Prevalence of Aflatoxins in Selected Dry Fruits, Impact of Storage Conditions on Contamination Levels and Associated Health Risks on Pakistani Consumers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Aflatoxins Extraction and Immuno-Affinity Cleanup
2.3. Derivatization
2.4. Chromatographic Analysis
2.5. Method Validation
2.6. Aflatoxins Exposure and Health Risk Assessment
2.7. Exposure Assessment
2.8. Health Risk Characterization
2.9. Effect of Storage Conditions on the Levels of Aflatoxins
2.10. Statistical Analysis
3. Results and Discussion
3.1. Aflatoxins Occurrence in Dates, Walnuts, and Pistachios
| Country (Region or City) | Pistachios | Walnuts | Dates | Reference | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 | AFT | AFB1 | AFT | AFB1 | AFT | ||||||||||||||
| Max | Mean | %P | Max | Mean | %P | Max | Mean | %P | Max | Mean | %P | Max | Mean | %P | Max | Mean | %P | ||
| Pakistan (South Pounjab) | 6.73 | 3.32 | 95 | 7.90 | 7.13 | 95 | 9.99 | 5.52 | 95 | 13.73 | 12.76 | 95 | 4.34 | 1.33 | 75 | 5.13 | 2.73 | 75 | This study |
| Pakistan (North Pujab) | - | 5.80 | 50 | 20.70 | 7.10 | 50 | - | - | - | - | - | - | - | 4.80 | 40 | 10.20 | 5.30 | 40 | [36] |
| Pakistan (North Pujab) | 13.67 | 6.47 | 70 | 21.50 | 7.53 | 70 | 11.50 | 4.80 | 45 | 15.78 | 5.43 | 45 | 9.80 | 4.50 | 60 | 18.79 | 6.32 | 60 | [37] |
| Spain | 1037.3 | 21.4 | 10 | 1134.5 | 34.5 | 10 | - | - | - | - | - | - | - | - | - | - | - | - | [56] |
| Italy | 354.5 | 53.3 | 50 | 387.3 | 70.5 | 50 | - | - | - | - | - | - | - | - | - | - | - | - | [57] |
| India | <LOD | - | - | 186.6 * | - | - | - | - | - | - | - | - | - | - | - | - | - | - | [68] |
| Morocco | 1430 | 158 | 45 | 1450 | 163 | 45 | 2500 | 360 | 30 | 4320 | 730 | 30 | - | - | - | - | - | - | [54] |
| Saudi arabia (Mekka) | 411 | - | - | 422 | 16.6 | 34 | 17.4 | - | - | 36.6 | 3.4 | 50 | - | - | - | - | - | - | [50] |
| Saudi arabia (Jeddah) | 4.28 | - | 45.5 | 7.13 | 1.86 | 63.6 | 3.85 | - | 66.7 | 4.39 | 1.16 | 75 | - | - | - | - | - | - | [58] |
| Iran | - | - | - | - | - | - | 2.08 | 0.48 | 2.3 | 38.1 | 14.4 | 90.7 | - | - | - | - | - | - | [55] |
| Iran | - | - | - | - | - | - | - | - | - | - | 6.0 | 2.1 | 40.9 | 8.1 | 2.6 | 40.9 | [28] | ||
