Factors Related to Anxiety, Depressive Symptoms and Quality of Life in Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Settings
2.2. Procedure
2.3. Methods and Variables
Statistical Analysis
3. Results
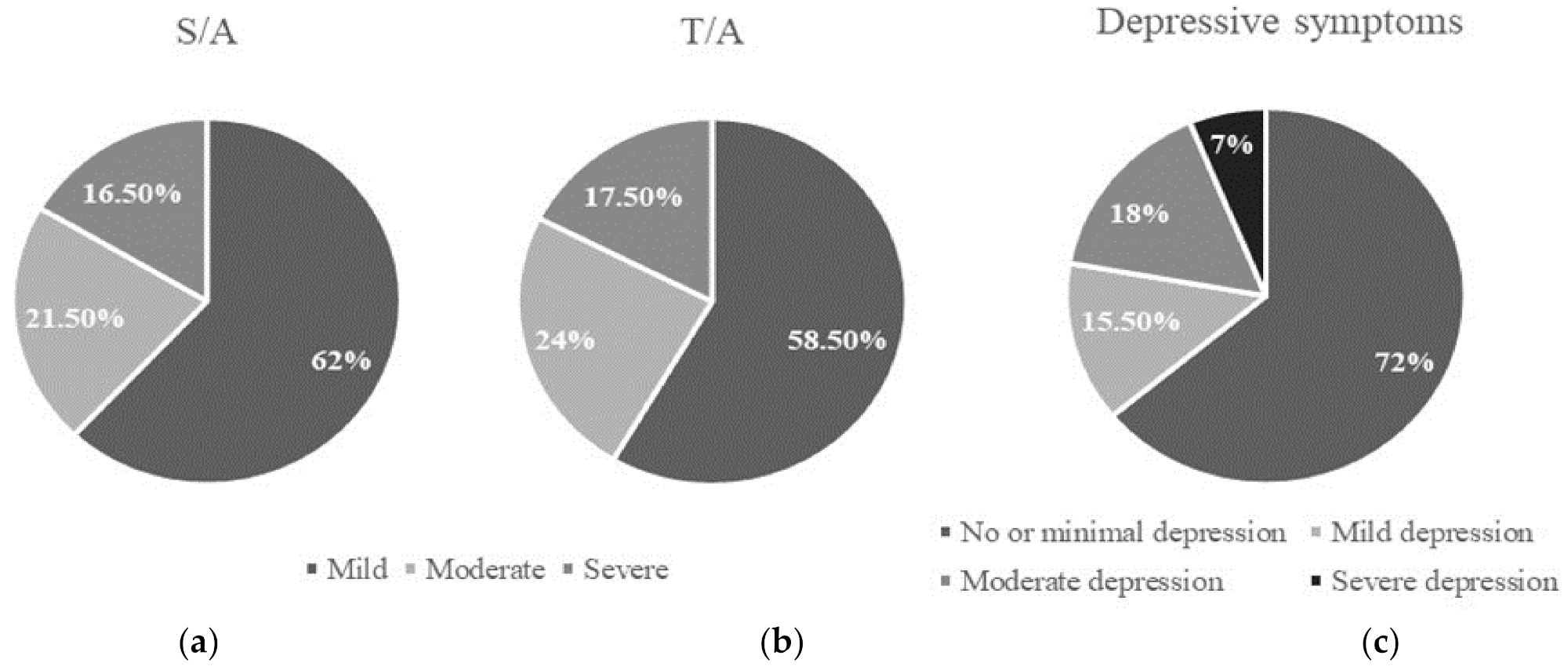
3.1. Anxiety, Depressive Symptoms, and Other Factors
3.2. Quality of Life
3.3. Anxiety, Depressive Symptoms and QoL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Las Cifras del Cáncer en España 2021. Available online: https://seom.org/images/Cifras_del_cancer_en_Espnaha_2021.pdf (accessed on 10 January 2022).
