Effects of Resistance Training in Hypobaric vs. Normobaric Hypoxia on Circulating Ions and Hormones
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Resistance Training
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Control Variables
3.2. Hormones and Circulating Ions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ramos-Campo, D.J.; Rubio-Arias, J.; Freitas, T.T.; Camacho, A.; Jiménez-Diaz, J.F.; Alcaraz, P.E. Acute Physiological and Performance Responses to High-Intensity Resistance Circuit Training in Hypoxic and Normoxic Conditions. J. Strength Cond. Res. 2017, 31, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Slattery, K.M.; Sculley, D.V.; Smith, S.M.; Peiffer, J.J.; Dascombe, B.J. Acute physiological and perceptual responses to high-load resistance exercise in hypoxia. Clin. Physiol. Funct. Imaging 2018, 38, 595–602. [Google Scholar] [CrossRef]
- Feriche, B.; Schoenfeld, B.J.; Bonitch-Gongora, J.; de la Fuente, B.; Almeida, F.; Argüelles, J.; Benavente, C.; Padial, P. Altitude-induced effects on muscular metabolic stress and hypertrophy-related factors after a resistance training session. Eur. J. Sport Sci. 2020, 20, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Ohiwa, N.; Honda, A.; Matsubayashi, T.; Ikeda, T.; Akimoto, T.; Suzuki, Y.; Hirano, Y.; Russell, A.P. Effects of systemic hypoxia on human muscular adaptations to resistance exercise training. Physiol. Rep. 2015, 3, e12267. [Google Scholar] [CrossRef][Green Version]
- Guardado, I.; Urena, B.; Cardenosa, A.; Cardenosa, M.; Camacho, G.; Andrada, R. Effects of strength training under hypoxic conditions on muscle performance, body composition and haematological variables. Biol. Sport 2020, 37, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Slattery, K.M.; Dascombe, B.J. Intermittent hypoxic resistance training: Is metabolic stress the key moderator? Med. Hypotheses 2015, 84, 145–149. [Google Scholar] [CrossRef]
- Feriche, B.; García-Ramos, A.; Morales-Artacho, A.J.; Padial, P. Resistance Training Using Different Hypoxic Training Strategies: A Basis for Hypertrophy and Muscle Power Development. Sports Med. Open 2017, 3, 12. [Google Scholar] [CrossRef]