| Iran | - | - | - | 5.8 | 1.5 | - | - | - | - | 3.6 | 1.8 | - | - | - | - | - | - | - | [61] |
| Algeria | 8.72 | 4.45 | 87.5 | 13.45 | 6.70 | 87.5 | 6.34 | 3.42 | 75 | 8.76 | 4.90 | 75 | - | - | - | - | - | - | [62] |
| Turkey | - | - | - | - | - | - | 5.06 | 0.86 | 20 | 10.3 | 1.68 | 64 | - | - | - | - | - | - | [60] |
| Turkey | 368 | 4.55 | 14.6 | 385 | 4.95 | 14.6 | - | - | - | - | - | - | - | - | - | - | - | - | [59] |
| Tunisia | - | - | - | - | - | - | - | - | - | - | - | - | ND | - | - | 3.04 | - | 46 | [65] |
| District | Product | Number of Samples | Positive Samples * (%P) | AFB1 > 2 ng/g | AFT > 4 ng/g | AFB1 > 8 ng/g | AFT > 20 ng/g | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||||
| Bahawalpur | Dates | 5 | 4 (80) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pistachios | 5 | 4 (80) | 4 | 80 | 03 | 60 | 0 | 0 | 0 | 0 | |
| Walnuts | 5 | 5 (100) | 4 | 80 | 04 | 80 | 0 | 0 | 0 | 0 | |
| Dera Ghazi Khan | Dates | 5 | 5 (100) | 4 | 80 | 04 | 80 | 0 | 0 | 0 | 0 |
| Pistachios | 5 | 5 (100) | 5 | 100 | 05 | 100 | 0 | 0 | 0 | 0 | |
| Walnuts | 5 | 5 (100) | 5 | 100 | 04 | 80 | 0 | 0 | 0 | 0 | |
| Multan | Dates | 5 | 3 (60) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pistachios | 5 | 4 (80) | 4 | 80 | 03 | 60 | 0 | 0 | 0 | 0 | |
| Walnuts | 5 | 4 (80) | 3 | 60 | 04 | 80 | 3 | 60 | 2 | 40 | |
| Rahim Yar Khan | Dates | 5 | 3 (60) | 1 | 20 | 01 | 20 | 0 | 0 | 0 | 0 |
| Pistachios | 5 | 5 (100) | 3 | 60 | 03 | 60 | 0 | 0 | 0 | 0 | |
| Walnuts | 5 | 5 (100) | 3 | 60 | 04 | 80 | 3 | 60 | 1 | 20 | |
| Overall | 60 | 52 (86.7) | 36 | 60 | 35 | 58.3 | 6 | 10 | 3 | 5 | |
3.2. Exposure Assessment and Health Risk Characterization of Aflatoxins
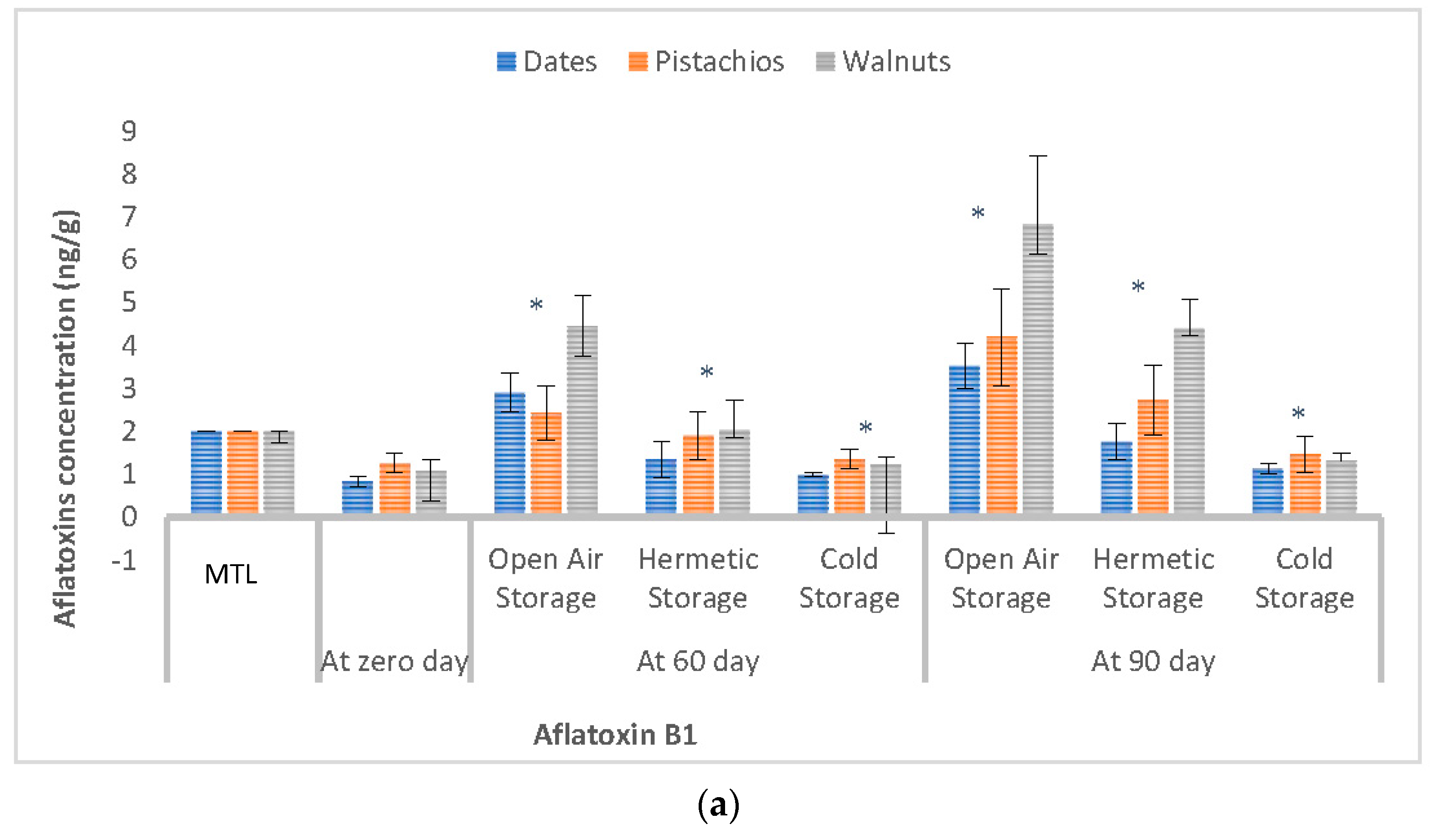
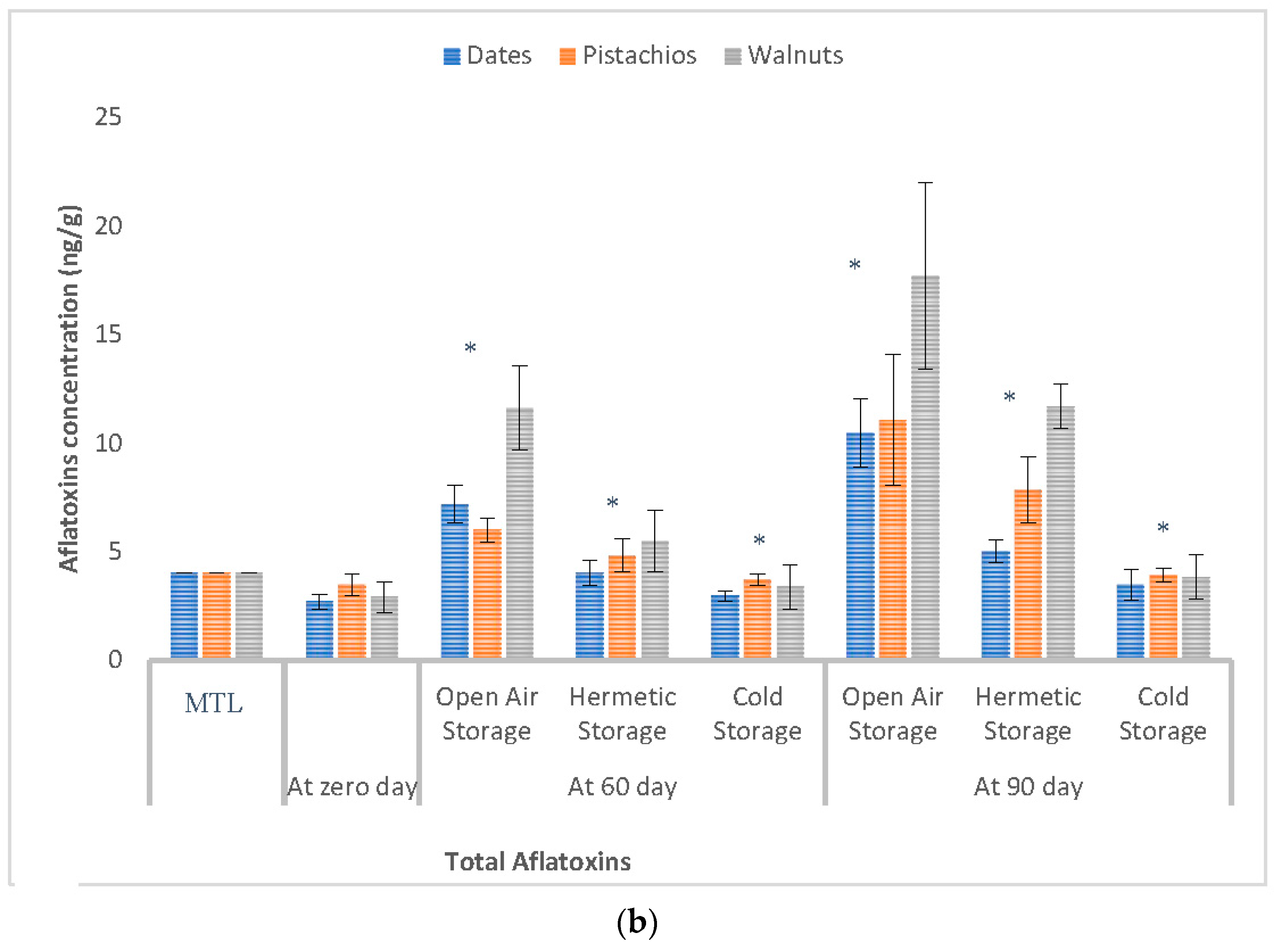
3.3. Effect of Storage Conditions on the Levels of Aflatoxins in Selected Dry Fruits
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, J.W.; Groopman, J.D. Aflatoxins. In Encyclopedia of Cancer, 3rd ed.; Boffetta, P., Hainaut, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 30–43. [Google Scholar]
