.5 | 3 | 2 | 81.8% |
| Difficulty judging distance | 2 | 2 | 2 | 2.5 | 63.6% |
| Sore eyes | 1 | 1.5 | 3 | 3.5 | 72.7% |
| Heavy eyes | 2 | 2.5 | 2 | 3 | 72.7% |
| Harder to do close work | 1 | 2 | 2 | 2.5 | 54.5% |
| Visual fatigue | 2 | 1 | 3 | 1.5 | 81.8% |
| Itchy eyes | 1 | 1 | 1 | 2.5 | 63.6% |
| Red eyes | 1 | 1.5 | 2 | 3 | 63.6% |
| Eye strain | 1 | 1.5 | 2 | 4 | 63.6% |
| Squinting | 1 | 2 | 2 | 3 | 54.5% |
| Gain Torsion 1 | Gain Neutral 2 | SPNTdiff 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Visual Symptom | AUC 4 | Se 5 | Sp 6 | AUC 4 | Se 5 | Sp 6 | AUC 4 | Se 5 | Sp 6 | |
| Intensity | Dizzy reading | 0.340 | 0.282 | 0.438 | 0.296 | 0.388 | 0.339 | 0.440 | 0.482 | 0.638 |
| Concentrating to read | 0.734 | 0.763 | 0.814 | 0.513 | 0.614 | 0.716 | 0.634 | 0.563 | 0.814 | |
| Words moving on page | 0.652 | 0.746 | 0.753 | 0.489 | 0.475 | 0.361 | 0.638 | 0.746 | 0.853 | |
| Blurred vision | 0.545 | 0.464 | 0.758 | 0.427 | 0.352 | 0.583 | 0.515 | 0.444 | 0.708 | |
| Difficulty judging distance | 0.578 | 0.611 | 0.893 | 0.353 | 0.407 | 0.693 | 0.498 | 0.611 | 0.893 | |
| Sore eyes | 0.704 | 0.667 | 0.909 | 0.525 | 0.375 | 0.667 | 0.644 | 0.667 | 0.809 | |
| Heavy eyes | 0.600 | 0.589 | 0.873 | 0.534 | 0.450 | 0.714 | 0.586 | 0.539 | 0.873 | |
| Harder to do close work | 0.493 | 0.328 | 0.838 | 0.389 | 0.448 | 0.762 | 0.481 | 0.310 | 0.638 | |
| Visual fatigue | 0.477 | 0.417 | 0.600 | 0.279 | 0.483 | 0.500 | 0.462 | 0.411 | 0.602 | |
| Itchy eyes | 0.297 | 0.225 | 0.683 | 0.348 | 0.319 | 0.702 | 0.255 | 0.264 | 0.483 | |
| Red eyes | 0.539 | 0.473 | 0.836 | 0.366 | 0.329 | 0.604 | 0.515 | 0.273 | 0.833 | |
| Eye strain | 0.629 | 0.518 | 0.867 | 0.431 | 0.455 | 0.758 | 0.525 | 0.618 | 0.859 | |
| Squinting | 0.366 | 0.436 | 0.606 | 0.415 | 0.401 | 0.723 | 0.301 | 0.236 | 0.703 | |
| Frequency | Dizzy reading | 0.412 | 0.406 | 0.716 | 0.176 | 0.273 | 0.408 | 0.347 | 0.209 | 0.581 |
| Concentrating to read | 0.626 | 0.718 | 0.640 | 0.486 | 0.519 | 0.572 | 0.614 | 0.518 | 0.713 | |
| Words moving on page | 0.536 | 0.520 | 0.687 | 0.361 | 0.242 | 0.400 | 0.622 | 0.608 | 0.748 | |
| Blurred vision | 0.682 | 0.606 | 0.833 | 0.466 | 0.145 | 0.681 | 0.693 | 0.515 | 0.815 | |
| Difficulty judging distance | 0.484 | 0.509 | 0.481 | 0.347 | 0.382 | 0.412 | 0.463 | 0.494 | 0.569 | |
| Sore eyes | 0.337 | 0.281 | 0.734 | 0.331 | 0.091 | 0.611 | 0.512 | 0.545 | 0.769 | |
| Heavy eyes | 0.433 | 0.373 | 0.538 | 0.308 | 0.182 | 0.749 | 0.376 | 0.242 | 0.675 | |
| Harder to do close work | 0.679 | 0.582 | 0.698 | 0.428 | 0.397 | 0.494 | 0.454 | 0.545 | 0.768 | |
| Visual fatigue | 0.572 | 0.573 | 0.644 | 0.473 | 0.432 | 0.532 | 0.472 | 0.114 | 0.651 | |
| Itchy eyes | 0.353 | 0.114 | 0.538 | 0.372 | 0.300 | 0.580 | 0.321 | 0.273 | 0.623 | |
| Red eyes | 0.357 | 0.327 | 0.645 | 0.480 | 0.361 | 0.544 | 0.357 | 0.364 | 0.722 | |
| Eye strain | 0.462 | 0.321 | 0.561 | 0.458 | 0.364 | 0.577 | 0.496 | 0.364 | 0.720 | |
| Squinting | 0.383 | 0.421 | 0.525 | 0.410 | 0.339 | 0.465 | 0.500 | 0.455 | 0.628 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majcen Rosker, Z.; Vodicar, M.; Kristjansson, E. Is Altered Oculomotor Control during Smooth Pursuit Neck Torsion Test Related to Subjective Visual Complaints in Patients with Neck Pain Disorders? Int. J. Environ. Res. Public Health 2022, 19, 3788. https://doi.org/10.3390/ijerph19073788
Majcen Rosker Z, Vodicar M, Kristjansson E. Is Altered Oculomotor Control during Smooth Pursuit Neck Torsion Test Related to Subjective Visual Complaints in Patients with Neck Pain Disorders? International Journal of Environmental Research and Public Health. 2022; 19(7):3788. https://doi.org/10.3390/ijerph19073788
Chicago/Turabian StyleMajcen Rosker, Ziva, Miha Vodicar, and Eythor Kristjansson. 2022. "Is Altered Oculomotor Control during Smooth Pursuit Neck Torsion Test Related to Subjective Visual Complaints in Patients with Neck Pain Disorders?" International Journal of Environmental Research and Public Health 19, no. 7: 3788. https://doi.org/10.3390/ijerph19073788
APA StyleMajcen Rosker, Z., Vodicar, M., & Kristjansson, E. (2022). Is Altered Oculomotor Control during Smooth Pursuit Neck Torsion Test Related to Subjective Visual Complaints in Patients with Neck Pain Disorders? International Journal of Environmental Research and Public Health, 19(7), 3788. https://doi.org/10.3390/ijerph19073788