- Sociedad Española de Oncologia Médica. Cifras del Cancer en España 2020; Sociedad Española de Oncologia Médica: Madrid, Spain, 2020; ISBN 9788409277049. [Google Scholar]
- Huang, H.-Y.; Tsai, W.-C.; Chou, W.-Y.; Hung, Y.-C.; Liu, L.-C.; Huang, K.-F.; Wang, W.-C.; Leung, K.-W.; Hsieh, R.-K.; Kung, P.-T. Quality of life of breast and cervical cancer survivors. BMC Women’s Health 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Whisenant, M.; Wong, B.; Mitchell, S.A.; Beck, S.L.; Mooney, K. Trajectories of Depressed Mood and Anxiety During Chemotherapy for Breast Cancer. Cancer Nurs. 2020, 43, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Dinapoli, L.; Colloca, G.; Di Capua, B.; Valentini, V. Psychological Aspects to Consider in Breast Cancer Diagnosis and Treatment. Curr. Oncol. Rep. 2021, 23, 38. [Google Scholar] [CrossRef]
- Izci, F.; Sarsanov, D.; Erdogan, Z.I.; Ilgun, A.S.; Celebi, E.; Alco, G.; Kocaman, N.; Ordu, C.; Ozturk, A.; Duymaz, T.; et al. Impact of Personality Traits, Anxiety, Depression and Hopelessness Levels on Quality of Life in the Patients with Breast Cancer. Eur. J. Breast Health 2018, 14, 105–111. [Google Scholar] [CrossRef] [Green Version]
- So, W.K.W.; Law, B.M.H.; Ng, M.S.N.; He, X.; Chan, D.N.S.; Chan, C.W.H.; McCarthy, A.L. Symptom clusters experienced by breast cancer patients at various treatment stages: A systematic review. Cancer Med. 2021, 10, 2531–2565. [Google Scholar] [CrossRef]
- Kim, J.H.; Paik, H.-J.; Jung, Y.J.; Kim, D.-I.; Jo, H.J.; Lee, S.; Kim, H.Y. A Prospective Longitudinal Study about Change of Sleep, Anxiety, Depression, and Quality of Life in Each Step of Breast Cancer Patients. Oncology 2019, 97, 245–253. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Wang, W.; Pang, R.; Liu, J.; Man, Q.; Zhang, A. Prevalence and risk factors of anxiety and depression among patients with breast cancer: A protocol for systematic review and meta-analysis. BMJ Open 2021, 11, e041588. [Google Scholar] [CrossRef]
- Gold, M.; Dunn, L.B.; Phoenix, B.; Paul, S.M.; Hamolsky, D.; Levine, J.D.; Miaskowski, C. Co-occurrence of anxiety and depressive symptoms following breast cancer surgery and its impact on quality of life. Eur. J. Oncol. Nurs. 2016, 20, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.M.W.; Lacroix, A.Z.; Li, W.; Zaslavsky, O.; Wassertheil-Smoller, S.; Weitlauf, J.C.; Brenes, G.A.; Nassir, R.; Ockene, J.K.; Caire-Juvera, G.; et al. Depression and quality of life before and after breast cancer diagnosis in older women from the Women’s Health Initiative. J. Cancer Surviv. 2015, 9, 620–629. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Chan, M.K.Y.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef]
- Zainal, N.Z.; Ng, C.G.; Wong, A.; Andrew, B.; Taib, N.A.M.; Low, S.-Y. Prevalence of depression, trait anxiety, and social support during the diagnostic phases of breast cancer. J. Taibah Univ. Med. Sci. 2021, 16, 497–503. [Google Scholar] [CrossRef]
- Okati-Aliabad, H.; Ansari-Moghadam, A.; Mohammadi, M.; Kargar, S.; Shahraki-Sanavi, F. The prevalence of anxiety and depression and its association with coping strategies, supportive care needs, and social support among women with breast cancer. Support. Care Cancer 2021, 30, 703–710. [Google Scholar] [CrossRef]
