Amino Acid-Enriched Formula for the Post-Operative Care of Extraction Sockets Evaluated by 3-D Intraoral Scanning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Subject Recruitment
2.3. Inclusion Criteria
- -
- Patients should be aged 18 years or older;
- -
- Patients should exhibit good general health with no systemic disorders which might affect the healing phases (such as diabetes, cardiovascular events, immuno-depression);
- -
- Patients do not require immediate rehabilitation (with an implant or other prosthetic technique) for the single edentulia before the 2 months of healing;
- -
- Compliance with the study protocol and follow-up recall and willingness to adhere to the hygiene instructions and the use of a domiciliary gel.
2.4. Exclusion Criteria
2.5. Study Design
- -
- Test group (n = 20), who were not using the intra-operative hyaluronic acid-based gel (Polifarma Benessere S.r.l. & Professional Dietetics Aminogam®) (15 mL). After the procedure, patients received motivations and instructions for domiciliary maintenance using the same gel for 15 days, once a day in the evening after common oral hygiene procedures, which included brushing their teeth from the second day after surgery and using a chlorhexidine 0.20 mouthwash.
- -
- Control group (n = 20), who were not using an intra-operative gel, nor were they performing domiciliary maintenance. These patients received motivations and instructions for common post-extractive oral hygiene procedures, which included brushing their teeth from the second day after surgery and using a chlorhexidine 0.20 mouthwash.
2.6. Surgical Procedures
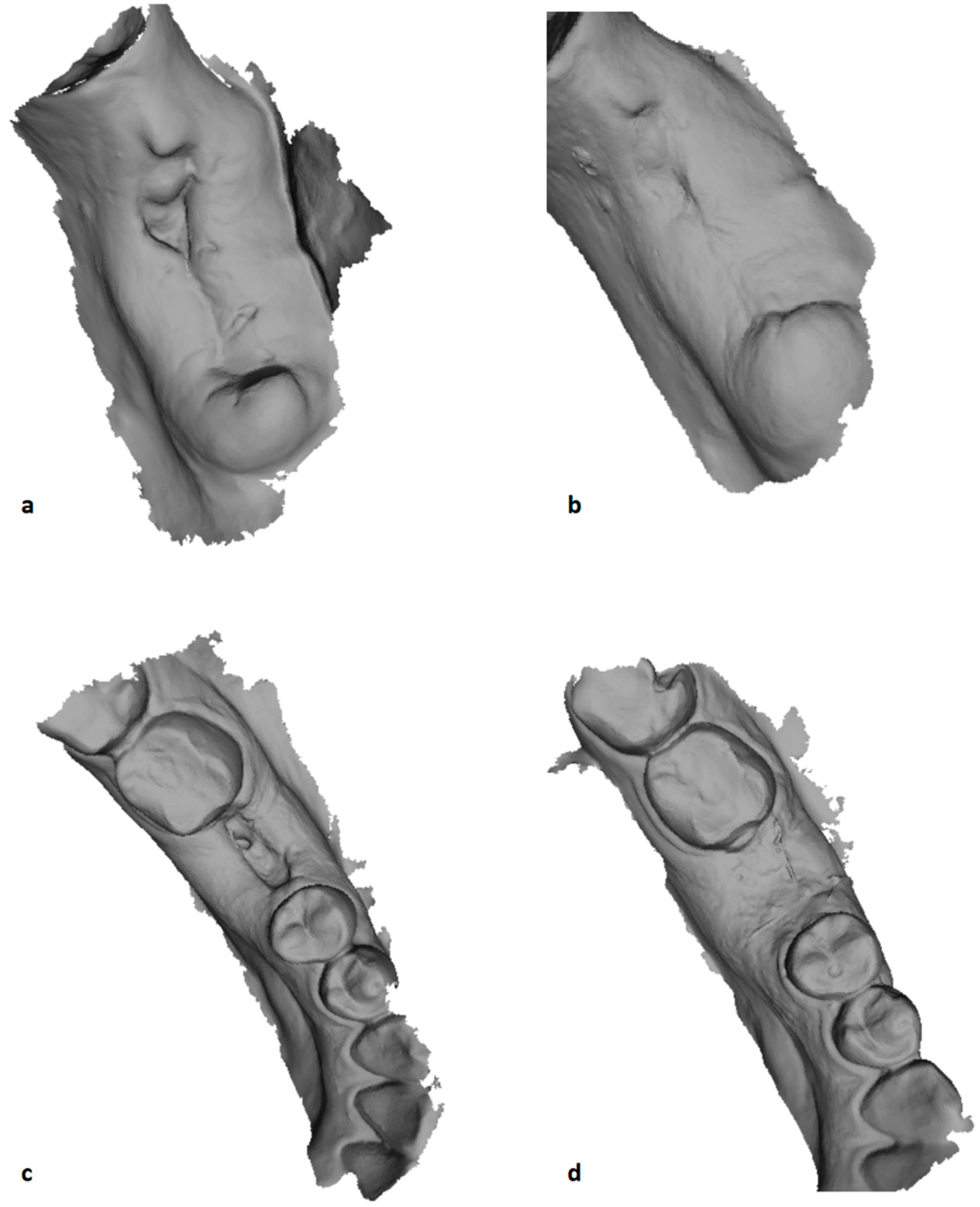
2.7. Outcome Assessment
2.8. Follow-Up Schedule
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabbiani, G.; Ryan, G.B.; Majne, G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Prunotto, M.; Desmouliere, A.; Varga, J.; De Wever, O.; Mareel, M.; Gabbiani, G. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. Am. J. Pathol. 2012, 180, 1340–1355. [Google Scholar] [PubMed]
- Van De Water, L.; Varney, S.; Tomasek, J.J. Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv. Wound Care (New Rochelle) 2013, 2, 122–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marconcini, S.; Denaro, M.; Cosola, S.; Gabriele, M.; Toti, P.; Mijiritsky, E.; Proietti, A.; Basolo, F.; Giammarinaro, E.; Covani, U. Myofibroblast Gene Expression Profile after Tooth Extraction in the Rabbit. Materials 2019, 12, 3697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurent, T.C.; Laurent, U.B.; Fraser, J.R. Functions of hyaluronan. Ann. Rheum. Dis. 1995, 54, 429–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jentsch, H.; Pomowski, R.; Kundt, G.; Göcke, R. Treatment of gingivitis with hyaluronan. J. Clin. Periodontol. 