- Kon, M.; Ikeda, T.; Homma, T.; Suzuki, Y. Effects of low-intensity resistance exercise under acute systemic hypoxia on hormonal responses. J. Strength Cond. Res. 2012, 26, 611–617. [Google Scholar] [CrossRef]
- Filopoulos, D.; Cormack, S.J.; Whyte, D.G. Normobaric hypoxia increases the growth hormone response to maximal resistance exercise in trained men. Eur. J. Sport Sci. 2017, 17, 821–829. [Google Scholar] [CrossRef]
- Davison, G.W.; Morgan, R.M.; Hiscock, N.; Garcia, J.M.; Grace, F.; Boisseau, N.; Davies, B.; Castell, L.; McEneny, J.; Young, I.S.; et al. Manipulation of systemic oxygen flux by acute exercise and normobaric hypoxia: Implications for reactive oxygen species generation. Clin. Sci. 2006, 110, 133–141. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Rubio-Arias, J.A.; Dufour, S.; Chung, L.; Ávila-Gandía, V.; Alcaraz, P.E. Biochemical responses and physical performance during high-intensity resistance circuit training in hypoxia and normoxia. Eur. J. Appl. Physiol. 2017, 117, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.Y.; Huang, T.Y.; Chien, Y.C.; Chen, Y.C.; Liu, S.Y. Effects of acute exposure to mild simulated hypoxia on hormonal responses to low-intensity resistance exercise in untrained men. Res. Sports Med. 2014, 22, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Coppel, J.; Hennis, P.; Gilbert-Kawai, E.; Grocott, M.P. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: A systematic review of crossover trials. Extrem Physiol. Med. 2015, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.P.; Debevec, T. CrossTalk proposal: Barometric pressure, independent of PO2, is the forgotten parameter in altitude physiology and mountain medicine. J. Physiol. 2020, 598, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Savourey, G.; Launay, J.C.; Besnard, Y.; Guinet, A.; Travers, S. Normo- and hypobaric hypoxia: Are there any physiological differences? Eur. J. Appl. Physiol. 2003, 89, 122–126. [Google Scholar] [CrossRef]
- Self, D.A.; Mandella, J.G.; Prinzo, O.V.; Forster, E.M.; Shaffstall, R.M. Physiological equivalence of normobaric and hypobaric exposures of humans to 25,000 feet (7620 m). Aviat. Space Environ. Med. 2011, 82, 97–103. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013, 43, 179–194. [Google Scholar] [CrossRef]
