Abstract
This is a case report of Basidiobolomycosis in a 65-year-old male patient from Jizan presenting with colonic perforation and concomitant liver involvement from February 2021 to July 2021. To control the infection, the patient underwent colonic resection and segmental liver resection, as well as three antifungal drugs. The treatment was successful, and the condition was completely resolved.
1. Introduction
Basidiobolomycosis is a rare granulomatous infection of the skin and subcutaneous tissue that can develop quickly and create a persistent infection of the subcutaneous tissue in immunocompetent individuals. It most usually affects infants under the age of one month and is rarely found in adults [1,2].
Even though gastrointestinal involvement is uncommon, it can affect the stomach, small intestine, colon, and liver. Basidiobolomycosis can cause symptoms that are similar to colonic or inflammatory bowel disease. Colonic bowel disease with concurrent liver involvement is uncommon, with only six cases reported. Colonic perforation caused by Basidiobolomycosis is extremely uncommon, with only two cases documented in the literature [2].
In the current case, Basidiobolomycosis was diagnosed in a 65-year-old male who had colonic perforation and liver involvement. A comprehensive literature review was conducted from 1964 to July 2021 to evaluate previously reported cases and gather information on symptoms, diagnostic method, and treatment choices.
2. Case
A 65-year-old man from Jizan (southwestern Saudi Arabia) presented with a one-month history of lower abdominal pain, constipation, and weight loss. No significant medical or surgical history was recorded. Ethical approval (HA-02-J-008) was obtained from the Unit of Biomedical Ethics Research Committee at the Faculty of Medicine in King Abdulaziz University, Saudi Arabia.
Abdominal examination revealed a palpable right-side-abdominal mass with mild tenderness. Laboratory tests revealed leukocytosis (white blood cell (WBC) count: 17,230/mcl), eosinophilia (2540/mcl (14.7%), microcytic hypochromic anemia (hemoglobin (Hb): 10.8 g/dL), and thrombocytosis (platelet count: 505,000/mcl). A computed tomography (CT) of the abdomen revealed circumferential intestinal wall thickening of the cecum and ascending colon, luminal constriction with enlargement of several mesenteric lymph nodes, and two lesions in the liver, segments five and seven, with peripheral enhancement and central hypodensity of 3 × 3 cm (Figure 1).
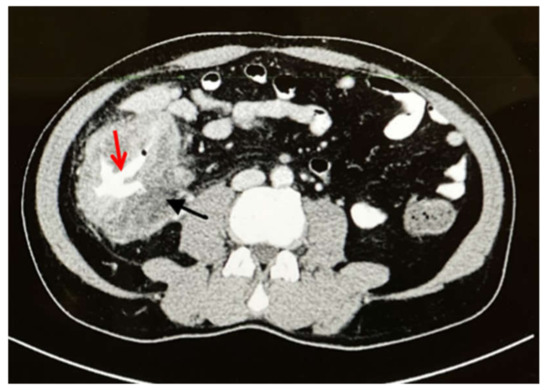
Figure 1.
Axial CT with IV and oral contrast showed wall thickening affecting the cecum and ascending colon (black arrow) with lumen narrowing (red arrow).
Colonoscopy revealed an ulcerated, partially obstructed large mass in the proximal ascending colon. A biopsy revealed active eosinophilic colitis with necrotic debris. A liver biopsy of tissue from the mass was performed and showed an eosinophilic abscess. The patient was diagnosed as a case of cecal mass with the possibility of a cancer, colon with liver metastasis need to be rolled out.
The patient returned to the emergency room two weeks later with intense abdominal pain that lasted a few hours. On examination, the patient’s temperature was 38 °C, his pulse was 110 beats/min, and the abdomen was tender with rigidity and an absence of bowel sounds. Laboratory tests revealed the following: WBC count, 13,700/mcl; Hb, 10.4 g/dL; neutrophil count, 9210/mcl (89%); and eosinophil count, 0/mcl (0%). A CT scan of the abdomen showed cecal perforation with free air in the peritoneum and an increase in the size of the liver masses (3 × 4 cm) (Figure 2). Emergency exploratory laparotomy revealed cecal perforation and pus in the peritoneum. Right hemicolectomy with end-to-end anastomosis was performed with drainage of the peritoneum. Intravenous vancomycin (1 g BID for 14 days) was administered to the patient for bacterial peritonitis secondary to colonic perforation. Three days postoperatively, histopathological examination of the resected specimen showed granulomatous inflammation containing multinucleated giant cells associated with eosinophilic infiltrate and micro abscess formation. There were fungal hyphae that look large, broad, and irregular with sparse septa and thin walls. The hyphae were highlighted with special stains that included Grocott methenamine silver (GMS) and periodic acid-Schiff (PAS) stains, the overall features are consistent with Basidobolomycosis infection (Figure 3a,b). Tissue culture from the edge of the perforation in the cecum revealed Escherichia coli, Candida albicans, and Basidiobolus spp. The patient was started on the antifungal medication voriconazole intravenously (200 mg BID) and was switched to an oral tablet (200 mg BID).