- Civilotti, C.; Botto, R.; Maran, D.; Leonardis, B.; Bianciotto, B.; Stanizzo, M. Anxiety and Depression in Women Newly Diagnosed with Breast Cancer and Waiting for Surgery: Prevalence and Associations with Socio-Demographic Variables. Medicina 2021, 57, 454. [Google Scholar] [CrossRef]
- Hashemi, S.-M.; Rafiemanesh, H.; Aghamohammadi, T.; Badakhsh, M.; Amirshahi, M.; Sari, M.; Behnamfar, N.; Roudini, K. Prevalence of anxiety among breast cancer patients: A systematic review and meta-analysis. Breast Cancer 2020, 27, 166–178. [Google Scholar] [CrossRef]
- Lee, L.J.; Ross, A.; Griffith, K.; Jensen, R.E.; Wallen, G.R. Symptom Clusters in Breast Cancer Survivors: A Latent Class Profile Analysis. Oncol. Nurs. Forum 2020, 47, 89–100. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Kwan, L.; Breen, E.C.; Cole, S.W. Inflammation and Behavioral Symptoms After Breast Cancer Treatment: Do Fatigue, Depression, and Sleep Disturbance Share a Common Underlying Mechanism? J. Clin. Oncol. 2011, 29, 3517–3522. [Google Scholar] [CrossRef] [Green Version]
- So, W.K.-W.; Marsh, G.; Ling, W.M.; Leung, F.Y.; Lo, J.C.K.; Yeung, M.; Li, G.K.H. The Symptom Cluster of Fatigue, Pain, Anxiety, and Depression and the Effect on the Quality of Life of Women Receiving Treatment for Breast Cancer: A Multicenter Study. Oncol. Nurs. Forum 2009, 36, E205–E214. [Google Scholar] [CrossRef]
- Berger, A.M.; Kumar, G.; LeVan, T.D.; Meza, J.L. Symptom Clusters And Quality Of Life Over 1 Year In Breast Cancer Patients Receiving Adjuvant Chemotherapy. Asia-Pac. J. Oncol. Nurs. 2020, 7, 134–140. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Feng, Z.; Xu, Y.; Zeng, G. Longitudinal Trends in Anxiety, Depression, and Quality of Life During Different Intermittent Periods of Adjuvant Breast Cancer Chemotherapy. Cancer Nurs. 2018, 41, 62–68. [Google Scholar] [CrossRef]
- Sanford, S.D.; Beaumont, J.L.; Butt, Z.; Sweet, J.J.; Cella, D.; Wagner, L.I. Prospective Longitudinal Evaluation of a Symptom Cluster in Breast Cancer. J. Pain Symptom Manag. 2014, 47, 721–730. [Google Scholar] [CrossRef]
- Salibasic, M.; Delibegovic, S. The Quality of Life and Degree of Depression of Patients Suffering from Breast Cancer. Med. Arch. 2018, 72, 202–205. [Google Scholar] [CrossRef]
- Williams, K.; Bergquist-Beringer, S. Symptoms and Health-Related Quality of Life in Patients Receiving Cancer Therapy Matched to Genomic Profiles. Oncol. Nurs. Forum 2018, 45, E125–E136. [Google Scholar] [CrossRef]
- Kim, E.; Andersen, M.R.; Standish, L.J. Comparison of Health-Related Quality of Life Between Adjuvant Breast Cancer Treatment Groups. Oncol. Nurs. Forum 2019, 46, 59–70. [Google Scholar] [CrossRef]
- Da Mata Tiezzi, M.F.B.; de Andrade, J.M.; Romão, A.P.M.S.; Tiezzi, D.G.; Lerri, M.R.; Carrara, H.A.H.; Lara, L.A.S. Quality of Life in Women With Breast Cancer Treated With or Without Chemotherapy. Cancer Nurs. 2017, 40, 108–116. [Google Scholar] [CrossRef]
- Akel, R.; El Darsa, H.; Anouti, B.; Mukherji, D.; Temraz, S.; Raslan, R.; Tfayli, A.; Assi, H. Anxiety, Depression and Quality of Life in Breast Cancer Patients in the Levant. Asian Pac. J. Cancer Prev. 2017, 18, 2809–2816. [Google Scholar] [CrossRef]