2003, 30, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Alcaide, L.M.; Molinero-Mourelle, P.; González-Serrano, J.; Rubio-Alonso, L.; Bornstein, M.M.; López-Quiles, J. Efficacy of a topical gel containing chitosan, chlorhexidine, allantoin and dexpanthenol for pain and inflammation control after third molar surgery: A randomized and placebo-controlled clinical trial. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e644–e651. [Google Scholar] [CrossRef] [PubMed]
- Baus, R.A.; Zahir-Jouzdani, F.; Dünnhaupt, S.; Atyabi, F.; Bernkop-Schnürch, A. Mucoadhesive hydrogels for buccal drug delivery: In vitro-in vivo correlation study. Eur. J. Pharm. Biopharm. 2019, 142, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Mariggiò, M.A.; Cassano, A.; Vinella, A.; Vincenti, A.; Fumarulo, R.; Lo Muzio, L.; Maiorano, E.; Ribatti, D.; Favia, G. Enhancement of fibroblast proliferation, collagen biosynthesis and production of growth factors as a result of combining sodium hyaluronate and aminoacids. Int. J. Immunopathol. Pharmacol. 2009, 22, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romeo, U.; Libotte, F.; Palaia, G.; Galanakis, A.; Gaimari, G.; Tenore, G.; Del Vecchio, A.; Polimeni, A. Oral soft tissue wound healing after laser surgery with or without a pool of amino acids and sodium hyaluronate: A randomized clinical study. Photomed. Laser Surg. 2014, 32, 10–16. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, X.; Qin, L.; Zhai, M.; Yuan, J.; Chen, J.; Li, D. Effect of hyaluronic acid in bone formation and its applications in dentistry. J. Biomed. Mater. Res. A 2016, 104, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Luraghi, G.; Scribante, A. Ozonized Water Administration in Peri-Implant Mucositis Sites: A Randomized Clinical Trial. Appl. Sci. 2021, 11, 7812. [Google Scholar] [CrossRef]
- Barone, A.; Marchionni, F.S.; Cinquini, C.; Cipolli Panattoni, A.; Toti, P.; Marconcini, S.; Covani, U.; Gabriele, M. Antibiotic treatment to prevent postextraction complications: A monocentric, randomized clinical trial. Preliminary outcomes. Minerva Stomatol. 