- Ismail, A.; Gonçalves, B.L.; de Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- IARC. Monographs on the evaluation of carcinogenic risks to humans. In Chemical Agents and Related Occupations. A Review of Human Carcinogens; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100. [Google Scholar]
- Ismail, A.; Naeem, I.; Gong, Y.Y.; Routledge, M.N.; Akhtar, S.; Riaz, M.; Ramalho, L.N.Z.; de Oliveira, C.A.F.; Ismail, Z. Early life exposure to dietary aflatoxins, health impact and control perspectives: A review. Trends Food Sci. Technol. 2021, 112, 212–224. [Google Scholar] [CrossRef]
- Carughi, A.; Feeney, M.J.; Kris-Etherton, P.; Fulgoni, V.; Kendall, C.W.C.; Bulló, M.; Webb, D. Pairing nuts and dried fruit for cardiometabolic health. Nutr. J. 2016, 15, 23. [Google Scholar] [CrossRef]
- Kalogiouri, P.N.; Manousi, N.; Rosenberg, E.; Zachariadis, A.G.; Samanidou, F.V. Advances in the chromatographic separation and determination of bioactive compounds for assessing the nutrient profile of nuts. Curr. Anal. Chem. 2021, 17, 495–511. [Google Scholar] [CrossRef]
- Alasalvar, C.; Salvadó, J.-S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, T.; Barreca, D.; Laganà, G.; Mandalari, G. Health Benefits Related to Tree Nut Consumption and Their Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 5960. [Google Scholar] [CrossRef]
- Sullivan, V.K.; Na, M.; Proctor, D.N.; Kris-Etherton, P.M.; Petersen, K.S. Consumption of dried fruits Is associated with greater intakes of underconsumed nutrients, higher total energy intakes, and better diet quality in US adults: A cross-sectional analysis of the national health and nutrition examination survey, 2007–2016. J. Acad. Nutr. Diet. 2021, 121, 1258–1272. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on the substantiation of health claims related to walnuts and maintenance of normal blood LDL-cholesterol concentrations (ID 1156, 1158) and improvement of endothelium-dependent vasodilation (ID 1155, 1157) pursuant to Article 13 (1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2074. [Google Scholar]
- FDA. Qualified Health Claims: Letter of Enforcement Discretion-Nuts and Coronary Heart Disease (Docket No 02P-0505). 2003. Available online: http://wayback.archive-it.org/7993/20171114183724/https://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm072926.htm (accessed on 1 January 2022).
