Heart Rate Variability in Hyperthyroidism: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
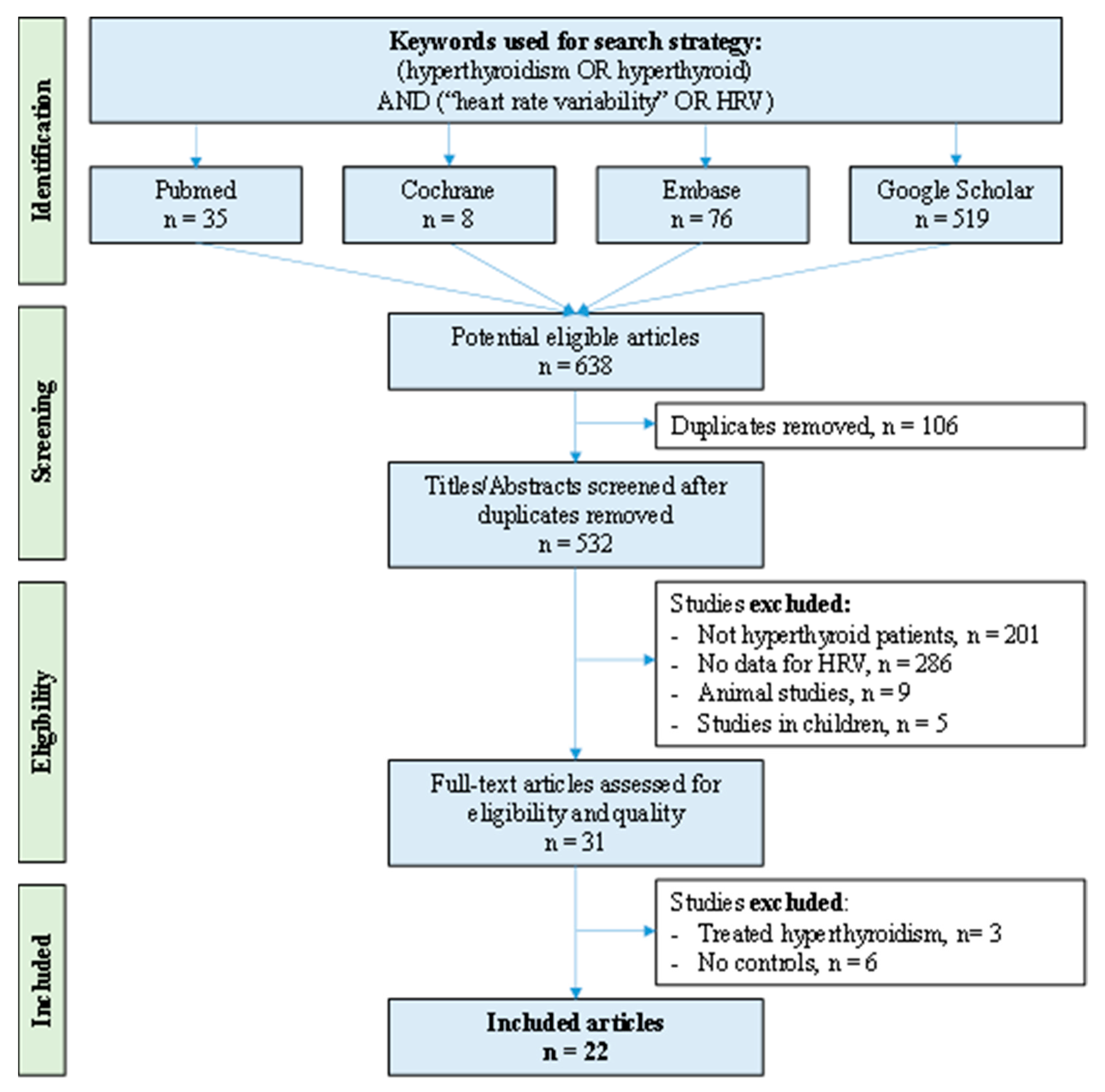
2. Methods
2.1. Literature Search
2.2. Data Extraction
2.3. Quality of Assessment
2.4. Statistical Considerations
3. Results
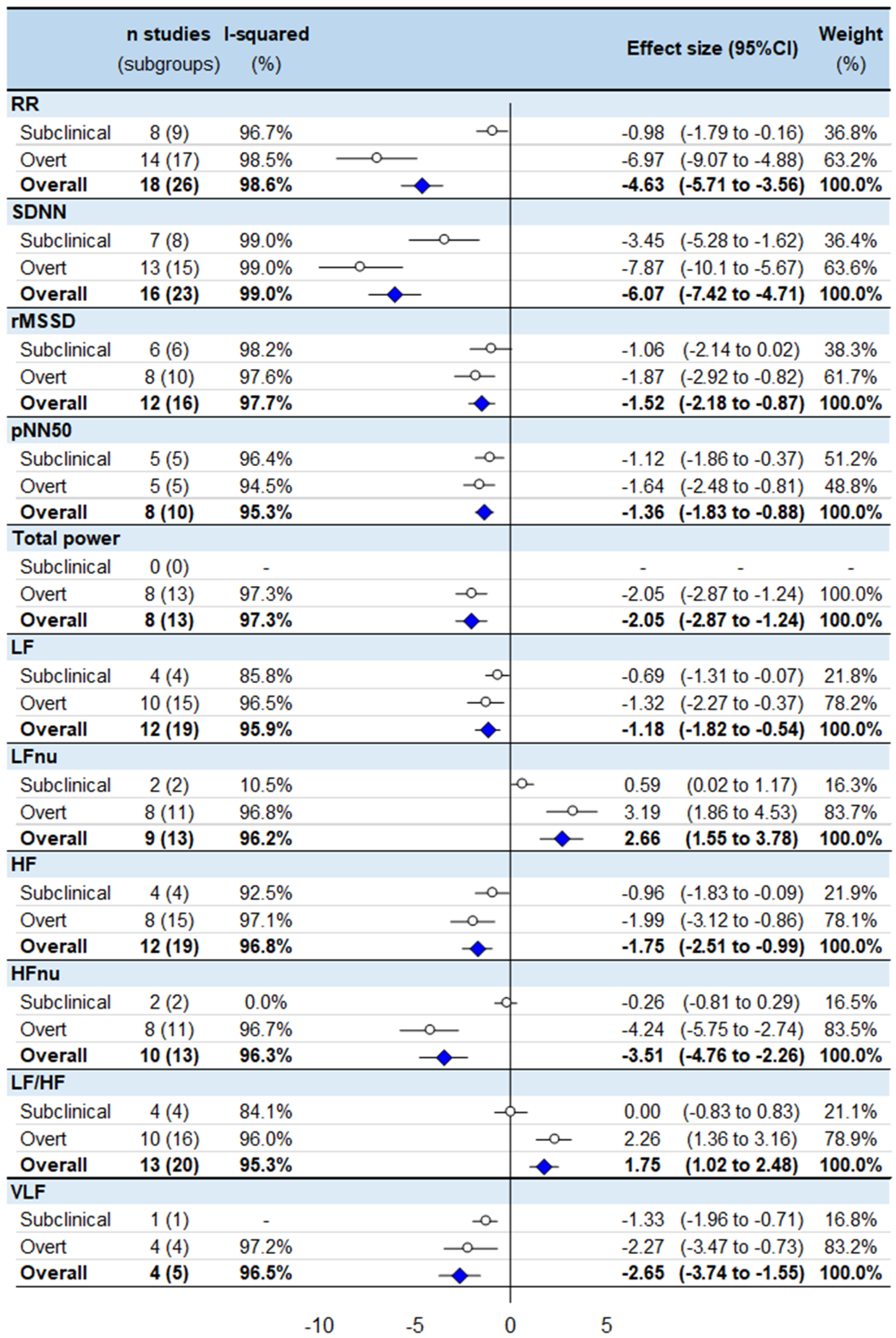
3.1. Meta-Analyses of HRV Values in Untreated Hyperthyroidism
3.2. Meta-Analysis Stratified by Subclinical or Overt Status
3.3. Meta-Regressions and Sensitivity Analyses
4. Discussion
4.1. Deleterious Effects of Thyroid Hyperfunction on HRV
4.2. Clinical Implications
4.3. Other Variables Related to HRV in Hyperthyroidism
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhat, A.N.; Kalsotra, L.; Yograj, S. Autonomic reactivity with altered thyroid status. JK Sci. 2007, 9, 70–74. [Google Scholar]
- Reeves, J.W.; Fisher, A.J.; Newman, M.G.; Granger, D.A. Sympathetic and hypothalamic-pituitary-adrenal asymmetry in generalized anxiety disorder: Sympathetic and HPA asymmetry in GAD. Psychophysiology 2016, 53, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Parle, J.V.; Maisonneuve, P.; Sheppard, M.C.; Boyle, P.; Franklyn, J.A. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: A 10-year cohort study. Lancet 2001, 358, 861–865. [Google Scholar] [CrossRef]
- Klein, I.; Ojamaa, K. Thyroid hormone and the cardiovascular system: From theory to practice. J. Clin. Endocrinol. Metab. 1994, 78, 1026–1027. [Google Scholar] [PubMed]
- Kahaly, G.J.; Dillmann, W.H. Thyroid hormone action in the heart. Endocr. Rev. 2005, 26, 704–728. [Google Scholar] [CrossRef] [Green Version]
- Florea, V.G.; Cohn, J.N. The autonomic nervous system and heart failure. Circ. Res. 2014, 114, 1815–1826. [Google Scholar] [CrossRef] [Green Version]
- Liggett, S.B.; Shah, S.D.; Cryer, P.E. Increased fat and skeletal muscle j3-adrenergic receptors but unaltered metabolic and hemodynamic sensitivity to epinephrine in vivo in experimental human thyrotoxicosis. J. Clin. Investig. 1989, 83, 803–809. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.S. Dysautonomias: Clinical disorders of the autonomic nervous system. Ann. Intern. Med. 2002, 137, 753. [Google Scholar] [CrossRef]
- Zaidi, M.; Robert, A.; Fesler, R.; Derwael, C.; Brohet, C. Dispersion of ventricular repolarisation: A marker of ventricular arrhythmias in patients with previous myocardial infarction. Heart 1997, 78, 371–375. [Google Scholar] [CrossRef]
- Algra, A.; Tijssen, J.G.; Roelandt, J.R.; Pool, J.; Lubsen, J. Heart rate variability from 24-hour electrocardiography and the 2-year risk for sudden death. Circulation 1993, 88, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Cygankiewicz, I.; Zareba, W. Heart rate variability. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 379–393. [Google Scholar]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Min, K.B.; Min, J.-Y.; Paek, D.; Cho, S.