Backward Walking Training Impacts Positive Effect on Improving Walking Capacity after Stroke: A Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Selection Strategy
2.2. Study Selection
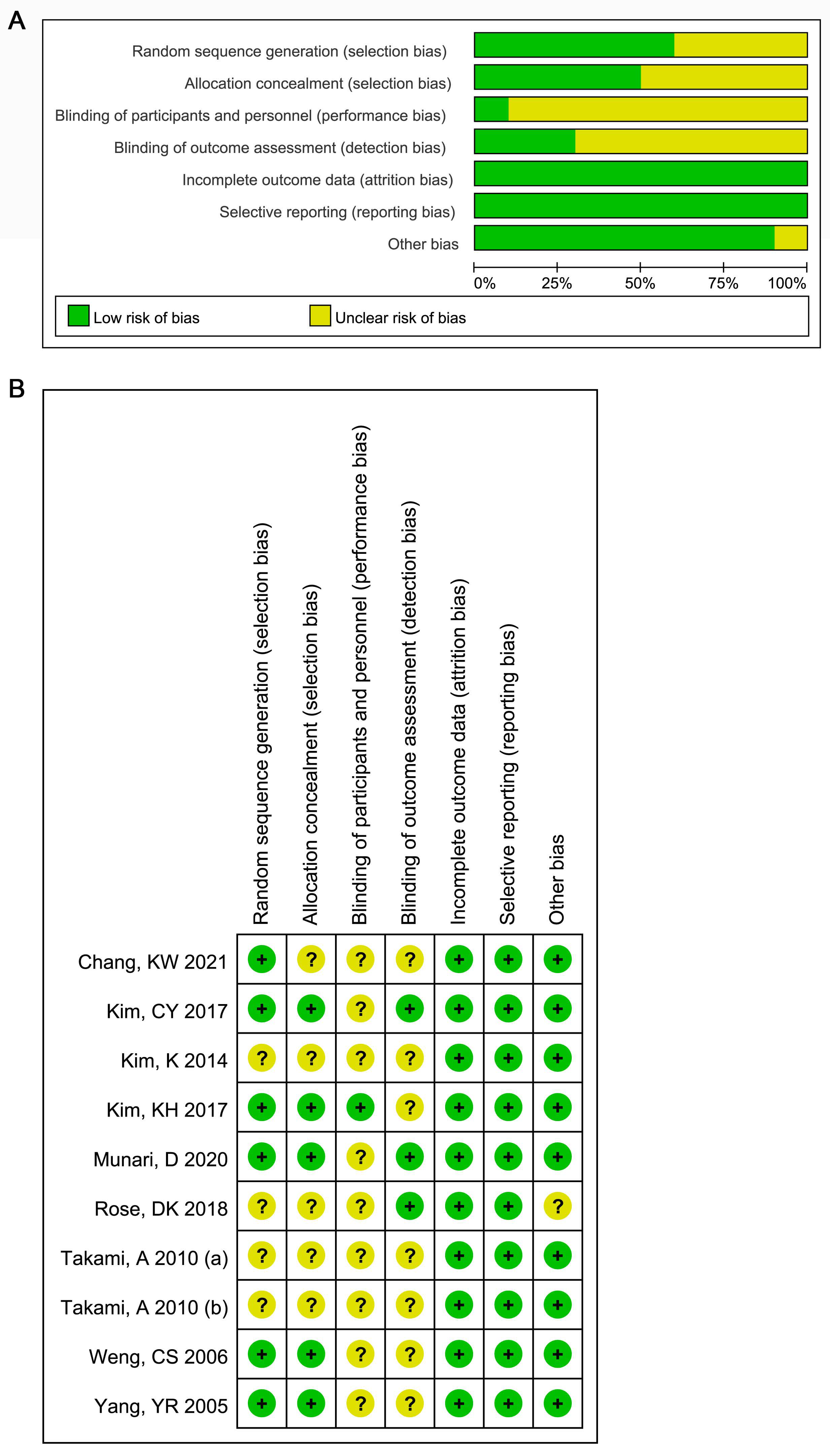
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
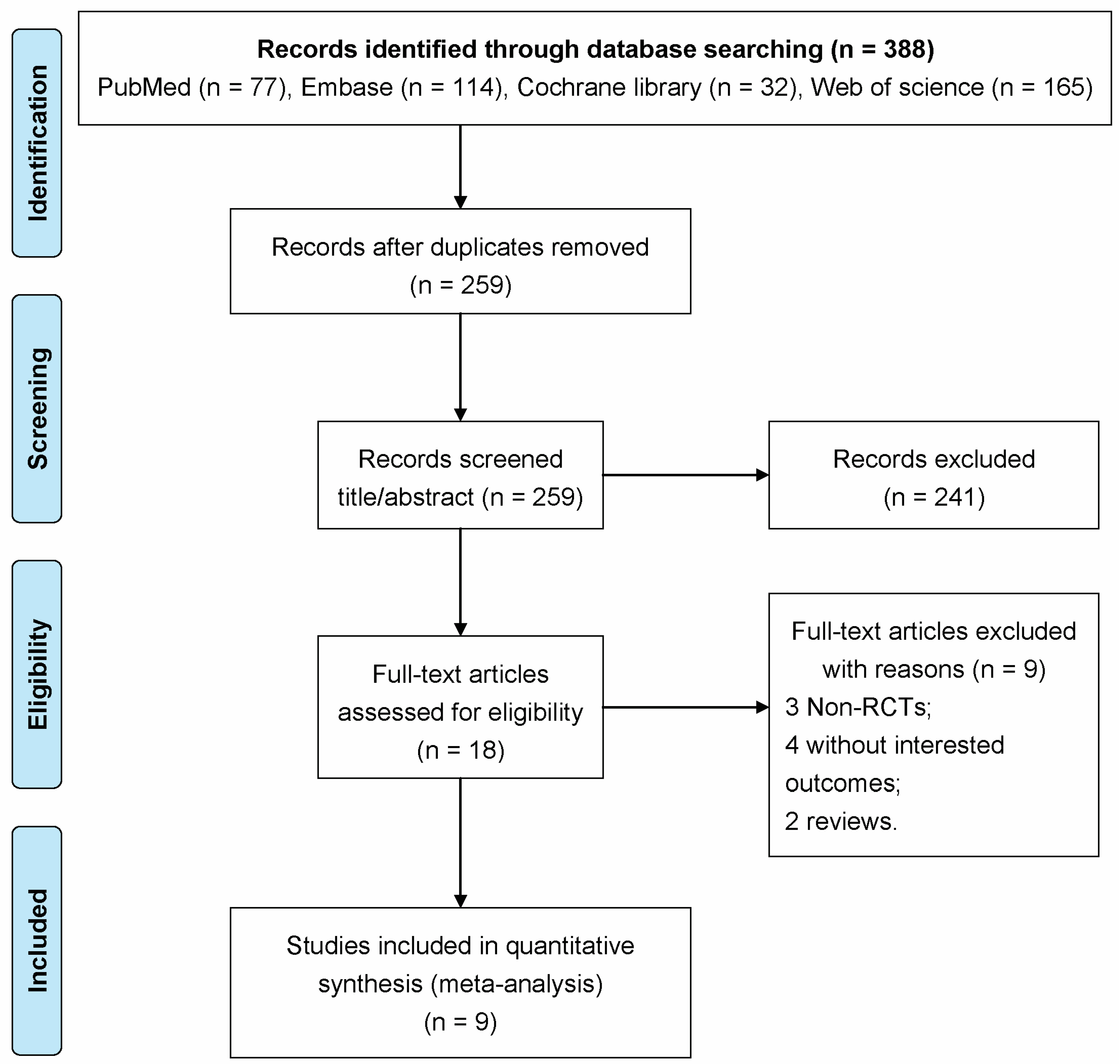
3.1. Literature Search
3.2. Characteristics of the Enrolled Studies
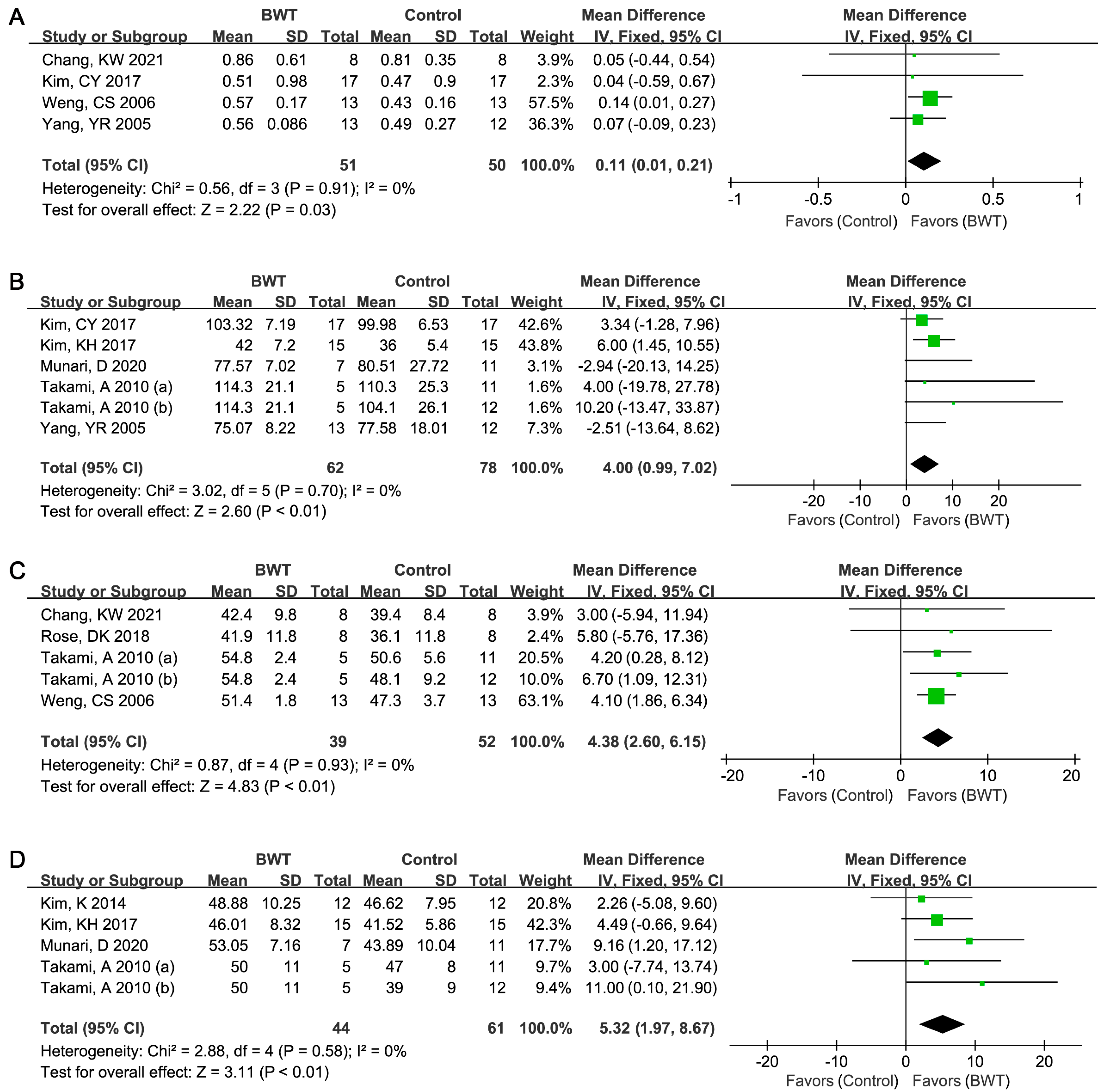
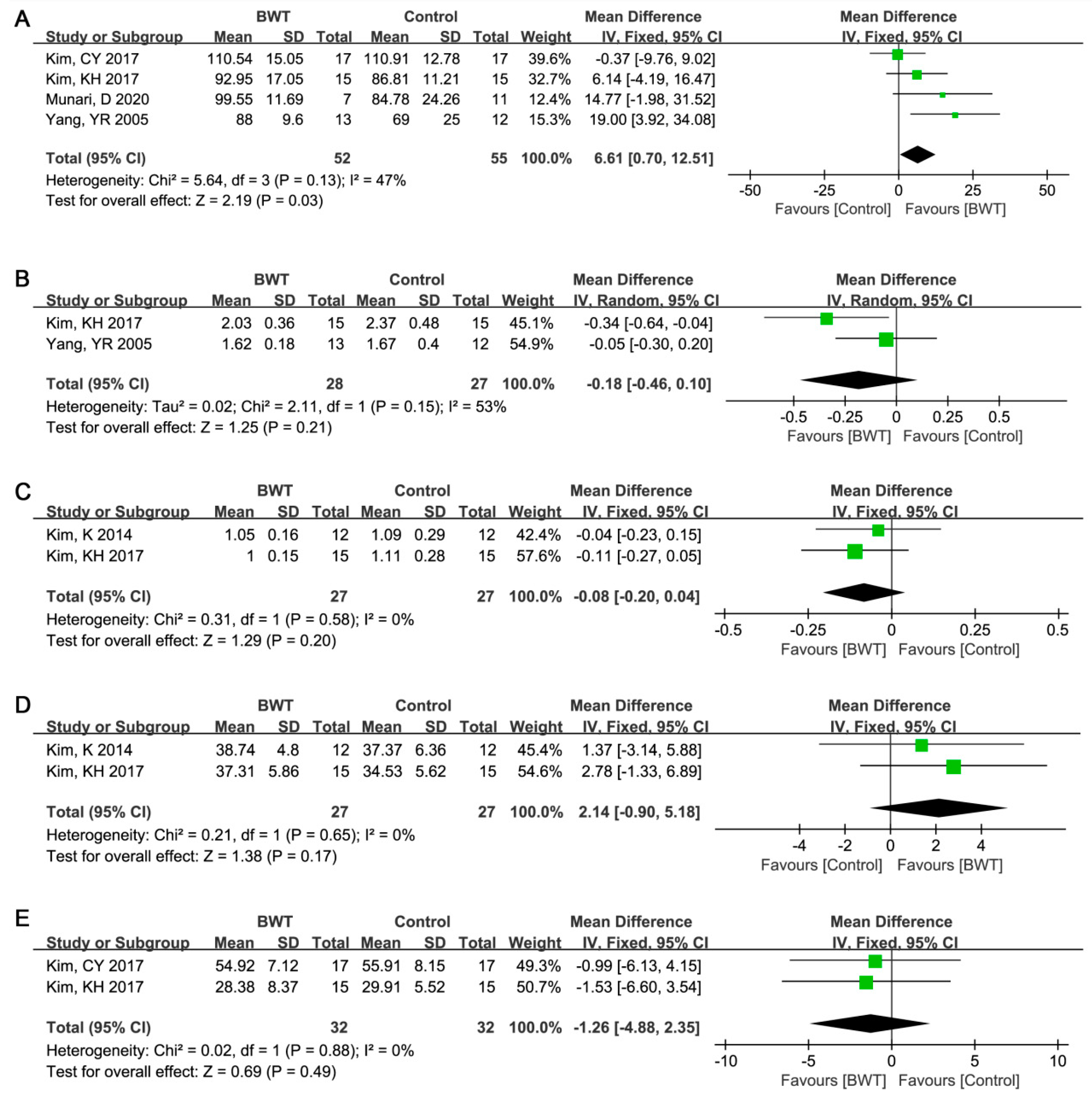
3.3. Results of Meta-Analysis
3.4. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dean, C.M.; Richards, C.L.; Malouin, F. Walking speed over 10 metres overestimates locomotor capacity after stroke. Clin. Rehabil. 2001, 15, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Mun, B.M.; Kim, T.H.; Lee, J.H.; Lim, J.Y.; Seo, D.K.; Lee, D.J. Comparison of Gait Aspects According to FES Stimulation Position Applied to Stroke Patients. J. Phys. Ther. Sci. 2014, 26, 563–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bland, M.D.; Sturmoski, A.; Whitson, M.; Connor, L.T.; Fucetola, R.; Huskey, T.; Corbetta, M.; Lang, C.E. Prediction of discharge walking ability from initial assessment in a stroke inpatient rehabilitation facility population. Arch. Phys. Med. Rehabil. 2012, 93, 1441–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, K.; Ellis, P.; Bernhardt, J.; Maggs, P.; Hull, S. Balance and mobility outcomes for stroke patients: A comprehensive audit. Aust. J. Physiother. 1997, 43, 173–180. [Google Scholar] [CrossRef]
- Hammami, N.; Coroian, F.O.; Julia, M.; Amri, M.; Mottet, D.; Herisson, C.; Laffont, I. Isokinetic muscle strengthening after acquired cerebral damage: A literature review. Ann. Phys. Rehabil. Med. 2012, 55, 279–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, D.; Taillon-Hobson, A.; Bilodeau, M.; Sveistrup, H.; Finestone, H. Virtual reality exercise improves mobility after stroke: An inpatient randomized controlled trial. Stroke 2014, 45, 1853–1855. [Google Scholar] [CrossRef] [PubMed]