- Institute for Health Metrics and Evaluation. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Ait Mimoune, N.; Arroyo-Manzanares, N.; Gámiz-Gracia, L.; García-Campaña, A.M.; Bouti, K.; Sabaou, N.; Riba, A. Aspergillus section Flavi and aflatoxins in dried figs and nuts in Algeria. Food Addit. Contam Part B 2018, 11, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xie, L.; Xu, H. Determination of toxigenic fungi and aflatoxins in nuts and dried fruits using imaging and spectroscopic techniques. Food Chem. 2018, 252, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Benkerroum, N. Aflatoxins: Producing-molds, structure, health issues and incidence in Southeast Asian and Sub-Saharan African countries. Int. J. Environ. Res. Public Health 2020, 17, 1215. [Google Scholar] [CrossRef] [PubMed]
- Golge, O.; Hepsag, F.; Kabak, B. Determination of aflatoxins in walnut sujuk and Turkish delight by HPLC-FLD method. Food Control 2016, 59, 731–736. [Google Scholar] [CrossRef]
- Iqbal, A.; Khalil, I.A.; Shah, H. Aflatoxin contents of stored and artificially inoculated cereals and nuts. Food Chem. 2006, 98, 699–703. [Google Scholar]
- EFSA. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. EFSA J. 2007, 5, 446. [Google Scholar]
- Basaran, P.; Ozcan, M. Occurrence of aflatoxins in various nuts commercialized in Turkey. J. Food Saf. 2009, 29, 95–105. [Google Scholar] [CrossRef]
- FAO/WHO. General Standard for Contaminants and Toxins in Food and Feed. Available online: http://www.fao.org/fao-who-codexalimentarius/thematic-areas/contaminants/en/ (accessed on 2 January 2022).
- NZFSA. Mycotoxin Surveillance Programme 2008–09 Aflatoxins and Ochratoxin A in Dried Fruits and Spices. 2009. Available online: https://www.mpi.govt.nz/dmsdocument/12912/direct (accessed on 31 December 2021).
- PFA. Punjab Pure Food Rules 2018. Lahore, Punjab; Punjab Food Authority: Punjab, Pakistan, 2018. [Google Scholar]
- Statista. Nuts Industry Worldwide, Statistics and Facts. 2021. Available online: https://www.statista.com/topics/5954/nut-industry-worldwide/ (accessed on 2 January 2022).
- FAO. FAOSTAT. 2019. Available online: https://www.fao.org/faostat/en/#data (accessed on 2 January 2022).
- Wamucii, S. Pakistan Market Trends and Insights. 2019. Available online: https://www.selinawamucii.com/insights/market/pakistan/nuts/ (accessed on 31 December 2021).
- Fatima, G.; Khan, I.A.; Buerkert, A. Socio-economic characterisation of date palm (Phoenix dactylifera L.) growers and date value chains in Pakistan. SpringerPlus 2016, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Diarra, M.; Amoah, R.S. Physical factors in the hermetic SuperGrainBag® and effect on the larger grain borer [Prostephanus truncatus (Horn)(Coleoptera: Bostrichidae)] and aflatoxin production by Aspergillus flavus during the storage of ‘Obatanpa’maize (Zea mays L.) variety. J. Stored Prod. Res. 2019, 83, 84–91. [Google Scholar] [CrossRef]
- Heshmati, A.; Zohrevand, T.; Khaneghah, A.M.; Nejad, A.S.M.; Sant’Ana, A.S. Co-occurrence of aflatoxins and ochratoxin A in dried fruits in Iran: Dietary exposure risk assessment. Food Chem. Toxicol. 2017, 106, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Khan, M.; Mehmood, Z.; Saeed, M.; Kalim, I.; Ahmad, I.; Malik, K. Determination of aflatoxins in spices and dried fruits. J. Sci. Res. 2018, 10, 315–321. [Google Scholar] [CrossRef]
- Al Ghamdi, F.L.; Bokhari, F.M.; Aly, M.M. Toxigenic fungi associated with dried fruits and fruit-based products collected from Jeddah province. J. Pharm. Biol. Sci. 2019, 14, 10–20. [Google Scholar]
- Kang, Y.-W.; Cho, T.-Y.; Park, H.-R.; Oh, K.-S.; Kim, D.-S. Analysis of total aflatoxins in spices and dried fruits. J. Food Hyg. Saf. 2010, 25, 65–72. [Google Scholar]
- Wang, Y.-J.; Nie, J.-Y.; Zhen, Y.; LI, Z.-X.; Cheng, Y.; Farooq, S. Multi-mycotoxin exposure and risk assessments for Chinese consumption of nuts and dried fruits. J. Integr. Agric. 2018, 17, 1676–1690. [Google Scholar] [CrossRef]