-I.; Son, M. Is 5-minute heart rate variability a useful measure for monitoring the autonomic nervous system of workers? Int. Heart J. 2008, 49, 175–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutheil, F.; Chambres, P.; Hufnagel, C.; Auxiette, C.; Chausse, P.; Ghozi, R.; Paugam, G.; Boudet, G.; Khalfa, N.; Naughton, G.; et al. ‘Do Well B.’: Design of Well Being monitoring systems. A study protocol for the application in autism. BMJ Open 2015, 5, e007716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hufnagel, C.; Chambres, P.; Bertrand, P.R.; Dutheil, F. The need for objective measures of stress in autism. Front. Psychol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Boudet, G.; Walther, G.; Courteix, D.; Obert, P.; Lesourd, B.; Pereira, B.; Chapier, R.; Vinet, A.; Chamoux, A.; Naughton, G.; et al. Paradoxical dissociation between heart rate and heart rate variability following different modalities of exercise in individuals with metabolic syndrome: The RESOLVE study. Eur. J. Prev. Cardiolog. 2017, 24, 281–296. [Google Scholar] [CrossRef]
- McMillan, D.E. Interpreting heart rate variability sleep/wake patterns in cardiac patients. J. Cardiovasc. Nurs. 2002, 17, 69–81. [Google Scholar] [CrossRef]
- Foley, C.M.; McAllister, R.M.; Hasser, E.M. Thyroid status influences baroreflex function and autonomic contributions to arterial pressure and heart rate. Am. J. Physiol. Circ. Physiol. 2001, 280, H2061–H2068. [Google Scholar] [CrossRef]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The incidence and prevalence of thyroid dysfunction in Europe: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef] [Green Version]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Saleem, S.; Hussain, M.M.; Majeed, S.M.I.; Khan, M.A. Gender differences of heart rate variability in healthy volunteers. J. Pak. Med. Assoc. 2012, 62, 422–425. [Google Scholar]
- Rothberg, L.J.; Lees, T.; Clifton-Bligh, R.; Lal, S. Association between heart rate variability measures and blood glucose levels: Implications for noninvasive glucose monitoring for diabetes. Diabetes Technol. Ther. 2016, 18, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuusela, T. Methodological aspects of heart rate variability analysis. In Heart Rate Variability (HRV) Signal Analysis Clinical Applications; Kamath, M.V., Watanabe, M., Upton, A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 9–42. [Google Scholar]
- Umetani, K.; Singer, D.H.; McCraty, R.; Atkinson, M. Twenty-four hour time domain heart rate variability and heart rate: Relations to age and gender over nine decades. J. Am. Coll. Cardiol. 1998, 31, 593–601. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Harbour, R.; Miller, J. A new system for grading recommendations in evidence based guidelines. BMJ 2001, 323, 334–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gotzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [Green Version]
- Benoist d’Azy, C.; Pereira, B.; Chiambaretta, F.; Dutheil, F. Oxidative and anti-oxidative stress markers in chronic glaucoma: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0166915. [Google Scholar]
- Benoist d’Azy, C.; Pereira, B.; Naughton, G.; Chiambaretta, F.; Dutheil, F. Antibioprophylaxis in prevention of endophthalmitis in intravitreal injection: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0156431. [Google Scholar] [CrossRef]