- Ozemek, C.; Erlandson, K.M.; Jankowski, C.M. Physical activity and exercise to improve cardiovascular health for adults living with HIV. Prog. Cardiovasc. Dis. 2020, 63, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Ussery, E.N.; Carlson, S.A.; Whitfield, G.P.; Watson, K.B.; Berrigan, D.; Fulton, J.E. Transportation and Leisure Walking Among U.S. Adults: Trends in Reported Prevalence and Volume, National Health Interview Survey 2005–2015. Am. J. Prev. Med. 2018, 55, 533–540. [Google Scholar] [CrossRef] [PubMed]
- DeMark, L.; Fox, E.J.; Spigel, P.M.; Osborne, J.; Rose, D.K. Clinical application of backward walking training to improve walking function, balance, and fall-risk in acute stroke: A case series. Top. Stroke Rehabil. 2019, 26, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Bae, Y. Backward walking observational training improves gait ability in patients with chronic stroke: Randomised controlled pilot study. Int. J. Rehabil. Res. 2019, 42, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Yen, J.G.; Wang, R.Y.; Yen, L.L.; Lieu, F.K. Gait outcomes after additional backward walking training in patients with stroke: A randomized controlled trial. Clin. Rehabil. 2005, 19, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, K.B.; Bae, Y.-H.; Fong, S.S.M.; Lee, S.M. Effects of progressive backward body weight suppoted treadmill training on gait ability in chronic stroke patients: A randomized controlled trial. Technol. Health Care 2017, 25, 867–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Xu, J.; An, R. Effectiveness of backward walking training on balance performance: A systematic review and meta-analysis. Gait Posture 2019, 68, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-Y.; Lee, J.