- Ali, S.; Ali, A.; Sartaj, A.; Ali, M.; Amjad, A. Natural occurrence of aflatoxin B1 In dry fruits of Gilgit-Baltistan, Pakistan. Fresenius Environ. Bull. 2020, 29, 2818–2822. [Google Scholar]
- Asghar, M.A.; Ahmed, A.; Zahir, E.; Asghar, M.A.; Iqbal, J.; Walker, G. Incidence of aflatoxins contamination in dry fruits and edible nuts collected from Pakistan. Food Control 2017, 78, 169–175. [Google Scholar] [CrossRef]
- Luttfullah, G.; Hussain, A. Studies on contamination level of aflatoxins in some dried fruits and nuts of Pakistan. Food Control 2011, 22, 426–429. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Mehmood, Z.; Asi, M.R.; Shahid, M.; Sehar, M.; Malik, N. Co-occurrence of aflatoxins and ochratoxin A in nuts, dry fruits, and nuty products. J. Food Saf. 2018, 38, e12462. [Google Scholar] [CrossRef]
- Masood, M.; Iqbal, S.Z.; Asi, M.R.; Malik, N. Natural occurrence of aflatoxins in dry fruits and edible nuts. Food Control 2015, 55, 62–65. [Google Scholar] [CrossRef]
- Saqib, F.; Gill, I.M. A historical analysis of emperature and rainfall patterns of Punjab—Pakistan. Pak. Geo. Rev. 2019, 74, 74–89. [Google Scholar]
- Javed, A.; Naeem, I.; Benkerroum, N.; Riaz, M.; Akhtar, S.; Ismail, A.; Sajid, M.; Tayyab Khan, M.; Ismail, Z. Occurrence and health risk assessment of aflatoxins through intake of Eastern herbal medicines collected from four districts of Southern Punjab—Pakistan. Int. J. Environ. Res. Public Health 2021, 18, 9531. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Analysis of Aflatoxin Liquid Chromatography with Post-Column Photochemical Derivatization; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- FAO/WHO. Safety evaluation of certain food additives and contaminants. In Safety Evaluation of Certain Food Additives: Sixty-Eighth Meeting of the Joint FAO/WHO Expert Committee on Food Additive: Sixty-Eighth Meeting of the Joint FAO/WHO Expert Committee on Food Additives; WHO, Ed.; WHO: Geneva, Switzerland, 2008; Volume 59, pp. 305–380. [Google Scholar]
- EFSA. Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to ochratoxin A in food. EFSA J. 2006, 4, 365. [Google Scholar] [CrossRef]
- Wang, X.; Lien, K.-W.; Ling, M.-P. Probabilistic health risk assessment for dietary exposure to aflatoxin in peanut and peanut products in Taiwan. Food Control 2018, 91, 372–380. [Google Scholar] [CrossRef]
- Saha Turna, N.; Wu, F. Estimation of Tolerable Daily Intake (TDI) for Immunological Effects of Aflatoxin. Available online: https://pubmed.ncbi.nlm.nih.gov/34147038/ (accessed on 28 January 2022).
- Tangendjaja, B.; Rachmawati, S.; Wina, E. Mycotoxin contamination on corn used by feed mills in indonesia. Indones. J. Agric. Sci. 2008, 9, 68–76. [Google Scholar] [CrossRef]
- Duman, A.D. Storage of red chili pepper under hermetically sealed or vacuum conditions for preservation of its quality and prevention of mycotoxin occurrence. J. Stored Prod. Res. 2010, 46, 155–160. [Google Scholar] [CrossRef]
- Namjoo, M.; Salamat, F.; Rajabli, N.; Hajihoseeini, R.; Niknejad, F.; Kohsar, F.; Joshaghani, H. Quantitative determination of aflatoxin by high performance liquid chromatography in wheat silos in Golestan province, North of Iran. Iran J. Public Health 2016, 45, 905–910. [Google Scholar] [PubMed]
- Pearson, S.M.; Candlish, A.A.G.; Aidoo, K.E.; Smith, J.E. Determination of aflatoxin levels in pistachio and cashew nuts using immunoaffinity column clean-up with HPLC and fluorescence detection. Biotechnol. Tech. 1999, 13, 97–99. [Google Scholar] [CrossRef]
- Abdullah AlFaris, N.; Zaidan Altamimi, J.; Alothman, Z.A.; Fahad Al Qahtani, S.; Wabaidur, S.M.; Ghfar, A.A.; Saleh Aldayel, T. Analysis of aflatoxins in foods retailed in Saudi Arabia using immunoaffinity column cleanup and high-performance liquid chromatography-fluorescence detection. J. King Saud Univ. Sci. 2020, 32, 1437–1443. [Google Scholar] [CrossRef]
- El tawila, M.M.; Neamatallah, A.; Serdar, S.A. Incidence of aflatoxins in commercial nuts in the holy city of Mekkah. Food Control 2013, 29, 121–124. [Google Scholar] [CrossRef]