- Jackson, I.; Rowan, P.; Padhye, N.; Hwang, L.; Vernon, S. Racial/ethnic differences in health-related quality of life among female breast cancer survivors: Cross-sectional findings from the Medical Expenditure Panel Survey. Public Health 2021, 196, 74–81. [Google Scholar] [CrossRef]
- Arraras, J.I.; Illarramendi, J.J.; Salgado, E.; De La Cruz, S.; Asin, G.; Manterola, A.; Ibañez, B.; Zarandona, U.; Dominguez, M.A.; Vera, R. An evaluation study of the determinants of future perspective and global Quality of Life in Spanish long-term premenopausal early-stage breast cancer survivors. Wspolczesna Onkol. 2016, 20, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Alquraan, L.; Alzoubi, K.H.; Rababa’H, S.; Karasneh, R.; Al-Azzam, S.; Al-Azayzih, A. Prevalence of Depression and the Quality-of-Life of Breast Cancer Patients in Jordan. J. Multidiscip. Health 2020, 13, 1455–1462. [Google Scholar] [CrossRef]
- Alonso-Molero, J.; Dierssen-Sotos, T.; Gomez-Acebo, I.; Baz, N.F.D.L.; Guevara, M.; Amiano, P.; Castaño-Vinyals, G.; Fernandez-Villa, T.; Moreno, V.; Bayo, J.; et al. Quality of Life in a Cohort of 1078 Women Diagnosed with Breast Cancer in Spain: 7-Year Follow-Up Results in the MCC-Spain Study. Int. J. Environ. Res. Public Health 2020, 17, 8411. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II; APA PsycTests: Washington, DC, USA, 1996. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, M.A.; Groenvold, M.; Arraras, J.I.; Franklin, J.; te Velde, A.; Muller, M.; Franzini, L.; Williams, A.; De Haes, H.C.; Hopwood, P.; et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: First results from a three-country field study. J. Clin. Oncol. 1996, 14, 2756–2768. [Google Scholar] [CrossRef] [PubMed]
- Cull, A.; Sprangers, M.; Bjordal, K.; Aaronson, N.; West, K.; Bottomley, A. Eortc Quality of Life Group Translation Procedure on Behalf of the Eortc Quality of Life Group; EORTC: Brussels, Belgium, 2002; ISBN 2930064323. [Google Scholar]
- Arraras, J.; Arias, F.; Tejedor, M.; Pruja, E.; Marcos, M.; Martinez, E.; Valerdi, J. The eortc QLQ-C30 (version 3.0) quality of life questionnaire: Validation study for Spain with head and neck cancer patients. Psycho-Oncology 2002, 11, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Arraras, J.I.; Tejedor, M.; Illaramendi, J.J.; Vera, R.; Pruja, E.; Marcos, M.; Arias, F.; Valerdi, J.J. The EORTC breast cancer quality questionnaire (QLQ-BR23): A psychometric study with Spanish patients. Psicol. Conduct. 2001, 9, 81–97. [Google Scholar]
- Fayers, P.; Bottomley, A.E.O.R.T.C.; EORTC Quality of Life Group. Quality of life research within the EORTC—The EORTC QLQ-C30. Eur. J. Cancer 2002, 38, S125–S133. [Google Scholar] [CrossRef]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. EORTC QLQ-C30 Scoring Manual, 3rd ed.; EORTC: Brussels, Belgium, 2001. [Google Scholar]
- Sanz, J.; Perdigón, A.L.; Vázquez, C. Adaptaci ó n espa ñ ola del Inventario para la Depresi ó n de Beck-II (BDI-II):2. Propiedades psicom é tricas en poblaci ó n general The spanish adaptation of Beck’ s Depression Inventory-II (BDI-II). Clín. Salud 2003, 14, 249–280. [Google Scholar]
- Buela-Casal, G.; Guillén-Riquelme, A.S.C.N. Cuestionario de Ansiedad Estado-Rasgo: Adaptación Española, 8th ed.; Hogrefe TEA: Madrid, Spain, 2011. [Google Scholar]