-S.; Kim, H.-D. Comparison of the Effect of Lateral and Backward Walking Training on Walking Function in Patients with Poststroke Hemiplegia A Pilot Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2017, 96, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Ye, X.L.; Chen, W.J.; Chen, G.Q.; Wu, J.T.; Wu, H.; Xu, X.M. Effectiveness of backward walking for people affected by stroke: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2020, 99, e20731. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Lee, S.; Lee, K. Effects of Progressive Body Weight Support Treadmill Forward and Backward Walking Training on Stroke Patients’ Affected Side Lower Extremity’s Walking Ability. J. Phys. Ther. Sci. 2014, 26, 1923–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, D.K.; DeMark, L.; Fox, E.J.; Clark, D.J.; Wludyka, P. A Backward Walking Training Program to Improve Balance and Mobility in Acute Stroke: A Pilot Randomized Controlled Trial. J. Neurol. Phys. Ther. 2018, 42, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Takami, A.; Wakayama, S. Effects of Partial Body Weight Support while Training Acute Stroke Patients to Walk Backwards on a Treadmill—A Controlled Clinical Trial Using Randomized Allocation. J. Phys. Ther. Sci. 2010, 22, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Weng, C.-S.; Wang, J.; Pan, X.-Y.; Yu, Z.-Z.; Wang, G.; Gao, L.-P.; Huo, C.-N. Effectiveness of backward walking treadmill training in lower extremity function after stroke. Zhonghua Yi Xue Za Zhi 2006, 86, 2635–2638. [Google Scholar] [PubMed]