- Lanhers, C.; Pereira, B.; Naughton, G.; Trousselard, M.; Lesage, F.-X.; Dutheil, F. Creatine supplementation and lower limb strength performance: A systematic review and meta-analyses. Sports Med. 2015, 45, 1285–1294. [Google Scholar] [CrossRef]
- Lanhers, C.; Pereira, B.; Naughton, G.; Trousselard, M.; Lesage, F.-X.; Dutheil, F. Creatine supplementation and upper limb strength performance: A systematic review and meta-analysis. Sports Med. 2017, 47, 163–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollier, M.; Chamoux, A.; Naughton, G.; Pereira, B.; Dutheil, F. Chest CT scan screening for lung cancer in asbestos occupational exposure. Chest 2014, 145, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Citrome, L. An effect size interpretation is required STAT!: Visualising effect size and an interview with Kristoffer Magnusson. Int. J. Clin. Pract. 2014, 68, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Burggraaf, J.; Tulen, J.H.M.; Lalezari, S.; Schoemaker, R.C.; De Meyer, P.H.; Meinders, A.E.; Cohen, A.F.; Pijl, H. Sympathovagal imbalance in hyperthyroidism. Am. J. Physiol. Endocrinol. Metab. 2001, 281, 190–195. [Google Scholar] [CrossRef]
- Cacciatori, V.; Bellavere, F.; Pezzarossa, A.; Dellera, A.; Gemma, M.L.; Thomaseth, K.; Castelló, R.; Moghetti, P.; Muggeo, M. Power spectral analysis of heart rate in hyperthyroidism. J. Clin. Endocrinol. Metab. 1996, 81, 2828–2835. [Google Scholar]
- Cai, Z.; Dai, M.; Zhang, Y.; Zhong, H.; Tan, T.; Bao, M. Imbalance of cardiac autonomic nervous activity and increase of ventricular repolarization dynamicity induced by thyroid hormones in hyperthyroidism. Auton. Neurosci. 2018, 213, 86–91. [Google Scholar] [CrossRef]
- Chen, J.-L.; Chiu, H.-W.; Tseng, Y.-J.; Chu, W.-C. Hyperthyroidism is characterized by both increased sympathetic and decreased vagal modulation of heart rate: Evidence from spectral analysis of heart rate variability. Clin. Endocrinol. 2006, 64, 611–616. [Google Scholar] [CrossRef]
- Chen, J.-L.; Tseng, Y.-J.; Chiu, H.-W.; Hsiao, T.-C.; Chu, W.-C. Nonlinear analysis of heart rate dynamics in hyperthyroidism. Physiol. Meas. 2007, 28, 427–437. [Google Scholar] [CrossRef]
- Chen, J.-L.; Shiau, Y.-H.; Tseng, Y.-J.; Chiu, H.-W.; Hsiao, T.-C.; Wessel, N.; Kurths, J.; Chu, W.-C. Concurrent sympathetic activation and vagal withdrawal in hyperthyroidism: Evidence from detrended fluctuation analysis of heart rate variability. Phys. A Stat. Mech. Its Appl. 2010, 389, 1861–1868. [Google Scholar] [CrossRef]
- Eustatia-Rutten, C.F.A.; Corssmit, E.P.M.; Heemstra, K.A.; Smit, J.W.A.; Schoemaker, R.C.; Romijn, J.A.; Burggraaf, J. Autonomic Nervous System Function in Chronic Exogenous Subclinical Thyrotoxicosis and the Effect of Restoring Euthyroidism. J. Clin. Endocrinol. Metab. 