- Thakur, M.; Sharma, R.; Mishra, A.K.; Singh, K.R. Prevalence and Psychobiological Correlates of Depression Among Breast Cancer Patients. Indian J. Surg. Oncol. 2021, 12, 251–257. [Google Scholar] [CrossRef]
- Pitman, A.; Suleman, S.; Hyde, N.; Hodgkiss, A. Depression and anxiety in patients with cancer. BMJ 2018, 361, 1–6. [Google Scholar] [CrossRef]
- Montazeri, A.; Vahdaninia, M.; Harirchi, I.; Ebrahimi, M.; Khaleghi, F.; Jarvandi, S. Quality of life in patients with breast cancer before and after diagnosis: An eighteen months follow-up study. BMC Cancer 2008, 8, 330. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Sereika, S.M.; Marsland, A.L.; Conley, Y.P.; Bender, C.M. Symptom Clusters in Women With Breast Cancer During the First 18 Months of Adjuvant Therapy. J. Pain Symptom Manag. 2020, 59, 233–241. [Google Scholar] [CrossRef]
- Tsaras, K.; Papathanasiou, I.V.; Mitsi, D.; Veneti, A.; Kelesi, M.; Zyga, S.; Fradelos, E.C. Assessment of Depression and Anxiety in Breast Cancer Patients: Prevalence and Associated Factors. Asian Pac. J. Cancer Prev. 2018, 19, 1661–1669. [Google Scholar] [CrossRef]
- Naser, A.Y.; Hameed, A.N.; Mustafa, N.; Alwafi, H.; Dahmash, E.Z.; Alyami, H.S.; Khalil, H. Depression and Anxiety in Patients With Cancer: A Cross-Sectional Study. Front. Psychol. 2021, 12, 585534. [Google Scholar] [CrossRef]
- Fradelos, E.C.; Papathanasiou, I.V.; Veneti, A.; Daglas, A.; Christodoulou, E.; Zyga, S.; Kourakos, M. Psychological Distress and Resilience in Women Diagnosed with Breast Cancer in Greece. Asian Pac. J. Cancer Prev. 2017, 18, 2545–2550. [Google Scholar] [CrossRef]
- Nakamura, Z.M.; Deal, A.M.; Nyrop, K.A.; Chen, Y.T.; Quillen, L.J.; Brenizer, T.; Muss, H.B. Serial Assessment of Depression and Anxiety by Patients and Providers in Women Receiving Chemotherapy for Early Breast Cancer. Oncologist 2021, 26, 147–156. [Google Scholar] [CrossRef]
- Oh, P.-J.; Cho, J.-R. Changes in Fatigue, Psychological Distress, and Quality of Life After Chemotherapy in Women with Breast Cancer. Cancer Nurs. 2020, 43, E54–E60. [Google Scholar] [CrossRef]
- Lim, C.C.; Devi, M.K.; Ang, E. Anxiety in women with breast cancer undergoing treatment: A systematic review. Int. J. Evid.-Based Health 2011, 9, 215–235. [Google Scholar] [CrossRef]
- Schwarz, R.; Krauss, O.; Höckel, M.; Meyer, A.; Zenger, M.; Hinz, A. The Course of Anxiety and Depression in Patients with Breast Cancer and Gynaecological Cancer. Breast Care 2008, 3, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Charalambous, A.; Kaite, C.P.; Charalambous, M.; Tistsi, T.; Kouta, C. The effects on anxiety and quality of life of breast cancer patients following completion of the first cycle of chemotherapy. SAGE Open Med. 2017, 5, 2050312117717507. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-H.; Chen, S.-J.; Liu, C.-Y. Adjuvant treatments of breast cancer increase the risk of depressive disorders: A population-based study. J. Affect. Disord. 2015, 182, 44–49. [Google Scholar] [CrossRef]
- Villar, R.R.; Fernández, S.P.; Garea, C.C.; Pillado, M.T.S.; Barreiro, V.B.; Martín, C.G. Quality of life and anxiety in women with breast cancer before and after treatment. Rev. Lat.-Am. Enferm. 2017, 25, e2958. [Google Scholar] [CrossRef]