- Rahmani, A.; Jinap, S.; Soleimany, F. Validation of the procedure for the simultaneous determination of aflatoxins ochratoxin A and zearalenone in cereals using HPLC-FLD. Food Addit. Contam. Part A 2010, 27, 1683–1693. [Google Scholar] [CrossRef]
- FAO/WHO. Evaluation of Certain Food Additives and Contaminants: Sixty-Eighth Report of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; Nebbia, C.; et al. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, e06040. [Google Scholar]
- Juan, C.; Zinedine, A.; Molto, J.; Idrissi, L.; Manes, J. Aflatoxins levels in dried fruits and nuts from Rabat-Salé area, Morocco. Food Control 2008, 19, 849–853. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Ashrafnejad, F.; Kazemi, A.; Sargheini, N.; Mahdavi, R.; Farshchian, M.; Mahluji, S. Aflatoxin in raw and salt-roasted nuts (pistachios, peanuts and walnuts) sold in markets of Tabriz, Iran. Jundishapur J. Microbiol. 2014, 7, e8674. [Google Scholar] [CrossRef] [PubMed]
- Fernane, F.; Cano-Sancho, G.; Sanchis, V.; Marín, S.; Ramos, A. Aflatoxins and ochratoxin A in pistachios sampled in Spain: Occurrence and presence of mycotoxigenic fungi. Food Add. Contam. 2010, 3, 185–192. [Google Scholar] [CrossRef]
- Diella, G.; Caggiano, G.; Ferrieri, F.; Ventrella, A.; Palma, M.; Napoli, C.; Rutigliano, S.; Lopuzzo, M.; Lovero, G.; Montagna, M. Aflatoxin contamination in nuts marketed in Italy: Preliminary results. Ann. Ig. 2018, 30, 401–409. [Google Scholar]
- Elzupir, A.O. Seasonal variation and health implications due to aflatoxins in nuts sold in Riyadh region. Rev. Fr. Allergol. 2019, 59, 15–21. [Google Scholar] [CrossRef]
- Hepsag, F.; Golge, O.; Kabak, B. Quantitation of aflatoxins in pistachios and groundnuts using HPLC-FLD method. Food Control 2014, 38, 75–81. [Google Scholar] [CrossRef]
- Yilmaz, S.Ö. The contamination rate of aflatoxins in ground red peppers, dried figs, walnuts without shell and seedless black raisins commercialized in Sakarya City Center, Turkey. Ital. J. Food Sci. 2017, 29, 591–598. [Google Scholar]
- Rezaei, M.; Karimi, F.; Parviz, M.; Behzadi, A.A.; Javadzadeh, M.; Mohammadpourfard, I.; Fallahzadeh, R.A.; Aghamirlou, H.M.; Malekirad, A.A. An empirical study on aflatoxin occurrence in nuts consumed in Tehran, Iran 2013. Health 2014, 6, 649–653. [Google Scholar] [CrossRef]
- Riba, A.; Matmoura, A.; Mokrane, S.; Mathieu, F.; Sabaou, N. Investigations on aflatoxigenic fungi and aflatoxins contamination in some nuts sampled in Algeria. Afr. J. Microbiol. Res. 2013, 7, 4974–4980. [Google Scholar]
- Ozer, H.; Oktay Basegmez, H.I.; Ozay, G. Mycotoxin risks and toxigenic fungi in date, prune and dried apricot among Mediterranean crops. Phytopathol. Mediterr. 2012, 51, 148–157. [Google Scholar]
- CFIA. Food safety action plan. Report 2009–2010 targeted surveys chemistry. In Aflatoxin in Dried Figs and Dried Dates; TS-CHEM-09/10-01; Canadian Food Inspection Agency: Ottawa, ON, Canada, 2012; p. 11. Available online: https://inspection.canada.ca/food-safety-for-industry/food-chemistry-and-microbiology/food-safety-testing-bulletin-and-reports/aflatoxins/eng/1348158204498/1348159550976 (accessed on 4 January 2022).
- Azaiez, I.; Font, G.; Mañes, J.; Fernández-Franzón, M. Survey of mycotoxins in dates and dried fruits from Tunisian and Spanish markets. Food Control 2015, 51, 340–346. [Google Scholar] [CrossRef]
- Abdel-Sater, M.A.; Saber, S.M. Mycoflora and mycotoxins of some Egyptian dried fruits. Bull. Fac. Sci. D Bot. Assiut. 1999, 28, 91–107. [Google Scholar]
- Zohri, A.A.; Abdel-Gawad, K.M. Survey of mycoflora and mycotoxins of some dried fruits in Egypt. J. Basic. Microbiol. 1993, 33, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Pandey, A.K.; Mishra, R.P. Qualitative and quantitative analysis of aflatoxins in dry fruits and nuts from central India. Def. Life Sci. J. 2020, 5, 278–282. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.F.; Rezaee, R.; Davarynejad, G.; Asili, J.; Nemati, S.H.; Goumenou, M.; Tsakiris, I.; Tsatsakis, A.M.; Shirani, K.; Karimi, G. Risk assessment of exposure to aflatoxin B1 and ochratoxin A through consumption of different pistachio (Pistacia vera L.) cultivars collected from four geographical regions of Iran. Environ. Toxicol. Pharmacol. 2018, 61, 61–66. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T. Food Safety: Mycotoxins and Phycotoxins in Perspective. Mycotoxins and Phycotoxins—Developments in Chemistry, Toxicology and Food Safety; Alaken Inc.: Fort Collins, CO, USA, 1998; pp. 25–48. [Google Scholar]