2008, 93, 2835–2841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcone, C.; Matrone, B.; Bozzini, S.; Guasti, L.; Falcone, R.; Benzi, A.; Colonna, A.; Savulescu, I.; Vailati, A.; Pelissero, G. Time-Domain Heart Rate Variability in Coronary Artery Disease Patients Affected by Thyroid Dysfunction. Int. Heart J. 2014, 55, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galetta, F.; Franzoni, F.; Fallahi, P.; Tocchini, L.; Graci, F.; Gaddeo, C.; Rossi, M.; Cini, G.; Carpi, A.; Santoro, G.; et al. Changes in autonomic regulation and ventricular repolarization induced by subclinical hyperthyroidism. Biomed. Pharmacother. 2010, 64, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Goichot, B.; Brandenberger, G.; Vinzio, S.; Perrin, E.; Geny, B.; Schlienger, J.L.; Simon, C. Sympathovagal response to orthostatism in overt and in subclinical hyperthyroidism. J. Endocrinol. Investig. 2004, 27, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.R.; Begum, N.; Ferdousi, S.; Begum, S.; Ali, T. Heart rate variability in hyperthyroidism. J. Bangladesh Soc. Physiol. 2009, 4, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Karthik, S.; Pal, G.K.; Nanda, N.; Hamide, A.; Bobby, Z.; Amudharaj, D.; Pal, P. Sympathovagal imbalance in thyroid dysfunctions in females: Correlation with thyroid profile, heart rate and blood pressure. Ind. J. Physiol. Pharmacol. 2009, 53, 243–252. [Google Scholar]
- Mavai, M.; Singh, Y.R.; Gupta, R.C.; Mathur, S.K.; Bhandari, B. Linear analysis of autonomic activity and its correlation with creatine kinase-MB in overt thyroid dysfunctions. Ind. J. Clin. Biochem. 2018, 33, 222–228. [Google Scholar] [CrossRef]
- Ngassam, E.; Azabji-Kenfack, M.; Tankeu, A.T.; Mfeukeu-Kuate, L.; Nganou-Gnindjio, C.-N.; Mba, C.; Katte, J.C.; Dehayem, M.Y.; Mbanya, J.C.; Sobngwi, E. Heart rate variability in hyperthyroidism on sub Saharan African patients: A case-control study. BMC Res. Notes 2018, 11, 814. [Google Scholar] [CrossRef] [Green Version]
- Osman, F.; Franklyn, J.A.; Daykin, J.; Chowdhary, S.; Holder, R.L.; Sheppard, M.C.; Gammage, M.D. Heart rate variability and turbulence in hyperthyroidism before, during, and after treatment. Am. J. Cardiol. 2004, 94, 465–469. [Google Scholar] [CrossRef]
- Peixoto de Miranda, É.J.F.; Hoshi, R.A.; Bittencourt, M.S.; Goulart, A.C.; Santos, I.S.; Brunoni, A.R.; Diniz, M.F.H.S.; Ribeiro, A.L.P.; Dantas, E.M.; Mill, J.G.; et al. Relationship between heart rate variability and subclinical thyroid disorders of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Braz. J. Med. Biol. Res. 2018, 51. [Google Scholar] [CrossRef]
- Petretta, M.; Bonaduce, D.; Spinelli, L.; Vicario, M.L.; Nuzzo, V.; Marciano, F.; Camuso, P.; De Sanctis, V.; Lupoli, G. Cardiovascular haemodynamics and cardiac autonomic control in patients with subclinical and overt hyperthyroidism. Eur. J. Endocrinol. 2001, 145, 691–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitzalis, M.V.; Mastropasqua, F.; Massari, F.; Ciampolillo, A.; Passantino, A.; Ognissanti, M.; Mannarini, A.; Zanna, D.; Giorgino, R.; Rizzon, P. Assessment of cardiac vagal activity in patients with hyperthyroidism. Int. J. Cardiol. 1998, 64, 145–151. [Google Scholar] [CrossRef]