- Scott, B.R.; Slattery, K.M.; Sculley, D.V.; Lockhart, C.; Dascombe, B.J. Acute Physiological Responses to Moderate-Load Resistance Exercise in Hypoxia. J. Strength Cond. Res. 2017, 31, 1973–1981. [Google Scholar] [CrossRef]
- Cintineo, H.; Freidenreich, D.; Blaine, C.; Cardaci, T.; Pellegrino, J.; Arent, S. Acute physiological responses to an intensity-and time-under-tension-equated single-vs. multiple-set resistance training bout in trained men. J. Strength Cond. Res. 2018, 32, 3310–3318. [Google Scholar] [CrossRef]
- De Salles, B.F.; Simão, R.; Miranda, F.; da Silva Novaes, J.; Lemos, A.; Willardson, J.M. Rest interval between sets in strength training. Sports Med. 2009, 39, 765–777. [Google Scholar] [CrossRef]
- Lockhart, C.; Scott, B.R.; Thoseby, B.; Dascombe, B.J. Acute Effects of Interset Rest Duration on Physiological and Perceptual Responses to Resistance Exercise in Hypoxia. J. Strength Cond. Res. 2020, 34, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.G.; Lane, C.J.; Schroeder, E.T. Influence of rest interval length on acute testosterone and cortisol responses to volume-load-equated total body hypertrophic and strength protocols. J. Strength Cond. Res. 2012, 26, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef]
- Leite, R.D.; Prestes, J.; Rosa, C.; De Salles, B.F.; Maior, A.S.; Miranda, H.; Simão, R. Acute effect of resistance training volume on hormonal responses in trained men. J. Sports Med. Phys. Fit. 2011, 51, 322–328. [Google Scholar]
- de Freitas, M.C.; Gerosa-Neto, J.; Zanchi, N.E.; Lira, F.S.; Rossi, F.E. Role of metabolic stress for enhancing muscle adaptations: Practical applications. World J. Methodol. 2017, 7, 46–54. [Google Scholar] [CrossRef]
- Henselmans, M.; Schoenfeld, B.J. The effect of inter-set rest intervals on resistance exercise-induced muscle hypertrophy. Sports Med. 2014, 44, 1635–1643. [Google Scholar] [CrossRef]
- Rahimi, R.; Ghaderi, M.; Mirzaei, B.; Ghaeni, S.; Faraji, H.; Vatani, D.S.; Rahmani-Nia, F. Effects of very short rest periods on immunoglobulin A and cortisol responses to resistance exercise in men. J. Hum. Sport Exerc. 2010, 5, 146–157. [Google Scholar] [CrossRef]