- Ali, M.; Idrees, M.; Ali, L.; Hussain, A.; Ur Rehman, I.; Saleem, S.; Afzal, S.; Butt, S. Hepatitis B virus in Pakistan: A systematic review of prevalence, risk factors, awareness status and genotypes. Virol. J. 2011, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, M.C.; Alika, O.P.; Awa, N.P.; Atanda, O.O.; Mwanza, M. Microbiological quality and risk assessment for aflatoxins in groundnuts and roasted cashew nuts meant for human consumption. J. Toxicol. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Navarro, H.; Navarro, S.; Finkelman, S. Hermetic and modified atmosphere storage of shelled peanuts to prevent free fatty acid and aflatoxin formation. Integr. Prot. Stored Prod. IOBC-WPRS Bull. 2012, 81, 183–192. [Google Scholar]
- Adetunji, M.; Atanda, O.; Ezekiel, C.N.; Sulyok, M.; Warth, B.; Beltrán, E.; Krska, R.; Obadina, O.; Bakare, A.; Chilaka, C.A. Fungal and bacterial metabolites of stored maize (Zea mays, L.) from five agro-ecological zones of Nigeria. Mycot. Res. 2014, 30, 89–102. [Google Scholar] [CrossRef]
- Walker, S.; Jaime, R.; Kagot, V.; Probst, C. Comparative effects of hermetic and traditional storage devices on maize grain: Mycotoxin development, insect infestation and grain quality. J. Stored Prod. Res. 2018, 77, 34–44. [Google Scholar] [CrossRef]
- Galván, A.I.; Rodríguez, A.; Martín, A.; Serradilla, M.J.; Martínez-Dorado, A.; Córdoba, M.d.G. Effect of temperature during drying and storage of dried figs on growth, gene expression and aflatoxin production. Toxins 2021, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Alsuhaibani, A. Effects of storage periods and temperature on mold prevalence and aflatoxin contamination in nuts. Pak. J. Nutr. 2018, 17, 219–227. [Google Scholar] [CrossRef][Green Version]
- Peromingo, B.; Rodríguez, A.; Bernáldez, V.; Delgado, J.; Rodríguez, M. Effect of temperature and water activity on growth and aflatoxin production by Aspergillus flavus and Aspergillus parasiticus on cured meat model systems. Meat. Sci. 2016, 122, 76–83. [Google Scholar] [CrossRef]
- Mousa, W.; Ghazali, F.; Jinap, S.; Ghazali, H.; Radu, S. Modelling the effect of water activity and temperature on growth rate and aflatoxin production by two isolates of Aspergillus flavus on paddy. J. Appl. Microbiol. 2011, 111, 1262–1274. [Google Scholar] [CrossRef]
| Aflatoxin | % Recovery (±RSD) * | LOD (ng/g) | LOQ (ng/g) | ||
|---|---|---|---|---|---|
| Dates | Walnuts | Pistachios | |||
| AFB1 | 92.6 (±7.6) | 95.7 (±5.2) | 96.4 (±5.5) | 0.07 | 0.21 |
| AFB2 | 94.8(±6.6) | 93.9 (±3.7) | 91.5 (±6.7) | 0.03 | 0.09 |
| AFG1 | 89.7 (±8.4) | 86.6 (±7.1) | 88.7 (±5.4) | 0.07 | 0.21 |
| AFG2 | 87.8 (±5.8) | 85.7 (±4.5) | 84.4 (±4.9) | 0.03 | 0.09 |
| Punjabi Districts | Product | AFB1 * | AFB2 * | AFG1 * | AFG2 * | AFT * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | Mean (± SD) | %P ** | Max | Mean (± SD) | %P | Max | Mean (± SD) | %P | Max | Mean (± SD) | %P | Max | Mean (± SD) | %P | ||
| Bahawalpur | Dates | 1.45 | 0.81 (±0.61) a,g | 80 | 0.85 | 0.20 (±0.37) d | 40 | 2.03 | 0.79 (±0.83) g | 80 | 0.18 | 0.06 (±0.08) j | 40 | 3.32 | 1.87 (±1.35) l | 80 |
| Pistachios | 5.67 | 2.97 (±2.06) b | 80 | 4.85 | 1.59 (±2.27) e,k | 40 | 2.98 | 1.25 (±1.36) h,j | 60 | 2.45 | 1.43 (±1.01) k | 80 | 13.8 | 7.25 (±5.65) m | 80 | |
| Walnuts | 7.62 | 5.31 (±2.95) c | 100 | 4.52 | 2.81 (±1.89) f | 80 | 4.59 | 2.32 (±1.71) i | 100 | 3.65 | 1.97 (±1.57) l,h | 80 | 19.67 | 12.40 (±7.80) n | 100 | |
| Dera Ghazi Khan | Dates | 3.98 | 2.83 (±0.88) a | 100 | 0.92 | 0.39 (±0.46) d,j | 60 | 1.98 | 1.46 (±0.31) g | 100 | 1.11 | 0.44 (±0.51) j | 60 | 7.2 | 5.13 (±1.40) o | 100 |