- Chang, K.W.; Lin, C.M.; Yen, C.W.; Yang, C.C.; Tanaka, T.; Guo, L.Y. The Effect of Walking Backward on a Treadmill on Balance, Speed of Walking and Cardiopulmonary Fitness for Patients with Chronic Stroke: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 2376. [Google Scholar] [CrossRef] [PubMed]
- Munari, D.; Serina, A.; Disarò, J.; Modenese, A.; Filippetti, M.; Gandolfi, M.; Smania, N.; Picelli, A. Combined effects of backward treadmill training and botulinum toxin type A therapy on gait and balance in patients with chronic stroke: A pilot, single-blind, randomized controlled trial. NeuroRehabilitation 2020, 46, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Kendall, B.J.; Gothe, N.P. Effect of Aerobic Exercise Interventions on Mobility among Stroke Patients: A Systematic Review. Am. J. Phys. Med. Rehabil. 2016, 95, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.Y.; Charlesworth, S.A.; Lau, R.W.; Chung, R.C. Using aerobic exercise to improve health outcomes and quality of life in stroke: Evidence-based exercise prescription recommendations. Cerebrovasc. Dis. 2013, 35, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Balasukumaran, T.; Olivier, B.; Ntsiea, M.V. The effectiveness of backward walking as a treatment for people with gait impairments: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Groff, B.R.; Antonellis, P.; Schmid, K.K.; Knarr, B.A.; Stergiou, N. Stride-time variability is related to sensorimotor cortical activation during forward and backward walking. Neurosci. Lett. 2019, 692, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.F.; Day, B.L. Visual guidance of the human foot during a step. J. Physiol. 2005, 569, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Kurz, M.J.; Wilson, T.W.; Arpin, D.J. Stride-time variability and sensorimotor cortical activation during walking. NeuroImage 2012, 59, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