- Woods, D.R.; O’Hara, J.P.; Boos, C.J.; Hodkinson, P.D.; Tsakirides, C.; Hill, N.E.; Jose, D.; Hawkins, A.; Phillipson, K.; Hazlerigg, A.; et al. Markers of physiological stress during exercise under conditions of normoxia, normobaric hypoxia, hypobaric hypoxia, and genuine high altitude. Eur. J. Appl. Physiol. 2017, 117, 893–900. [Google Scholar] [CrossRef]
- Richalet, J.P.; Letournel, M.; Souberbielle, J.C. Effects of high-altitude hypoxia on the hormonal response to hypothalamic factors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1685–R1692. [Google Scholar] [CrossRef]
- Balog, E.M.; Fruen, B.R.; Kane, P.K.; Louis, C.F. Mechanisms of P(i) regulation of the skeletal muscle SR Ca2+ release channel. Am. J. Physio.l Cell Physiol. 2000, 278, C601–C611. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Westerblad, H. Role of phosphate and calcium stores in muscle fatigue. J. Physiol. 2001, 536 Pt 3, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-J.; Li, Q.; Guan, Y.; Shi, M.; Yang, J.; Li, D.-P.; Zhang, Y. Chronic intermittent hypobaric hypoxia ameliorates ischemia/reperfusion-induced calcium overload in heart via Na/Ca2+ exchanger in developing rats. Cell Physiol. Biochem. 2014, 34, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Place, N.; Westerblad, H. Molecular Basis for Exercise-Induced Fatigue: The Importance of Strictly Controlled Cellular Ca2+ Handling. Cold Spring Harb. Perspect. Med. 2018, 8, a029710. [Google Scholar] [CrossRef]
- Juel, C. Regulation of pH in human skeletal muscle: Adaptations to physical activity. Acta Physiol. 2008, 193, 17–24. [Google Scholar] [CrossRef]
- Lühker, O.; Berger, M.M.; Pohlmann, A.; Hotz, L.; Gruhlke, T.; Hochreiter, M. Changes in acid-base and ion balance during exercise in normoxia and normobaric hypoxia. Eur. J. Appl. Physiol. 2017, 117, 2251–2261. [Google Scholar] [CrossRef]
- McLester, J.R. Muscle contraction and fatigue. The role of adenosine 5′-diphosphate and inorganic phosphate. Sports Med. 1997, 23, 287–305. [Google Scholar] [CrossRef]
- Willardson, J.M. A brief review: Factors affecting the length of the rest interval between resistance exercise sets. J. Strength Cond. Res. 2006, 20, 978–984. [Google Scholar] [CrossRef]