- Portella, R.B.; Pedrosa, R.C.; Coeli, C.M.; Buescu, A.; Vaisman, M. Altered cardiovascular vagal responses in nonelderly female patients with subclinical hyperthyroidism and no apparent cardiovascular disease. Clin. Endocrinol. 2007, 67, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; Subramanian, M.; Ramasamy, N.; Thangaraj, P.; Murugaiyan, J.; Selvaraj, V. Comparative study on spectral analysis of heart rate variability in hyperthyroid patients and euthyroids. Natl. J. Physiol. Pharm. Pharmacol. 2015, 5, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Tobaldini, E.; Porta, A.; Bulgheroni, M.; Pecis, M.; Muratori, M.; Bevilacqua, M.; Montano, N. Increased complexity of short-term heart rate variability in hyperthyroid patients during orthostatic challenge. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 1988–1991. [Google Scholar]
- Tudoran, C.; Tudoran, M.; Vlad, M.; Balas, M.; Ciocarlie, T.; Parv, F. Alterations of heart rate variability and turbulence in female patients with hyperthyroidism of various severities. Niger. J. Clin. Pract. 2019, 22, 1349–1355. [Google Scholar] [CrossRef]
- Johnson, J. Atrial fibrillation and hyperthyroidism. Indian Pacing Electrophysiol. J. 2005, 5, 305–311. [Google Scholar]
- Klein, I.; Danzi, S. Thyroid disease and the heart. Circulation 2007, 116, 1725–1735. [Google Scholar] [CrossRef] [Green Version]
- Polikar, R.; Burger, A.G.; Scherrer, U.; Nicod, P. The thyroid and the heart. Circulation 1993, 87, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Tankeu, A.T.; Azabji-Kenfack, M.; Nganou, C.-N.; Ngassam, E.; Kuate-Mfeukeu, L.; Mba, C.; Dehayem, M.Y.; Mbanya, J.-C.; Sobngwi, E. Effect of propranolol on heart rate variability in hyperthyroidism. BMC Res. Notes 2018, 11, 151. [Google Scholar] [CrossRef]
- Levey, G.S.; Klein, I. Catecholamine-thyroid hormone interactions and the cardiovascular manifestations of hyperthyroidism. Am. J. Med. 1990, 88, 642–646. [Google Scholar] [CrossRef]
- Coulombe, P.; Dussault, J.H.; Letarte, J.; Simard, S.J. Catecholamines Metabolism in Thyroid Diseases. I. Epinephrine Secretion Rate in Hyperthyroidism and Hypothyroidism. J. Clin. Endocrinol. Metab. 1976, 42, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.; Taha, W.; Kundumadam, S.; Khan, M. Atrial fibrillation and hyperthyroidism: A literature review. Indian Hear. J. 2017, 69, 545–550. [Google Scholar] [CrossRef]
- Maciel, B.C.; Gallo, L.; Neto, J.A.M.; Maciel, L.M.Z.; Alves, M.L.D.; Paccola, G.M.F.; Iazigi, N. The role of the autonomic nervous system in the resting tachycardia of human hyperthyroidism. Clin. Sci. 1987, 72, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Insel, P. Adrenergic receptors. Evolving concepts on structure and function. Am. J. Hypertens. 1989, 3 Pt 2, 112–118. [Google Scholar] [CrossRef]
- Malliani, A. Heart rate variability: From bench to bedside. Eur. J. Intern. Med. 2005, 16, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Tandon, O.P.; Awashi, R.; Sekhri, T.; Sircar, S.S. Correlation of autonomic indices with thyroid status. Indian J. Physiol. Pharmacol. 2003, 47, 164–170. [Google Scholar] [PubMed]