| Study | Area | Group | Intervention | Follow Up |
|---|---|---|---|---|
| Chang, KW 2021 | China | BWT | 30 min conventional walking training + backward treadmill training | 3 times/week, 4 weeks |
| Control | 30 min strengthening, function and mobility activities, gait training | |||
| Kim, CY 2017 | Korea | BWT | 30 min Backward Walking Training | 3 times/week, 3 weeks |
| Control | 30 min Standing Balance Training | |||
| Kim, K 2014 | Korea | BWT | 30 min Progressive Body Weight Supported backward walking treadmill training | 6 times/week, 6 weeks |
| FWT | 30 min Progressive Body Weight Supported forward walking treadmill training | |||
| Kim, KH 2017 | Korea | BWT | 30 min Progressive Body Weight Supported backward walking treadmill training | 5 times/week, Four weeks |
| FWT | 30 min Progressive Body Weight Supported forward walking treadmill training | |||
| Munari, D 2020 | Italy | BWT | 40 min backward walking treadmill training | 3 times/week, 4 weeks |
| FWT | 40 min forward walking treadmill training | |||
| Rose, DK 2018 | USA | BWT | 30 min Backward Walking Training | 8 sessions during the inpatient period |
| Control | 30 min standing Balance Training | |||
| Takami, A 2010 | Japan | BWT | 30 min conventional walking training and 10 min backward treadmill training | 6 times/week, 3 weeks |
| FWT | 30 min conventional walking training and 10 min forward treadmill training | |||
| Control | 40 min overground walk training | |||
| Weng, CS 2006 | China | BWT | 30 min conventional walking training and 30 min backward walking training | 5 times/week, 3 weeks |
| Control | 60 min conventional walking training | |||
| Yang, YR 2005 | China | BWT | 30 min backward Walking Training and 40 min conventional training | 3 times/week, 3 weeks |