- Separovic, R.; Silovski, T.; Vuger, A.T.; Baji, Ž.; Silovski, H.; Juri, A. Association of Breast Cancer Symptoms with Patients’ Quality of Life and Depression; a Croatian Cross-Sectional Study. Psychiatr. Danub. 2019, 6, 92–98. [Google Scholar]
- Słowik, A.J.; Jablonski, M.; Michałowska-Kaczmarczyk, A.M.; Jach, R. Evaluation of quality of life in women with breast cancer, with particular emphasis on sexual satisfaction, future perspectives and body image, depending on the method of surgery. Psychiatr. Polska 2017, 51, 871–888. [Google Scholar] [CrossRef]
- Chu, W.-O.; Dialla, P.O.; Roignot, P.; Bone-Lepinoy, M.-C.; Poillot, M.-L.; Coutant, C.; Arveux, P.; Dabakuyo-Yonli, T.S. Determinants of quality of life among long-term breast cancer survivors. Qual. Life Res. 2016, 25, 1981–1990. [Google Scholar] [CrossRef]
- Cáceres, M.C.; Pérez-Civantos, D.; Guerrero-Martín, J.; Delgado, M.N.; Jurado, C.L.; Durán-Gómez, N. Depressive Symptoms and Quality of Life Associated With the Use of Monoclonal Antibodies in Breast Cancer Treatment. Oncol. Nurs. Forum 2021, 48, 535–545. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S. Do COVID-19–Related Treatment Changes Influence Fear of Cancer Recurrence, Anxiety, and Depression in Breast Cancer Patients? Cancer Nurs. 2021, 45, E628–E638. [Google Scholar] [CrossRef]
- Lovelace, D.L.; McDaniel, L.R.; Golden, D. Long-Term Effects of Breast Cancer Surgery, Treatment, and Survivor Care. J. Midwifery Women’s Health 2019, 64, 713–724. [Google Scholar] [CrossRef]
| Variable | Categories | N (%)/M ± SD |
|---|---|---|
| Age | 53.05 ± 10.71 | |
| Marital status | Married | 145 (72.5) |
| Single | 21 (10.5) | |
| Divorced | 15 (7.5) | |
| Widowed | 19 (9.5) | |
| Education level | No studies | 15 (7.5) |
| Elementary school | 59 (34) | |
| Middle school | 27 (13.5) | |
| High school | 44 (22.0) | |
| Higher education | 55 (27.5) | |
| Employment situation | Currently in employment | 22 (11) |
| Temporary sick leave | 80 (40) | |
| Permanent sick leave | 16 (8) | |
| Unemployed | 50 (25) | |
| Retired | 32 (16) | |
| Tumor staging | 0 | 10 (5) |
| I | 62 (31) | |
| II | 68 (34) | |
| III | 33 (16.5) | |
| IV | 27 (13.5) | |
| Grade | 1 | 34 (17) |
| 2 | 57 (28.5) | |
| 3 | 109 (54.5) | |
| Molecular subtype | Luminal A/Luminal B HER2 negative-like | 106 (53.0) |
| Luminal B HER2 positive-like/HER2-type | 73 (36.5) | |
| Triple negative | 21 (10.5) | |
| Current situation | Initial treatment | 147 (73.5) |
| Relapse | 34 (17) | |
| Check-ups | 19 (9.5) | |
| Therapy | Neoadjuvant | 22 (11) |
| Adjuvant | 178 (89) | |
| Menopause | Natural | 83 (41.5) |
| Drug-induced menopause | 60 (30) | |
| Intervention-induced menopause | 13 (6.5) | |
| Reproductive stage | 44 (22) | |
| Treatment | Surgery | 159 (79.5) |
| Chemotherapy | 172 (76) | |
| Radiotherapy | 90 (45) | |
| Hormonotherapy | 75 (37.5) | |
| Immunotherapy | 42 (21) | |
| Treatment combinations | Chemotherapy | 23 (11.5) |
| Surgery | 8 (4) | |
| Surgery and chemotherapy | 35 (17.5) | |
| Surgery, chemotherapy and radiotherapy | 21 (10.5) | |
| Surgery, chemotherapy, radiotherapy and hormonotherapy | 30 (15) | |
| Surgery, chemotherapy, radiotherapy, hormonotherapy and immunotherapy | 12 (6) | |