| Pistachios | 6.73 | 4.05 (±1.70) b | 100 | 1.24 | 0.49 (±0.67) e | 40 | 33.76 | 2.06 (±1.06) h,k | 100 | 2.45 | 1.29 (±0.89) k | 80 | 13.39 | 7.90 (±3.16) m | 100 | |
| Walnuts | 6.98 | 4.44 (±1.82) c | 100 | 3.78 | 2.35 (±1.61) f,i | 80 | 55.12 | 2.96 (±1.46) i | 3.44 | 1.68 (±1.23) l | 80 | 17.85 | 11.44 (±5.40) n | 100 | ||
| Multan | Dates | 1.12 | 0.64 (±0.59) a,g | 60 | 0.85 | 0.17 (±0.38) d,i | 20 | 1.09 | 0.49 (±0.53) g | 60 | 0.84 | 0.17 (±0.38) j | 20 | 3.02 | 1.47 (±1.39) l | 60 |
| Pistachios | 6.01 | 3.52 (±2.51) b | 80 | 4.85 | 1.59 (±2.27) e | 40 | 3.02 | 1.17 (±1.21) h,k | 80 | 3.15 | 1.03 (±1.47) k | 40 | 13.72 | 7.32 (±5.74) m | 80 | |
| Walnuts | 9.99 | 6.12 (±4.81) c | 80 | 4.52 | 2.81 (±1.89) f,h | 80 | 5.63 | 2.73 (±2.23) i | 80 | 4.63 | 2.06 (±1.73) l | 80 | 24.2 | 13.73 (±10.02) n | 80 | |
| Rahim Yar Khan | Dates | 4.34 | 1.04 (±1.86) a | 60 | 1.43 | 0.36 (±0.61) d | 60 | 2.09 | 0.52 (±0.89) g | 60 | 1.76 | 0.52 (±0.78) j,g | 40 | 9.62 | 2.44 (±4.08) l | 60 |
| Pistachios | 4.98 | 2.72 (±1.33) b | 100 | 2.12 | 1.04 (±0.76) e,k | 80 | 3.24 | 1.23 (±1.29) h | 80 | 3.15 | 1.03 (±1.47) k | 40 | 10.34 | 6.03 (±3.17) m | 100 | |
| Walnuts | 9.99 | 6.22 (±4.67) c | 100 | 4.52 | 2.81 (±1.89) f,i | 80 | 4.87 | 2.73 (±1.95) i | 80 | 3.45 | 1.70 (±1.29) l | 80 | 22.26 | 13.47 (±9.59) n | 100 | |
| Overall *** | 9.99 | 3.39 (±2.96) | 86.7 | 4.85 | 1.39 (±1.68) | 58.3 | 5.63 | 1.63 (±1.48) | 81.7 | 4.63 | 1.12 (±1.23) | 60 | 24.2 | 7.54 (±6.68) | 86.7 | |
| Product | Mean Concentration (ng/g) * ±SD | Average CR (g/Person/Day) ± SD | EDI (ng/kg bw/Day) | MoE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AFB1 | AFT | AFB1 | AFT | |||||||||
| AFB1 | TAF | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| Dates | 1.33 ± 1.36 | 2.73 ± 2.62 | 50 ± 6.19 | 40 ± 7.59 | 0.98 | 0.96 | 2.00 | 1.98 | 174.14 | 176.5 | 84.83 | 85.99 |
| Pistachios | 3.32 ± 1.87 | 7.13 ± 4.29 | 1.67 ± 0.32 | 0.99 ± 0.46 | 0.08 | 0.06 | 0.17 | 0.13 | 2088.7 | 2857.1 | 972.56 | 1330.39 |
| Walnuts | 5.52 ± 3.54 | 12.76 ± 7.76 | 4.56 ± 0.72 | 3.43 ± 0.77 | 0.37 | 0.34 | 0.85 | 0.79 | 460.1 | 496.0 | 199.02 | 214.56 |
| Overall | 3.39 ± 2.96 | 7.54 ± 6.65 | 18.7 ± 22.4 | 14.8 ± 18.4 | 0.93 | 0.91 | 2.07 | 2.02 | 182.80 | 186.81 | 82.13 | 84.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naeem, I.; Ismail, A.; Rehman, A.U.; Ismail, Z.; Saima, S.; Naz, A.; Faraz, A.; de Oliveira, C.A.F.; Benkerroum, N.; Aslam, M.Z.; et al. Prevalence of Aflatoxins in Selected Dry Fruits, Impact of Storage Conditions on Contamination Levels and Associated Health Risks on Pakistani Consumers. Int. J. Environ. Res. Public Health 2022, 19, 3404. https://doi.org/10.3390/ijerph19063404
Naeem I, Ismail A, Rehman AU, Ismail Z, Saima S, Naz A, Faraz A, de Oliveira CAF, Benkerroum N, Aslam MZ, et al. Prevalence of Aflatoxins in Selected Dry Fruits, Impact of Storage Conditions on Contamination Levels and Associated Health Risks on Pakistani Consumers. International Journal of Environmental Research and Public Health. 2022; 19(6):3404. https://doi.org/10.3390/ijerph19063404
Chicago/Turabian StyleNaeem, Iqra, Amir Ismail, Awais Ur Rehman, Zubair Ismail, Shehzadi Saima, Ambreen Naz, Asim Faraz, Carlos Augusto Fernandes de Oliveira, Noreddine Benkerroum, Muhammad Zahid Aslam, and et al. 2022. "Prevalence of Aflatoxins in Selected Dry Fruits, Impact of Storage Conditions on Contamination Levels and Associated Health Risks on Pakistani Consumers" International Journal of Environmental Research and Public Health 19, no. 6: 3404. https://doi.org/10.3390/ijerph19063404
APA StyleNaeem, I., Ismail, A., Rehman, A. U., Ismail, Z., Saima, S., Naz, A., Faraz, A., de Oliveira, C. A. F., Benkerroum, N., Aslam, M. Z., & Aslam, R. (2022). Prevalence of Aflatoxins in Selected Dry Fruits, Impact of Storage Conditions on Contamination Levels and Associated Health Risks on Pakistani Consumers. International Journal of Environmental Research and Public Health, 19(6), 3404. https://doi.org/10.3390/ijerph19063404








