Feasibility and Efficacy of Telehealth-Based Resistance Exercise Training in Adolescents with Cystic Fibrosis and Glucose Intolerance
Abstract
:1. Background
2. Materials and Methods
2.1. Participants and Study Design
2.2. Body Composition Assessments
2.3. Physical Fitness Assessments
2.4. Resistance Exercise Training Intervention
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
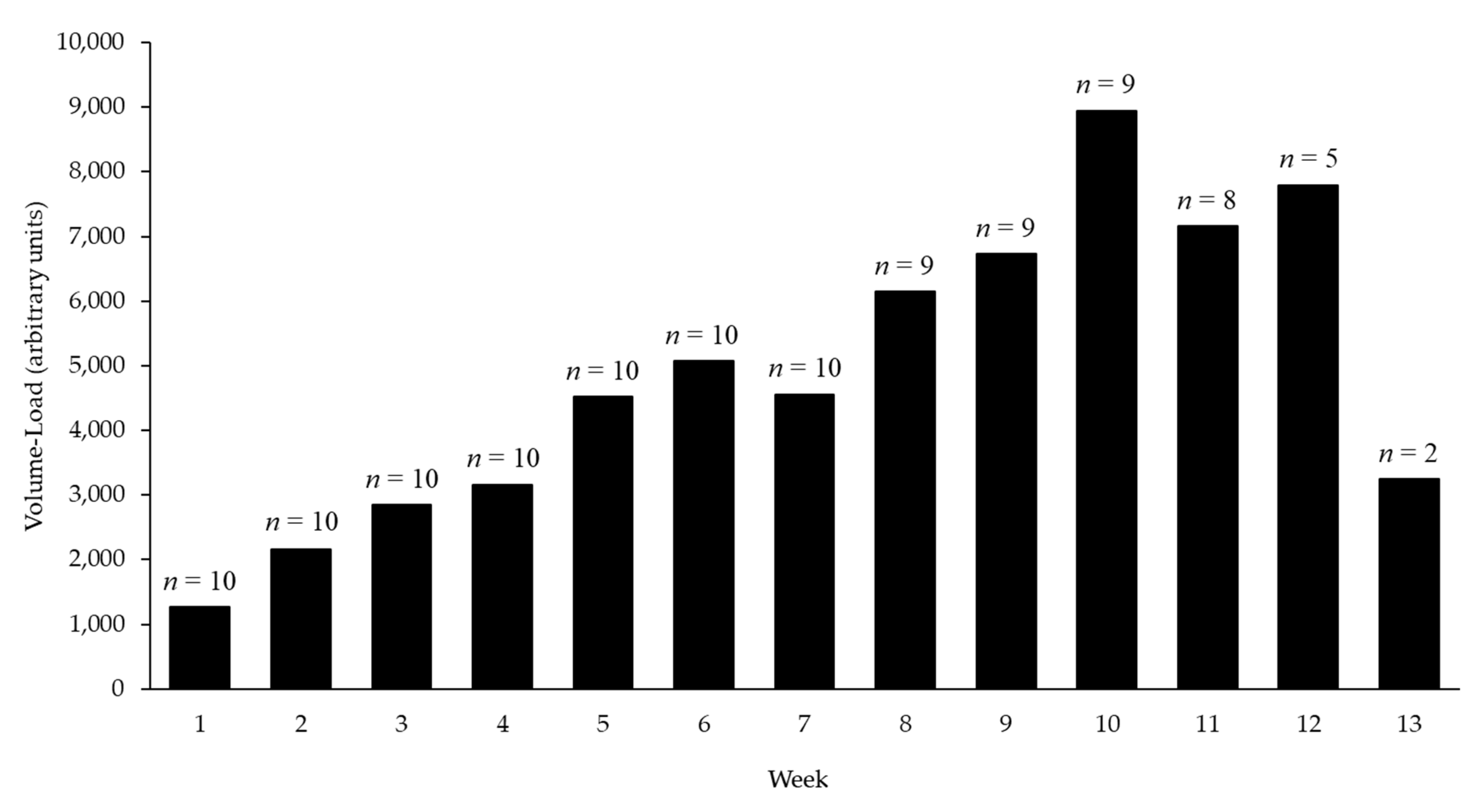
3.2. Resistance Exercise Training
3.3. Metabolic Responses, Body Composition, and Physical Fitness Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scotet, V.; L’Hostis, C.; Férec, C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef]
- Enright, S.; Chatham, K.; Ionescu, A.A.; Unnithan, V.B.; Shale, D.J. The influence of body composition on respiratory muscle, lung function and diaphragm thickness in adults with cystic fibrosis. J. Cyst. Fibros. 2007, 6, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Granados, A.; Chan, C.L.; Ode, K.L.; Moheet, A.; Moran, A.; Holl, R. Cystic fibrosis related diabetes: Pathophysiology, screening and diagnosis. J. Cyst. Fibros. 2019, 18, S3–S9. [Google Scholar] [CrossRef] [Green Version]
- Lanng, S.; Thorsteinsson, B.; Nerup, J.; Koch, C. Influence of the development of diabetes mellitus on clinical status in patients with cystic fibrosis. Eur. J. Pediatr. 1992, 151, 684–687. [Google Scholar] [CrossRef]
- Cerny, F. Exercise and Cystic Fibrosis (CF) 2.0. Pediatr. Exerc. Sci. 2013, 25, 616–623. [Google Scholar] [CrossRef]
- Radtke, T.; Nolan, S.N.; Hebestreit, H.; Kriemler, S. Physical exercise training for cystic fibrosis. Cochrane Database Syst. Rev. 2015, 11, CD002768. [Google Scholar] [CrossRef] [Green Version]
- Hickson, R.C.; Rosenkoetter, M.A.; Brown, M.M. Strength training effects on aerobic power and short-term endurance. Med. Sci. Sports Exerc. 1980, 12, 336–339. [Google Scholar] [CrossRef]
- Keeler, L.K.; Finkelstein, L.H.; Miller, W.; Fernhall, B. Early-Phase Adaptations of Traditional-Speed vs. Superslow Resistance Training on Strength and Aerobic Capacity in Sedentary Individuals. J. Strength Cond. Res. 2001, 15, 309–314. [Google Scholar] [CrossRef]
- Shaw, B.; Shaw, I. Compatibility of concurrent aerobic and resistance training on maximal aerobic capacity in sedentary males. Cardiovasc. J. Afr. 2009, 20, 104–106. [Google Scholar]
- Vendrusculo, F.M.; Heinzmann-Filho, J.P.; Da Silva, J.S.; Ruiz, M.P.; Donadio, M.V.F. Peak Oxygen Uptake and Mortality in Cystic Fibrosis: Systematic Review and Meta-Analysis. Respir. Care 2018, 64, 91–98. [Google Scholar] [CrossRef]
- McGinley, S.K.; Armstrong, M.J.; Boule, N.G.; Sigal, R.J. Effects of exercise training using resistance bands on glycaemic control and strength in type 2 diabetes mellitus: A meta-analysis of randomised controlled trials. Acta Diabetol. 2014, 52, 221–230. [Google Scholar] [CrossRef]