- Straznicky, N.E.; Eikelis, N.; Lambert, E.A.; Esler, M.D. Mediators of sympathetic activation in metabolic syndrome obesity. Curr. Hypertens. Rep. 2008, 10, 440–447. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, S.-A.; Chen, Y.-J.; Chang, M.-S.; Chan, P.; Lin, C.-I. Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. J. Am. Coll. Cardiol. 2002, 39, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Lip, G.Y.H.; Shantsila, A. Heart rate variability in atrial fibrillation: The balance between sympathetic and parasympathetic nervous system. Eur. J. Clin. Investig. 2019, 49, e13174. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, F. Autonomic nervous system and paroxysmal atrial fibrillation: A study based on the analysis of RR interval changes before, during and after paroxysmal atrial fibrillation. Eur. Heart J. 2004, 25, 1242–1248. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, G.; Makowski, K.; Michałkiewicz, D.; Kowal, J.; Ruchala, M.; Szczepanek, E.; Gielerak, G. The influence of subclinical hyperthyroidism on blood pressure, heart rate variability, and prevalence of arrhythmias. Thyroid 2012, 22, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.; Pal, P.; Nanda, N. Integrated regulation of cardiovascular functions. In Comprehensive Textbook of Medical Physiology; Jaypee Brothers Medical Publishers: New Delhi, India, 2007; pp. 654–657. [Google Scholar]
- Haensel, A.; Mills, P.J.; Nelesen, R.A.; Ziegler, M.G.; Dimsdale, J.E. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology 2008, 33, 1305–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Rovere, M.T.; Bigger, J.T., Jr.; Marcus, F.I.; Mortara, A.; Schwartz, P.J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998, 351, 478–484. [Google Scholar] [CrossRef]
- Dekker, J.M.; Crow, R.S.; Folsom, A.R.; Hannan, P.J.; Liao, D.; Swenne, C.A.; Schouten, E.G. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC study. Circulation 2000, 102, 1239–1244. [Google Scholar] [CrossRef]
- Bigger, J.T., Jr.; Fleiss, J.L.; Steinman, R.C.; Rolnitzky, L.M.; Kleiger, R.E.; Rottman, J.N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992, 85, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Bigger, J.; Fleiss, J.L.; Rolnitzky, L.M.; Steinman, R.C. Frequency domain measures of heart period variability to assess risk late after myocardial infarction. J. Am. Coll. Cardiol. 1993, 21, 729–736. [Google Scholar] [CrossRef] [Green Version]
- Tulppo, M.P.; Hughson, R.; Mäkikallio, T.H.; Airaksinen, K.E.J.; Seppänen, T.; Huikuri, H.V. Effects of exercise and passive head-up tilt on fractal and complexity properties of heart rate dynamics. Am. J. Physiol. Circ. Physiol. 2001, 280, H1081–H1087. [Google Scholar] [CrossRef]
- Mercuro, G.; Panzuto, M.G.; Bina, A.; Leo, M.; Cabula, R.; Petrini, L.; Pigliaru, F.; Mariotti, S. Cardiac function, physical exercise capacity, and quality of life during long-term thyrotropin- suppressive therapy with levothyroxine: Effect of individual dose tailoring. J. Clin. Endocrinol. Metab. 2000, 85, 159–164. [Google Scholar] [CrossRef]