- Debevec, T.; Pialoux, V.; Saugy, J.J.; Schmitt, L.; Cejuela, R.; Mury, P.; Ehrström, S.; Faiss, R.; Millet, G.P. Prooxidant/Antioxidant Balance in Hypoxia: A Cross-Over Study on Normobaric vs. Hypobaric “Live High-Train Low”. PLoS ONE 2015, 10, e0137957. [Google Scholar] [CrossRef]
| Groups | Inter-Set Rest (min) | SpO2 (%) | Lactate (mmol/L) | RPE |
|---|---|---|---|---|
| Hypobaric hypoxia (HH) (n = 9) | 1 | 92.44 ± 2.0 | 19.55 ± 3.5 | 8.22 ± 1.09 |
| 2 | 92.77 ± 1.5 | 15.98 ± 3.89 | 6.44 ± 1.58 + | |
| Total | 92.61 ± 1.7 * | 17.7 ± 4.0 * | 7.33 ± 1.6 | |
| Normobaric hypoxia (NH) (n = 7) | 1 | 95.01 ± 1.9 | 15.30 ± 3.3 | 7.8 ± 1.21 |
| 2 | 93.5 ± 2.1 | 12.47 ± 3.2 | 6.0 ± 1.73 + | |
| Total | 94.28 ± 2.0 * | 13.8 ± 3.4 * | 6.92 ± 1.7 | |
| p-value | 0.020 * | 0.007 * | 0.500 |
| Hypobaric Hypoxia | Within-Group Factor | Normobaric Hypoxia | Within-Group Factor | Between-Group Factors | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rest (min) | Baseline | At 5 min | At 10 min | At 30 min | p | ES | At 5 min | At 10 min | At 30 min | p | ES | Env (HH vs. NH) | Rest (1 m vs. 2 m) | Env. x Rest | |
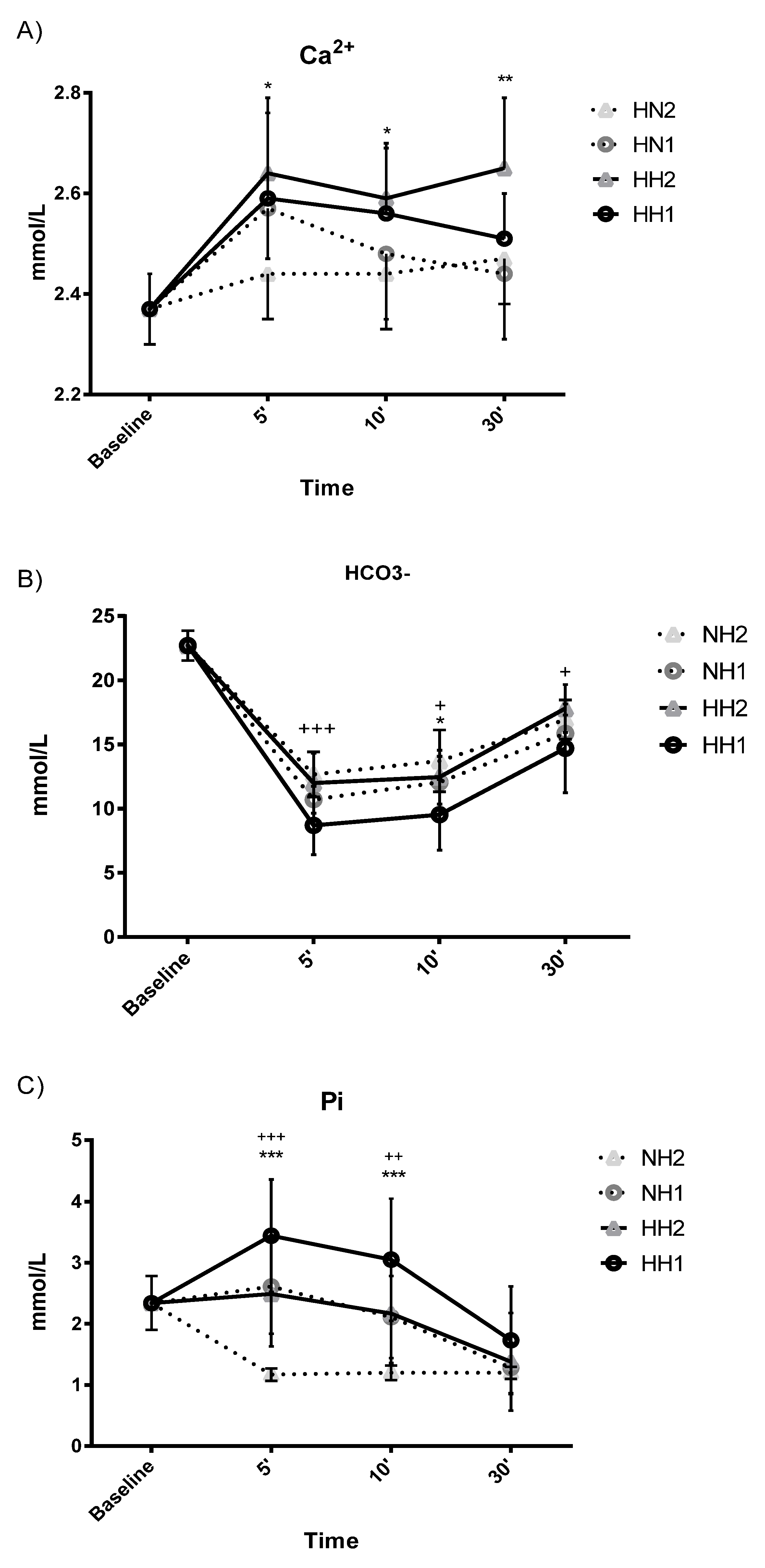
| Ca2+ (mmol/L) | 1 | 2.37 ± 0.07 | 2.59 ± 0.17 aa | 2.56 ± 0.13 aa | 2.51 ± 0.09 aa | 0.004 ** | 0.599 | 2.57 ± 0.10 a | 2.48 ± 0.13 | 2.44 ± 0.13 | 0.040 * | 0.548 | 0.038 * | 0.197 | 0.497 |
| 2 | 2.37 ± 0.07 | 2.64 ± 0.15 aa | 2.59 ± 0.11 aa | 2.65 ± 0.14 aa | 0.001 ** | 0.751 | 2.44 ± 0.09 | 2.44 ± 0.11 | 2.47 ± 0.09 | 0.333 | 0.278 | ||||
| HCO3− (mmol/L) | 1 | 22.70 ± 1.17 | 8.70 ± 2.28 aaccdd | 9.53 ± 2.75 aabbdd | 14.70 ± 3.44 aabbcc | 0.001 ** | 0.971 | 10.70 ± 1.04 aacdd | 12.05 ± 2.02 aabdd | 15.86 ± 1.42 aabbcc | 0.001 ** | 0.987 | 0.034 * | 0.003 ** | 0.733 |
| 2 | 22.70 ± 1.17 | 11.99 ± 2.33 aacdd | 12.46 ± 2.10 aabdd | 17.80 ± 1.86 aabbcc | 0.001 ** | 0.965 | 12.67 ± 1.76 aaccdd | 13.72 ± 2.40 aabbdd | 16.94 ± 1.52 aabbcc | 0.001 ** | 0.983 | ||||
| Pi (mmol/L) | 1 | 2.34 ± 0.44 | 3.44 ± 0.92 accdd | 3.05 ± 1.00 bbdd | 1.73 ± 0.88 bbcc | 0.001 ** | 0.818 | 2.61 ± 0.77 acdd | 2.11 ± 0.67 bdd | 1.28 ± 0.41 aabbcc | 0.001 ** | 0.974 | 0.001 ** | 0.001 ** | 0.211 |
| 2 | 2.34 ± 0.44 | 2.49 ± 0.86 ccdd | 2.17 ± 0.81 bbdd | 1.38 ± 0.80 abbcc | 0.001 ** | 0.688 | 1.17 ± 0.10 a | 1.20 ± 0.12 aa | 1.20 ± 0.10 aa | 0.001 ** | 0.719 | ||||