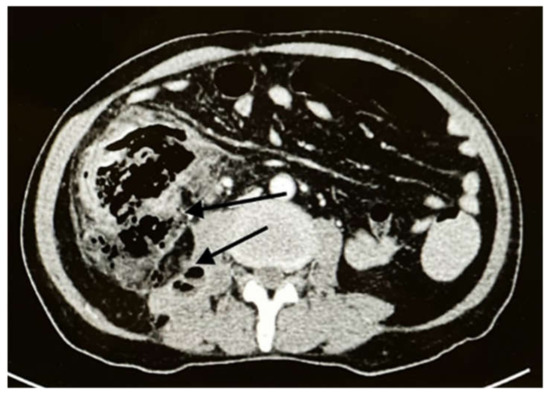
Figure 2.
Axial CT with IV contrast showed cecal perforation with free air around the colon (black arrows).
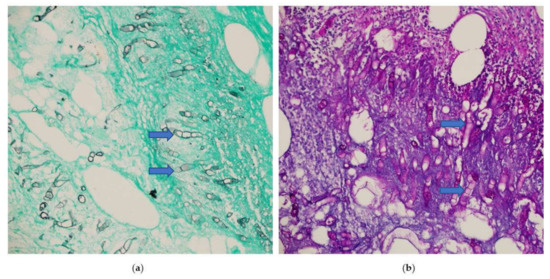
Figure 3.
Microscopic sections of the colonic specimen, (a) Grocott methenamine silver (blue arrow) (GMS), (b) periodic acid-Schiff (PAS) stains shows fungal hyphae that look large, broad, and irregular with sparse septa and thin walls (blue arrow). (Magnification ×400).
Two weeks later, the patient developed a fever and right-side abdominal pain. Laboratory tests revealed the following: WBC count, 17,230/mcl; neutrophil count, 11,350/mcl (65.9%); eosinophil count 2540/mcl (14.7%); and Hb, 10.8 g/dL. Percutaneous CT-guided drainage was used to drain the abdominal collections. The pleural effusion was aspirated, and percutaneous liver abscess drainage was attempted but failed due to the thick nature of the abscess. Another antifungal medication, itraconazole (200 mg PO. BID), was administered alongside voriconazole (200 mg IV BID) to control sepsis. The patient improved and was discharged from the hospital.
Two months later, the patient presented with fever and right upper abdominal pain despite being treated with oral voriconazole (200 mg BID). Upon examination, there was tenderness in the right upper abdomen with low-grade fever. A CT scan of the abdomen showed a liver abscess in segments six and seven (3 × 4 cm and 4 × 2 cm respectively) (Figure 4). Intravenous amphotericin B (225 mg Q24 h) was administered to the patient. Another attempt at percutaneous CT-guided liver drainage failed to drain the abscess. As a result, segments six and seven of the liver were resected segmentally. The patient made a full recovery and was discharged on long-term voriconazole treatment.
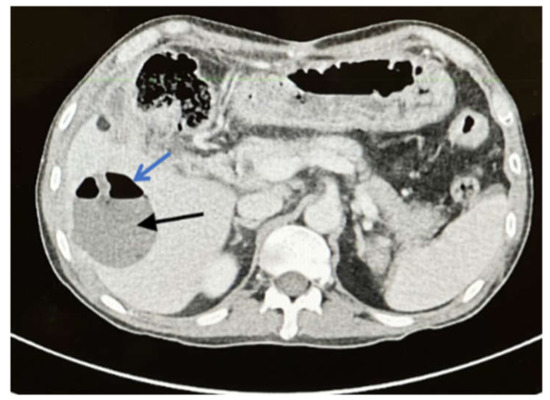
Figure 4.
Axial CT scan with delayed contrast phase shows 4 × 3 cm hypo-dense lesion (black arrow) with air fluid level (blue arrow) in segment six which is characteristic of liver abscess.