| Control | 40 min strengthening, function and mobility activities, gait training |
| Study | Group | N | Sex, M/F | Age, Years | Post Stroke Duration | Affected Side, L/R | Ischemic/ Hemorrhage | Severity of Stroke Patients |
|---|---|---|---|---|---|---|---|---|
| Chang, K.W., 2021 | BWT | 8 | 6/2 | 52.39 ± 6.06 | 22.93 ± 13.7 months | 5/3 | 1/7 | Hemiplegia; BMS of lower extremity ≥4; ability to walk at least 11 m; no visual defects or hemianopia |
| Control | 8 | 5/3 | 54.38 ± 14.05 | 43.64 ± 32.69 months | 1/7 | 5/3 | ||
| Kim, C.Y., 2017 | BWT | 17 | 7/10 | 63.83 ± 7.27 | 7.99 ± 3.58 months | 10/7 | 8/9 | Lower-extremity BMS of 3 or 4; ability to walk 14 m; hemiparesis |
| Control | 17 | 9/8 | 63.33 ± 11.60 | 7.12 ± 2.32 months | 8/9 | 11/6 | ||
| Kim, K., 2014 | BWT | 12 | 9/3 | 50.25 ± 16.69 | 11.83 ± 3.46 months | 4/8 | NR | No joint contracture, fractures, or hemianopia; functional gait index scores exceeding three points |
| FWT | 12 | 8/4 | 52.75 ± 9.21 | 11.00 ± 4.22 months | 6/6 | |||
| Kim, K.H., 2017 | BWT | 15 | 11/4 | 48.27 ± 16.05 | 10.93 ± 3.67 months | 10/5 | 4/11 | No joint contracture, pain, fractures, or hemianopia; FAC scores exceeding four and five points |
| FWT | 15 | 7/8 | 50.73 ± 13.50 | 11.27 ± 4.10 months | 8/7 | 6/9 | ||
| Munari, D., 2020 | BWT | 7 | 6/1 | 58.29 ± 10.14 | 84 ± 40.8 months | 2/5 | NR | Ability to walk backward and forward for more than 5 m without a brace or other aid |
| FWT | 11 | 7/4 | 64.73 ± 8.32 | 84 ± 44.4 months | 6/5 | |||
| Rose, D.K., 2018 | BWT | 8 | 4/4 | 53.8 ± 12.1 | 8.5 ± 4.2 days | 5/3 | NR | Able to maintain upright standing posture with moderate assistance; vision within functional limits |
| Control | 8 | 2/6 | 66.6 ± 7.3 * | 7.8 ± 3.3 days | 5/3 | |||