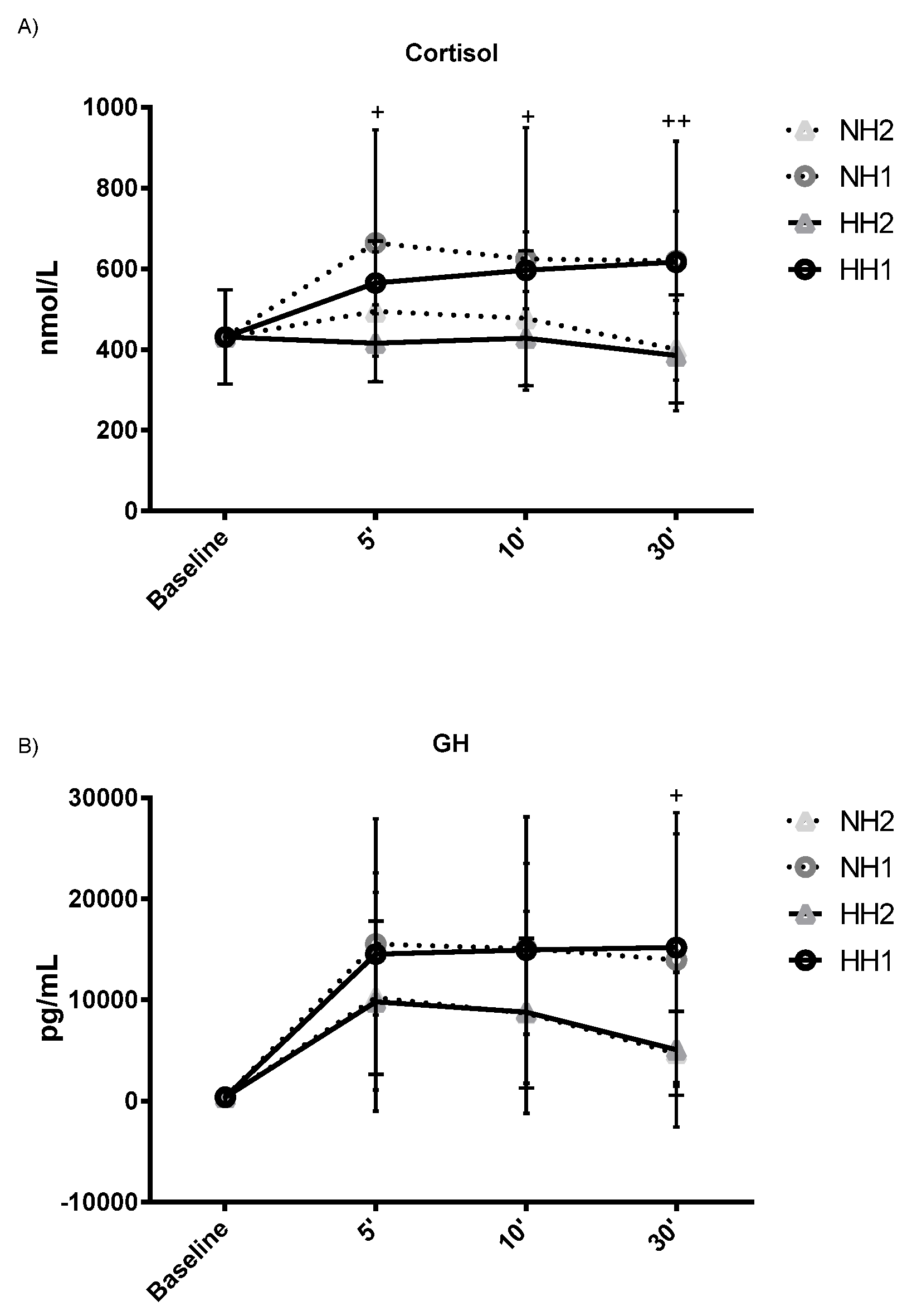
| C (nmol/L) | 1 | 431 ± 117 | 565 ± 76 a | 596 ± 95 a | 616 ± 126 a | 0.015* | 0.428 | 664 ± 280 a | 624 ± 325 | 620 ± 295 | 0.047 * | 0.495 | 0.083 | 0.006 ** | 0.899 |
| 2 | 431 ± 117 | 416 ± 95 | 428 ± 116 d | 385 ± 137 c | 0.056 | 0.407 | 494 ± 174 d | 477 ± 167 d | 401 ± 134 bc | 0.370 | 0.259 | ||||
| GH (pg/mL) | 1 | 398 ± 597 | 14542 ± 13420 a | 14950 ± 13174 aa | 15198 ± 13360 aa | 0.007 ** | 0.568 | 15559 ± 7026 aa | 15091 ± 8464 aa | 13971 ± 12473 a | 0.003 ** | 0.734 | 0.441 | 0.049 * | 0.997 |
| 2 | 398 ± 597 | 9833 ± 10883 a | 8798 ± 9908 | 5105 ± 7656 | 0.028 * | 0.466 | 10248 ± 7572 ad | 8716 ± 7404 | 4733 ± 4141 b | 0.012 * | 0.650 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timon, R.; Olcina, G.; Padial, P.; Bonitch-Góngora, J.; Martínez-Guardado, I.; Benavente, C.; de la Fuente, B.; Feriche, B. Effects of Resistance Training in Hypobaric vs. Normobaric Hypoxia on Circulating Ions and Hormones. Int. J. Environ. Res. Public Health 2022, 19, 3436. https://doi.org/10.3390/ijerph19063436
Timon R, Olcina G, Padial P, Bonitch-Góngora J, Martínez-Guardado I, Benavente C, de la Fuente B, Feriche B. Effects of Resistance Training in Hypobaric vs. Normobaric Hypoxia on Circulating Ions and Hormones. International Journal of Environmental Research and Public Health. 2022; 19(6):3436. https://doi.org/10.3390/ijerph19063436
Chicago/Turabian StyleTimon, Rafael, Guillermo Olcina, Paulino Padial, Juan Bonitch-Góngora, Ismael Martínez-Guardado, Cristina Benavente, Blanca de la Fuente, and Belen Feriche. 2022. "Effects of Resistance Training in Hypobaric vs. Normobaric Hypoxia on Circulating Ions and Hormones" International Journal of Environmental Research and Public Health 19, no. 6: 3436. https://doi.org/10.3390/ijerph19063436
APA StyleTimon, R., Olcina, G., Padial, P., Bonitch-Góngora, J., Martínez-Guardado, I., Benavente, C., de la Fuente, B., & Feriche, B. (2022). Effects of Resistance Training in Hypobaric vs. Normobaric Hypoxia on Circulating Ions and Hormones. International Journal of Environmental Research and Public Health, 19(6), 3436. https://doi.org/10.3390/ijerph19063436