2017, 66, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Menchini-Fabris, G.B.; Toti, P.; Crespi, R.; Crespi, G.; Cosola, S.; Covani, U. A Retrospective Digital Analysis of Contour Changing after Tooth Extraction with or without Using Less Traumatic Surgical Procedures. J. Clin. Med. 2022, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Toti, P.; Marconcini, S.; Derchi, G.; Saverio, M.; Covani, U. Esthetic Outcome of Implants Placed in Fresh Extraction Sockets by Clinicians with or without Experience: A Medi-um-Term Retrospective Evaluation. Int. J. Oral Maxillofac. Implants 2016, 31, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- De Santis, D.; Sinigaglia, S.; Pancera, P.; Faccioni, P.; Portelli, M.; Luciano, U.; Cosola, S.; Penarrocha, D.; Bertossi, D.; Nocini, R.; et al. An overview of socket preservation. J. Biol. Regul. Homeost. Agents 2019, 33 (Suppl. 1), 55–59. [Google Scholar]
- Covani, U.; Giammarinaro, E.; Marconcini, S. Alveolar socket remodeling: The tug-of-war model. Med. Hypotheses 2020, 142, 109746. [Google Scholar] [CrossRef]
- Capodiferro, S.; Tempesta, A.; Bucci, S.; Maiorano, E.; Favia, G.; Limongelli, L. Aminogam® Gel Allows Faster Wound Healing after Oral Surgery by Formation of Mature Connective Tis-sue with Low Vascular Density and Reducing Inflammatory Infiltration. A Retrospective Study on 580 Cases with Histological and Confocal Laser Investigation. Appl. Sci. 2020, 10, 1105. [Google Scholar] [CrossRef] [Green Version]
- La Gatta, A.; D’Agostino, A.; Schiraldi, C.; Colella, G.; Cirillo, N. A biophysically-defined hyaluronic acid-based compound accelerates migration and stimulates the production of keratinocyte-derived neuromodulators. Cell Adh. Migr. 2019, 13, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Canciani, E.; Sirello, R.; Pellegrini, G.; Henin, D.; Perrotta, M.; Toma, M.; Khomchyna, N.; Dellavia, C. Effects of Vitamin and Amino Acid-Enriched Hyaluronic Acid Gel on the Healing of Oral Mucosa: In Vivo and In Vitro Study. Medicina 2021, 57, 285. [Google Scholar] [CrossRef]
- Colella, G.; Vicidomini, A.; Soro, V.; Lanza, A.; Cirillo, N. Molecular insights into the effects of sodium hyaluronate preparations in keratinocytes. Clin. Exp. Dermatol. 2012, 37, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Favia, G.; Mariggio, M.A.; Maiorano, F.; Cassano, A.; Capodiferro, S.; Ribatti, D. Accelerated wound healing of oral soft tissues and angiogenic effect induced by a pool of aminoacids combined to sodium hyaluronate (AMINOGAM). J. Biol. Regul. Homeost. Agents 2008, 22, 109–116. [Google Scholar] [PubMed]