- Biondi, B.; Fazio, S.; Cuocolo, A.; Sabatini, D.; Nicolai, E.; Lombardi, G.; Salvatore, M.; Saccà, L. Impaired cardiac reserve and exercice capacity in patients receiving long-term thyrotropin suppressive therapy with levothyroxine. J. Clin. Endocrinol. Metab. 1996, 81, 4224–4228. [Google Scholar]
- Olson, B.R.; Klein, I.; Benner, R.; Burdett, R.; Trzepacz, P.; Levey, G.S. Hyperthyroid Myopathy and the Response to Treatment. Thyroid 1991, 1, 137–141. [Google Scholar] [CrossRef]
- Byrne, E.A.; Fleg, J.L.; Vaitkevicius, P.V.; Wright, J.; Porges, S.W. Role of aerobic capacity and body mass index in the age-associated decline in heart rate variability. J. Appl. Physiol. 1996, 81, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Venditti FJJr Manders, E.S.; Evans, J.C.; Larson, M.G.; Feldman, C.L.; Levy, D. Determinants of heart rate variability. J. Am. Coll. Cardiol. 1996, 28, 1539–1546. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, M.A.; Weinberg, C.; Cook, D.; Best, J.; Reenan, A.; Halter, J.B. Differential changes of autonomic nervous system function with age in man. Am. J. Med. 1983, 75, 249–258. [Google Scholar] [CrossRef]
- Wang, W.; Rong, M. Cardiac sympathetic afferent reflexes in heart failure. Heart Fail. Rev. 2000, 5, 57–71. [Google Scholar] [CrossRef]
- Askin, L.; Cetin, M.; Turkmen, S. Ambulatory blood pressure results and heart rate variability in patients with premature ventricular contractions. Clin. Exp. Hypertens. 2018, 40, 251–256. [Google Scholar] [CrossRef]
- de Andrade, P.E.; do Amaral, J.A.T.; Paiva, L.D.S.; Adami, F.; Raimudo, J.Z.; Valenti, V.E.; Abreu, L.C.; Raimundo, R.D. Reduction of heart rate variability in hypertensive elderly. Blood Press. 2017, 26, 350–358. [Google Scholar] [CrossRef]
- Schroeder, E.B.; Liao, D.; Chambless, L.E.; Prineas, R.J.; Evans, G.W.; Heiss, G. Hypertension, blood pressure, and heart rate variability: The atherosclerosis risk in communities (ARIC) study. Hypertension 2003, 42, 1106–1111. [Google Scholar] [CrossRef] [Green Version]
- Al-Trad, B.A.; Mansi, M.; Alaraj, M.; Al-Hazimi, A. Cardiac autonomic dysfunction in young obese males is not associated with disturbances in pituitary-thyroid axis hormones. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1689–1695. [Google Scholar]
- Mustafa, G.; Kursat, F.M.; Ahmet, T.; Alparslan, G.F.; Omer, G.; Sertoglu, E.; Erkan, S.; Ediz, Y.; Turker, T.; Ayhan, K. The relationship between erythrocyte membrane fatty acid levels and cardiac autonomic function in obese children. Rev. Port. Cardiol. 2017, 36, 499–508. [Google Scholar] [CrossRef]
- Wu, E.C.-H.; Huang, Y.-T.; Chang, Y.-M.; Chen, I.-L.; Yang, C.-L.; Leu, S.-C.; Su, H.-L.; Kao, J.-L.; Tsai, S.-C.; Jhen, R.-N.; et al. The Association between Nutritional Markers and Heart Rate Variability Indices in Patients Undergoing Chronic Hemodialysis. J. Clin. Med. 2019, 8, 1700. [Google Scholar] [CrossRef] [Green Version]