3. Discussion
Basidiobolomycosis is a rare fungal infection caused by Basidiobolus ranarum (B. ranarum). It belongs to the family Zygomycetes. The Zygomycetes family includes two orders, Mucorales and Entomophthorales. Mucorales cause infection in immunocompromised patients such as diabetics and post-transplants. Entomophthorales, which include Basiodiobolus, cause infection in immunocompetent individuals [3].
Basiodiobolus spp are filamentous fungi that were first identified in 1886 in frogs [4]. They are an environmental saprophyte found in soil and decaying vegetable materials and are occasionally found as commensals in the gastrointestinal tract of amphibians, reptiles, fish, dogs, frogs, and bats.
In 1956, the first case of B. ranarum infection in a human (a subcutaneous infection) was reported in Indonesia [1]. Since then, a few cases of subcutaneous, nose, and sinus infections have been reported. Most of the reported cases are from the warmer climates of tropical and subtropical regions, with the majority of reports coming from the southern region of Saudi Arabia, Arizona in the USA, and from Iran [5].
Previous patients were mostly male, with ages ranging from one month to 80 years old [6]. Gastrointestinal Basidiobolomycosis (GIB) is rare. It was first reported in 1964, and there have been 174 cases reported to July 2021. Colonic involvement was reported in 111 cases [7], colonic with liver involvement was reported in six cases [8], and colonic perforation was reported in two cases. The exact route of entry is unknown, but the involvement of the intestine indicates that it is due to the ingestion of food contaminated by infected soil. Table 1 provides a summary of the reported case of Basidiobolomycosis with colonic and liver involvement.

Table 1.
Summary of the reported cases of Basidiobolomycosis with colonic and liver diseases.
The most common presenting symptoms of GIB are abdominal pain and fever; however, some reported cases presented with colonic obstruction [13], lower GI bleeding, abdominal mass (retroperitoneal or colonic), and colonic perforation (the symptoms in most cases mimicked colonic cancer (abdominal pain, abdominal mass, weight loss), or inflammatory bowel disease (abdominal pain, fever, vomiting, and diarrhea); thus, it is usually misdiagnosed [14].
Leukocytosis, eosinophilia, and anemia were present in most of the cases. Radiological investigation in most of the patients revealed either gastrointestinal wall thickening resembling inflammatory bowel disease, or a colonic mass that mimicked colonic cancer, with or without a concomitant liver mass.
Endoscopy and biopsy are inconclusive in nearly all reported cases. Therefore, a mucosal biopsy is usually performed, and B. ranarum grows in the submucosa, so the histological examination of the biopsy commonly reveals inflammatory changes with eosinophilic infiltration and, in some cases, the presence of granulomas.
All reported cases were diagnosed either by excisional biopsy of tissue from the mass or histological examination of the resected segment of the colon, which is considered the gold standard for diagnosis. Histological diagnosis usually reveals the diagnostic features of this fungal infection, namely the presence of fungal hyphae with Splendore–Hoeppli bodies, granuloma with abundant eosinophilia, and intensely radiating eosinophilic granular material. A few reported cases were diagnosed by polymerase chain reaction (PCR) and molecular methods such as DNA sequencing of samples obtained from equivocal tissue specimens.
Treatment with antifungal therapy without surgical resection of the affected part has been successfully reported in a few cases [15]. In these cases, antifungal therapy was administered for an extended period. In most cases, antifungal therapy was administered for 6 months or more. Mortality from GIB is rare and usually in pediatric patients, except for two reported cases of a 61-year-old and 18-year-old with disseminated disease, they died despite antifungal therapy and surgery [7,9]. Awareness of the disease amongst physicians and pathologists would contribute to the successful treatment of more recent cases and would result in a significant reduction in the disease’s morbidity and mortality rates. Further research on the risk factors that lead to GIB are highly recommended.
4. Conclusions
GIB can be associated with a variety of signs and symptoms, and it can affect people of all ages. Most GIB cases presented with colonic involvement, few with liver involvement, and very rare cases presented with colonic perforation and concomitant liver involvement, as in this study. The diagnosis of GIB requires a high index of suspicion, and we recommend its inclusion in the differential diagnosis of abdominal masses associated with fever, weight loss, and eosinophilia, especially in endemic regions.
Author Contributions
Study concept and design, M.S.A., S.M.A. and J.A.; methodology, M.S.A., S.M.A. and M.M.A.; investigation, S.M.A., M.S.A. and H.A.E.; resources, S.M.A., H.A.E. and M.S.A.; data curation, M.S.A., S.M.A. and M.A.A.; writing—original draft preparation, M.S.A., S.M.A., J.A. and M.M.A.; writing—review and editing, S.M.A., M.S.A. and J.A.; visualization, H.A.E. and M.A.A.; supervision, S.M.A. and M.S.A.; project administration, M.S.A. and S.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under Grant no. FP-218-43.