| Takami, A., 2010 | BWT | 12 | 6/6 | 66.1 ± 6.3 | 13.2 ± 8.4 days | 5/7 | 7/5 | Success walking 10 m using braces or canes; Functional Independence Measure-Locomotion score of 5 or lower |
| FWT | 12 | 9/3 | 71.1 ± 10.6 | 14.7 ± 8.1 days | 7/5 | 11/1 | ||
| Control | 12 | 5/7 | 66.9 ± 10.6 | 13.7 ± 8.9 days | 2/10 | 11/1 | ||
| Weng, C.S., 2006 | BWT | 13 | 8/5 | 51 ± 12 | 62 ± 24 days | 6/7 | 8/5 | Lower-extremity BMS of 3 or 4; no joint contracture; ability to walk at least 10 m without assistance or ankle-foot orthosis |
| Control | 13 | 9/4 | 50 ± 14 | 63 ± 34 days | 7/6 | 6/7 | ||
| Yang, Y.R., 2005 | BWT | 13 | 10/3 | 63.38 ± 7.7 | 5.45 ± 3.03 months | 5/8 | NR | Hemiplegia; lower-extremity BMS at 3 or 4; ability to walk 11 m with/without a walking aid or orthosis |
| Control | 12 | 9/3 | 63.42 ± 11.06 | 7.33 ± 2.42 months | 4/8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.; Wang, M. Backward Walking Training Impacts Positive Effect on Improving Walking Capacity after Stroke: A Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 3370. https://doi.org/10.3390/ijerph19063370
Wen H, Wang M. Backward Walking Training Impacts Positive Effect on Improving Walking Capacity after Stroke: A Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(6):3370. https://doi.org/10.3390/ijerph19063370
Chicago/Turabian StyleWen, Hongwei, and Min Wang. 2022. "Backward Walking Training Impacts Positive Effect on Improving Walking Capacity after Stroke: A Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 6: 3370. https://doi.org/10.3390/ijerph19063370
APA StyleWen, H., & Wang, M. (2022). Backward Walking Training Impacts Positive Effect on Improving Walking Capacity after Stroke: A Meta-Analysis. International Journal of Environmental Research and Public Health, 19(6), 3370. https://doi.org/10.3390/ijerph19063370