- Rodrigues Neves, C.; Buskermolen, J.; Roffel, S.; Waaijman, T.; Thon, M.; Veerman, E.; Gibbs, S. Human saliva stimulates skin and oral wound healing in vitro. J. Tissue Eng. Regen. Med. 2019, 13, 1079–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voinchet, V.; Vasseur, P.; Kern, J. Efficacy and safety of hyaluronic acid in the management of acute wounds. Am. J. Clin. Dermatol. 2006, 7, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Boink, M.A.; Roffel, S.; Breetveld, M.; Thon, M.; Haasjes, M.S.P.; Waaijman, T.; Scheper, R.J.; Blok, C.S.; Gibbs, S. Comparison of advanced therapy medicinal product gingiva and skin substitutes and their in vitro wound healing potentials. J. Tissue Eng. Regen. Med. 2018, 12, e1088–e1097. [Google Scholar] [CrossRef] [PubMed]
- Touyz, L.Z.G.; Afrashtehfar, K.I. Implications of bisphosphonate calcium ion depletion interfering with desmosome epithelial seal in osseointegrated implants and pressure ulcers. Med. Hypotheses 2017, 107, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Afrashtehfar, K.I.; Kurtzman, G.M.; Mahesh, L. Improving oral rehabilitation through the preservation of the tissues through alveolar preservation. J. Adv. Prosthodont. 2012, 4, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Toti, P.; Quaranta, A.; Alfonsi, F.; Cucchi, A.; Negri, B.; Di Felice, R.; Marchionni, S.; Calvo-Guirado, J.L.; Covani, U.; et al. Clinical and Histological changes after ridge preservation with two xenografts: Preliminary results from a multicentre randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 204–214. [Google Scholar] [CrossRef]
- Glim, J.E.; Everts, V.; Niessen, F.B.; Ulrich, M.M.; Beelen, R.H. Extracellular matrix components of oral mucosa differ from skin and resemble that of foetal skin. Arch. Oral Biol. 2014, 59, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, F.; Röggla, J.; Idrees, M.A.; Griessinger, J. Chemical modification of hyaluronic acid for intraoral application. J. Pharm. Sci. 2014, 103, 2414–2423. [Google Scholar] [CrossRef]
- Koray, M.; Ofluoglu, D.; Onal, E.A.; Ozgul, M.; Ersev, H.; Yaltirik, M.; Tanyeri, H. Efficacy of hyalu-ronic acid spray on swelling, pain, and trismus after surgical extraction of impacted mandibu-lar third molars. Int. J. Oral Maxillofac. Surg. 2014, 43, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef] [PubMed]
| Treatment Group | Test | Control |
|---|---|---|
| N patients | 20 | 20 |
| Female | 11 | 9 |
| Male | 10 | 10 |
| Age (mean ± SD) | 45.30 ± 10.00 | 47.75 ± 10.00 |
| Upper teeth extracted | 10 | 9 |
| Lower teeth extracted | 10 | 11 |
| Second premolars | 8 | 9 |
| First molars | 9 | 10 |
| Second molars | 3 | 1 |
| Tooth extracted for mobility | 6 | 4 |
| Tooth extracted for fracture | 12 | 13 |
| Tooth extracted for a failed endodontic treatment | 2 | 3 |