| Other combinations | 71 (35.5) | |
| Surgical treatment | Conservative surgery | 110 (55) |
| Uni- or bilateral mastectomy | 49 (24.5) | |
| Without surgical treatment | 41 (20.5) | |
| Chemotherapy cycles | Chemotherapy cycles < 4 | 72 (41.9) |
| Chemotherapy cycles ≥ 4 | 100 (58.1) |
| S/A M ± SD | T/A M ± SD | BDI M ± SD | |
|---|---|---|---|
| Stage 0 | 11.00 ± 1.32 a | 19.20 ± 11.02 | 9.50 ± 7.15 |
| Stage I | 16.24 ± 11.19 a,b | 20.69 ± 9.72 | 9.95 ± 8.72 |
| Stage II | 20.68 ± 11.51 a,b | 23.82 ± 9.76 | 9.63 ± 5.92 |
| Stage III | 23.30 ± 13.23 b | 24.94 ± 8.89 | 13.24 ± 10.55 |
| Stage IV | 19.22 ± 13.26 a,b | 21.67 ± 10.03 | 12.11 ± 9.28 |
| p = 0.011 | p = 0.149 | p = 0.220 |
| S/A | T/A | BDI | ||||||
|---|---|---|---|---|---|---|---|---|
| M ± SD | p | M ± SD | p | M ± SD | p | |||
| Treatment | Surgery | No | 18.88 ± 10.50 | 0.917 | 23.24 ± 10.72 | 0.594 | 10.29 ± 9.41 | 0.754 |
| Yes | 19.10 ± 12.62 | 22.33 ± 9.55 | 10.75 ± 7.99 | |||||
| Radiotherapy | No | 18.03 ± 10.82 | 0.188 | 21.68 ± 9.32 | 0.184 | 10.09 ± 8.71 | 0.288 | |
| Yes | 20.31 ± 13.63 | 23.53 ± 10.27 | 11.34 ± 7.71 | |||||
| Hormonotherapy | No | 17.87 ± 11.07 | 0.076 | 22.53 ± 9.57 | 0.981 | 10.19 ± 8.18 | 0.308 | |
| Yes | 21.03 ± 13.70 | 22.49 ± 10.18 | 11.43 ± 8.43 | |||||
| Immunotherapy | No | 18.47 ± 11.93 | 0.192 | 22.39 ± 9.90 | 0.719 | 10.35 ± 8.25 | 0.310 | |
| Yes | 21.24 ± 13.03 | 23.00 ± 9.43 | 11.81 ± 8.37 | |||||
| Surgical treatment | Surgical treatment: mastectomy | 17.31 ± 12.32 | 0.593 | 21.67 ± 10.98 | 0.661 | 10.29 ± 7.83 | 0.194 | |
| Surgical treatment: conservative surgery | 19.90 ± 12.71 | 22.62 ± 8.88 | 10.95 ± 8.09 | |||||
| Without surgery | 18.88 ± 10.50 | 23.24 ± 10.72 | 10.29 ± 9.41 | |||||
| Chemotherapy cycles | Chemotherapy cycles < 4 | 18.33 ± 11.47 | 0.260 | 21.73 ± 9.81 | 0.126 | 9.53 ± 7.35 | 0.009 | |
| Chemotherapy cycles ≥ 4 | 20.37 ± 13.37 | 23.94 ± 9.64 | 12.70 ± 9.46 | |||||
| r | p | r | p | r | p | |||
| Number of chemotherapy cycles | 0.082 | 0.247 | 0.028 | 0.692 | 0.153 | 0.031 | ||
| EORTC QLQ-C30 | N = 200 |
|---|---|
| Mean ± SD | |
| Global health status/QoL | 63.1 ± 25.1 |
| Functional scales | |
| Physical functioning (PF) | 78.34 ± 21.83 |
| Role functioning (RF) | 77.18 ± 28.12 |
| Emotional functioning (EF) | 72.56 ± 23.78 |
| Cognitive functioning (CF) | 80.57 ± 27.26 |
| Social functioning (SF) | 76.57 ± 26.79 |
| Symptom scales/items | |
| Fatigue (FA) | 31.23 ± 27.40 |
| Nausea and vomiting (NV) | 7.11 ± 17.22 |
| Pain (PA) | 28.81 ± 29.25 |
| Dyspnea (DY) | 9.98 ± 24.99 |
| Insomnia (SL) | 35.81 ± 36.44 |
| Appetite loss (AP) | 12.16 ± 23.91 |
| Constipation (CO) | 21.49 ± 30.97 |
| Diarrhea (DI) | 8.82 ± 20.44 |
| Financial difficulties (FI) | 16.82 ± 28.93 |
| EORTC QLQ-BR23 | N = 200 |
| Mean ± SD | |
| Functional scales | |
| Body image (BRBI) | 77.03 ± 26.76 |
| Sexual functioning (BRSEF) | 80.60 ± 25.31 |
| Sexual enjoyment (BRSEE) | 77.33 ± 34.53 |
| Future perspective (BRFU) | 53.15 ± 34.60 |
| Symptom scales/items | |
| Systemic therapy side effects (BRST) | 26.65 ± 20.35 |
| Breast symptoms (BRBS) | 18.24 ± 21.59 |
| Arm symptoms (BRAS) | 18.09 ± 23.08 |
| Upset by hair loss (BRHL) | 20.54 ± 35.14 |