- Nevill, A.M.; Stewart, A.; Olds, T.; Holder, R. Relationship between adiposity and body size reveals limitations of BMI. Am. J. Phys. Anthr. 2005, 129, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Dong, S.Y.; Sun, X.N.; Xie, J.; Cui, Y. Percent body fat is a better predictor of cardiovascular risk factors than body mass index. Braz. J. Med. Biol. Res. 2012, 45, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Esco, M.R.; Williford, H.N.; Olson, M.S. Skinfold Thickness is Related to Cardiovascular Autonomic Control as Assessed by Heart Rate Variability and Heart Rate Recovery. J. Strength Cond. Res. 2011, 25, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Millis, R.M.; Austin, R.E.; Hatcher, M.D.; Bond, V.; Faruque, M.U.; Goring, K.L.; Hickey, B.M.; DeMeersman, R.E. Association of body fat percentage and heart rate variability measures of sympathovagal balance. Life Sci. 2010, 86, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Brunová, J.; Kasalický, P.; Lánská, V. The assessment of body composition using DEXA in patients with thyroid dysfunction. Cas. Lek. Cesk. 2007, 5, 497–502. [Google Scholar]
- LeLorier, J.; Grégoire, G.; Benhaddad, A.; Lapierre, J.; Derderian, F. Discrepancies between Meta-Analyses and Subsequent Large Randomized, Controlled Trials. N. Engl. J. Med. 1997, 337, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Dickersin, K.; Min, Y.I. Publication bias: The problem that won’t go away. Ann. N. Y. Acad. Sci. 1993, 703, 135–146. [Google Scholar] [CrossRef]
- Lee, Y.H. An overview of meta-analysis for clinicians. Korean J. Intern. Med. 2018, 33, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Copas, J.; Shi, J.Q. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics 2000, 1, 247–262. [Google Scholar] [CrossRef] [Green Version]
- Ernst, G. Heart-rate variability—More than heart beats? Front. Public Health 2017, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Piskorski, J.; Guzik, P. Filtering Poincaré plots. Comput. Methods Sci. Technol. 2005, 11, 39–48. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brusseau, V.; Tauveron, I.; Bagheri, R.; Ugbolue, U.C.; Magnon, V.; Bouillon-Minois, J.-B.; Navel, V.; Dutheil, F. Heart Rate Variability in Hyperthyroidism: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 3606. https://doi.org/10.3390/ijerph19063606
Brusseau V, Tauveron I, Bagheri R, Ugbolue UC, Magnon V, Bouillon-Minois J-B, Navel V, Dutheil F. Heart Rate Variability in Hyperthyroidism: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(6):3606. https://doi.org/10.3390/ijerph19063606
Chicago/Turabian StyleBrusseau, Valentin, Igor Tauveron, Reza Bagheri, Ukadike Chris Ugbolue, Valentin Magnon, Jean-Baptiste Bouillon-Minois, Valentin Navel, and Frédéric Dutheil. 2022. "Heart Rate Variability in Hyperthyroidism: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 6: 3606. https://doi.org/10.3390/ijerph19063606
APA StyleBrusseau, V., Tauveron, I., Bagheri, R., Ugbolue, U. C., Magnon, V., Bouillon-Minois, J.-B., Navel, V., & Dutheil, F. (2022). Heart Rate Variability in Hyperthyroidism: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(6), 3606. https://doi.org/10.3390/ijerph19063606