| N° patients smoking (<6 cigarettes) | 3 | 2 |
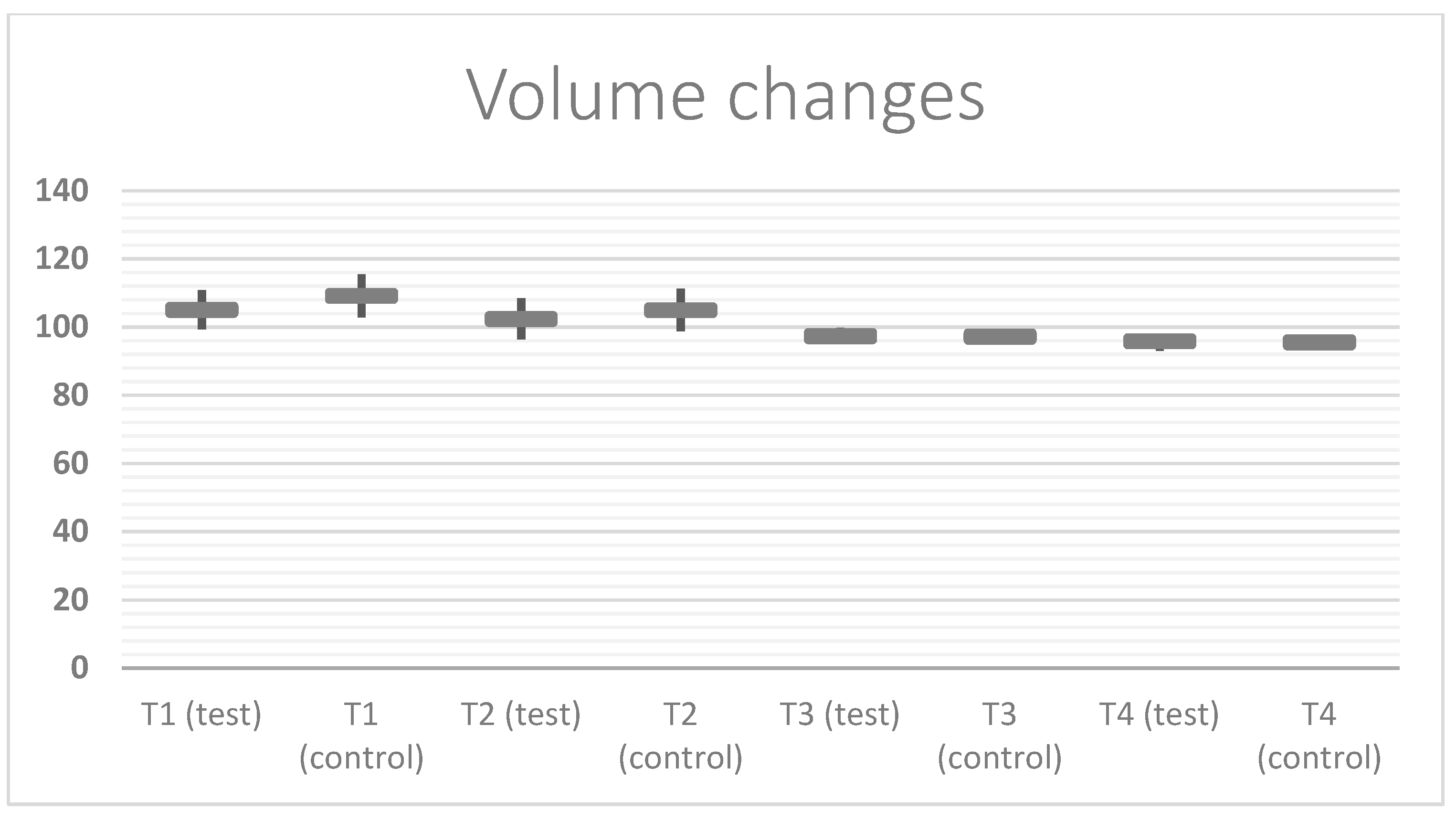
| Volume (Baseline: 100%) Mean Value ± SD (Standard Deviation) | T0 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| Test (N patients = 20) | 100% ± 0.00 | 105.05% ± 5.74 | 102.35% ± 6.05 | 97.35% ± 2.34 | 95.85% ± 1.81 |
| Control (N patients = 20) | 100% ± 0.00 | 109.15% ± 6.30 | 104.95% ± 6.26 | 97.20% ± 2.26 | 95.55% ± 1.88 |
| p value | 0.0380 | 0.1895 | 0.1895 | 0.8380 | |
| VAS score (0–10) Mean value ± SD (standard deviation) | T0 | T1 | T2 | T3 | T4 |
| Test (N patients = 20) | 3.9 ± 2.02 | 1.65 ± 0.75 | 1.1 ± 0.91 | 0.1 ± 0.31 | 0 ± 0 |
| Control (N patients = 20) | 4.5 ± 2.06 | 2.7 ± 1.49 | 1.35 ± 1.04 | 0.2 ± 0.41 | 0 ± 0 |
| p value | 0.82 | 0.04 | 0.86 | 0.94 | / |
| Number of painkillers taken Mean value ± SD (standard deviation) | T0 | T1 | T2 | T3 | Total |
| Test (N patients = 20) | 1.1 ± 1.24 | 1.3 ± 0.98 | 0.25 ± 0.44 | 0 ± 0 | 1.55 ± 1.10 |
| Control (N patients = 20) | 0.95 ± 1.05 | 1.8 ± 1.10 | 0.55 ± 0.60 | 0 ± 0 | 2.35 ± 1.18 |
| p value | 0.49 | 0.0382 | 0.1735 | / | 0.0327 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosola, S.; Oldoini, G.; Boccuzzi, M.; Giammarinaro, E.; Genovesi, A.; Covani, U.; Marconcini, S. Amino Acid-Enriched Formula for the Post-Operative Care of Extraction Sockets Evaluated by 3-D Intraoral Scanning. Int. J. Environ. Res. Public Health 2022, 19, 3302. https://doi.org/10.3390/ijerph19063302
Cosola S, Oldoini G, Boccuzzi M, Giammarinaro E, Genovesi A, Covani U, Marconcini S. Amino Acid-Enriched Formula for the Post-Operative Care of Extraction Sockets Evaluated by 3-D Intraoral Scanning. International Journal of Environmental Research and Public Health. 2022; 19(6):3302. https://doi.org/10.3390/ijerph19063302
Chicago/Turabian StyleCosola, Saverio, Giacomo Oldoini, Michela Boccuzzi, Enrica Giammarinaro, Annamaria Genovesi, Ugo Covani, and Simone Marconcini. 2022. "Amino Acid-Enriched Formula for the Post-Operative Care of Extraction Sockets Evaluated by 3-D Intraoral Scanning" International Journal of Environmental Research and Public Health 19, no. 6: 3302. https://doi.org/10.3390/ijerph19063302
APA StyleCosola, S., Oldoini, G., Boccuzzi, M., Giammarinaro, E., Genovesi, A., Covani, U., & Marconcini, S. (2022). Amino Acid-Enriched Formula for the Post-Operative Care of Extraction Sockets Evaluated by 3-D Intraoral Scanning. International Journal of Environmental Research and Public Health, 19(6), 3302. https://doi.org/10.3390/ijerph19063302