| EORTC QLQ-C30 | S/A | T/A | BDI | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Global health status/QoL | −0.386 | 0.000 | −0.333 | 0.000 | −0.546 | 0.000 |
| Physical functioning (PF) | −0.291 | 0.000 | −0.279 | 0.000 | −0.561 | 0.000 |
| Role functioning (RF) | −0.358 | 0.000 | −0.361 | 0.000 | −0.540 | 0.000 |
| Emotional functioning (EF) | −0.590 | 0.000 | −0.566 | 0.000 | −0.616 | 0.000 |
| Cognitive functioning (CF) | −0.415 | 0.000 | −0.355 | 0.000 | −0.539 | 0.000 |
| Social functioning (SF) | −0.296 | 0.000 | −0.317 | 0.000 | −0.476 | 0.000 |
| Fatigue (FA) | 0.471 | 0.000 | 0.457 | 0.000 | 0.641 | 0.000 |
| Nausea and vomiting (NV) | −0.008 | 0.907 | 0.014 | 0.841 | 0.200 | 0.005 |
| Pain (PA) | 0.286 | 0.000 | 0.347 | 0.000 | 0.560 | 0.000 |
| Dyspnea (DY) | 0.090 | 0.206 | 0.161 | 0.022 | 0.356 | 0.000 |
| Insomnia (SL) | 0.434 | 0.000 | 0.446 | 0.000 | 0.440 | 0.000 |
| Appetite loss (AP) | 0.244 | 0.000 | 0.264 | 0.000 | 0.448 | 0.000 |
| Constipation (CO) | 0.176 | 0.012 | 0.265 | 0.000 | 0.359 | 0.000 |
| Diarrhea (DI) | 0.085 | 0.229 | 0.203 | 0.004 | 0.253 | 0.000 |
| Financial difficulties (FI) | 0.245 | 0.000 | 0.283 | 0.000 | 0.336 | 0.000 |
| EORTC QLQ-BR23 | S/A | T/A | BDI | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Body image (BRBI) | −0.390 | 0.000 | −0.396 | 0.000 | −0.583 | 0.000 |
| Sexual functioning (BRSEF) | −0.097 | 0.171 | 0.022 | 0.759 | 0.006 | 0.935 |
| Sexual enjoyment (BRSEE) | −0.022 | 0.753 | 0.045 | 0.525 | 0.047 | 0.508 |
| Future perspective (BRFU) | −0.385 | 0.000 | −0.453 | 0.000 | −0.327 | 0.000 |
| Systemic therapy side effects (BRST) | 0.248 | 0.000 | 0.403 | 0.000 | 0.563 | 0.000 |
| Breast symptoms (BRBS) | 0.297 | 0.000 | 0.162 | 0.022 | 0.314 | 0.000 |
| Arm symptoms (BRAS) | 0.270 | 0.000 | 0.180 | 0.011 | 0.359 | 0.000 |
| Upset by hair loss (BRHL) | 0.227 | 0.000 | 0.163 | 0.021 | 0.242 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres, M.C.; Nadal-Delgado, M.; López-Jurado, C.; Pérez-Civantos, D.; Guerrero-Martín, J.; Durán-Gómez, N. Factors Related to Anxiety, Depressive Symptoms and Quality of Life in Breast Cancer. Int. J. Environ. Res. Public Health 2022, 19, 3547. https://doi.org/10.3390/ijerph19063547
Cáceres MC, Nadal-Delgado M, López-Jurado C, Pérez-Civantos D, Guerrero-Martín J, Durán-Gómez N. Factors Related to Anxiety, Depressive Symptoms and Quality of Life in Breast Cancer. International Journal of Environmental Research and Public Health. 2022; 19(6):3547. https://doi.org/10.3390/ijerph19063547
Chicago/Turabian StyleCáceres, Macarena C., Marta Nadal-Delgado, Casimiro López-Jurado, Demetrio Pérez-Civantos, Jorge Guerrero-Martín, and Noelia Durán-Gómez. 2022. "Factors Related to Anxiety, Depressive Symptoms and Quality of Life in Breast Cancer" International Journal of Environmental Research and Public Health 19, no. 6: 3547. https://doi.org/10.3390/ijerph19063547
APA StyleCáceres, M. C., Nadal-Delgado, M., López-Jurado, C., Pérez-Civantos, D., Guerrero-Martín, J., & Durán-Gómez, N. (2022). Factors Related to Anxiety, Depressive Symptoms and Quality of Life in Breast Cancer. International Journal of Environmental Research and Public Health, 19(6), 3547. https://doi.org/10.3390/ijerph19063547