Institutional Review Board Statement
The authors declare that the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national). Biomedical Ethics Research Committee at Faculty of Medicine in King Abdulaziz University, Saudi Arabia (13 January 2022; Reference No 50-22, Ethical Approval Number HA-02-J-008) and with the Helsinki Declaration of 1975, as revised in 2000.
Informed Consent Statement
The patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published. This report does not contain any personal information that could lead to the identification of the patient.
Acknowledgments
Authors acknowledge the DSR’s technical and financial support.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Al-Shanafey, S.; AlRobean, F.; Hussain, I.B. Surgical management of gastrointestinal Basidiobolomycosis in pediatric patients. J. Pediatr. Surg. 2012, 47, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Alsharidah, A.; Mahli, Y.; Alshabyli, N.; Alsuhaibani, M. Invasive Basidiobolomycosis Presenting as Retroperitoneal Fibrosis: A Case Report. Int. J. Environ. Res. Public Health 2020, 17, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohta, A.; Neogi, S.; Das, S. Gastrointestinal mucormycosis in an infant. Indian J. Pathol. Microbiol. 2011, 54, 664. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Hussain, K.; Petraitiene, R.; Schuetz, A.; Walsh, T. Entomophthoramycosis: A neglected tropical mycosis. Clin. Microbiol. Infect. 2016, 22, 688–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethy, M.; Sahu, S.; Sachan, S. Basidiobolomycosis: Case report and literature overview. Indian Dermatol. Online J. 2021, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Albishri, A.; Shoukeer, M.A.; Hader, H.; Ashour, M.H.M.; Alsherbiny, H.; Ghazwani, E.; Alkedassy, K. Gastrointestinal Basidiobolomycosis. J. Pediatr. Surg. Case Rep. 2020, 55, 101411. [Google Scholar] [CrossRef]
- Takrouni, A.O.; Schammut, M.H.; Al-Otaibi, M.; Al-Mulla, M.; Privitera, A. Disseminated intestinal Basidiobolomycosis with mycotic aneurysm mimicking obstructing colon cancer. BMJ Case Rep. CP 2019, 12, e225054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, R.; Chaharsoghi, M.A.; Khorvash, F.; Kaleidari, B.; Sanei, M.; Ahangarkani, F.; Abtahian, Z.; Meis, J.F.; Badali, H. An unusual case of gastrointestinal Basidiobolomycosis mimicking colon cancer; literature and review. J. Mycol. Med. 2019, 29, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Van den Berk, G.E.; Noorduyn, L.A.; van Ketel, R.J.; van Leeuwen, J.; Bemelman, W.A.; Prins, J.M. A fatal pseudo-tumour: Disseminated Basidiobolomycosis. BMC Infect. Dis. 2006, 6, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, M.A.; Al Khuwaitir, T.S.; Attia, T.H. Gastrointestinal Basidiobolomycosis with hepatic dissemination: A case report. JMM Case Rep. 2014, 1, e003269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejtehadi, F.; Anushiravani, A.; Bananzadeh, A.; Geramizadeh, B. Gastrointestinal Basidiobolomycosis accompanied by liver involvement: A case report. Iran. Red Crescent Med. J. 2014, 16, e14109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zekavat, O.R.; Abdolkarimi, B.; Pouladfar, G.; Fathpour, G.; Mokhtari, M.; Shakibazad, N. Colonic Basidiobolomycosis with liver involvement masquerading as gastrointestinal lymphoma: A case report and literature review. Rev. Soc. Bras. Med. Trop. 2017, 50, 712–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Shabrawi, M.H.; Kamal, N.M. Gastrointestinal Basidiobolomycosis in children: An overlooked emerging infection? J. Med. Microbiol. 2011, 60, 871–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shreef, K.; Saleem, M.; Saeedd, M.A.; Eissa, M. Gastrointestinal Basidiobolomycosis: An emerging, and a confusing, disease in children (A multicenter experience). Eur. J. Pediatr. Surg. 2018, 28, 194–199. [Google Scholar] [PubMed]
- Almoosa, Z.; Alsuhaibani, M.; AlDandan, S.; Alshahrani, D. Pediatric gastrointestinal Basidiobolomycosis mimicking malignancy. Med. Mycol. Case Rep. 2017, 18, 31–33. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).