- Russell, R.D.; Hu, D.; Greenaway, T.; Blackwood, S.J.; Dwyer, R.M.; Sharman, J.E.; Jones, G.; Squibb, K.A.; Brown, A.A.; Otahal, P.; et al. Skeletal Muscle Microvascular-Linked Improvements in Glycemic Control From Resistance Training in Individuals With Type 2 Diabetes. Diabetes Care 2017, 40, 1256–1263. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Scott, C.A.; Mao, C.; Tang, J.; Farmer, A.J. Resistance exercise versus aerobic exercise for type 2 diabetes: A sys-tematic review and meta-analysis. Sports Med. 2014, 44, 487–499. [Google Scholar] [CrossRef]
- Ishiguro, H.; Kodama, S.; Horikawa, C.; Fujihara, K.; Hirose, A.S.; Hirasawa, R.; Yachi, Y.; Ohara, N.; Shimano, H.; Hanyu, O.; et al. In Search of the Ideal Resistance Training Program to Improve Glycemic Control and its Indication for Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Sports Med. 2015, 46, 67–77. [Google Scholar] [CrossRef]
- Eriksson, J.; Taimela, S.; Eriksson, K.; Parviainen, S.; Peltonen, J.; Kujala, U. Resistance Training in the Treatment of Non-Insulin-Dependent Diabetes Mellitus. Int. J. Sports Med. 1997, 18, 242–246. [Google Scholar] [CrossRef]
- Prevention, CfDCa. COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 13 December 2021).
- Bland, K.A.; Bigaran, A.; Campbell, K.L.; Trevaskis, M.; Zopf, E.M. Exercising in Isolation? The Role of Telehealth in Exercise Oncology During the COVID-19 Pandemic and Beyond. Phys. Ther. 2020, 100, 1713–1716. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Cox, N.S.; Alison, J.A.; Rasekaba, T.; Holland, A. Telehealth in cystic fibrosis: A systematic review. J. Telemed. Telecare 2011, 18, 72–78. [Google Scholar] [CrossRef]
- Tomlinson, O.W.; Shelley, J.; Trott, J.; Bowhay, B.; Chauhan, R.; Sheldon, C.D. The feasibility of online video calling to en-gage patients with cystic fibrosis in exercise training. J. Telemed. Telecare 2020, 26, 356–364. [Google Scholar] [CrossRef]
- Slentz, C.A.; Tanner, C.J.; Bateman, L.A.; Durheim, M.T.; Huffman, K.M.; Houmard, J.A.; Kraus, W.E. Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care 2009, 32, 1807–1811. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, N.; Bunout, D.; Hirsch, S.; Barrera, G.; Leiva, L.; Henríquez, S.; De la Maza, M. Premature loss of muscle mass and function in type 2 diabetes. Diabetes Res. Clin. Pract. 2016, 117, 32–38. [Google Scholar] [CrossRef]
- Julian, V.; Blondel, R.; Pereira, B.; Thivel, D.; Boirie, Y.; Duclos, M. Body Composition Is Altered in Pre-Diabetic Patients With Impaired Fasting Glucose Tolerance: Results From the NHANES Survey. J. Clin. Med. Res. 2017, 9, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Srikanthan, P.; Karlamangla, A.S. Relative Muscle Mass Is Inversely Associated with Insulin Resistance and Prediabetes. Findings from The Third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef] [Green Version]
- Moorcroft, A.J.; Dodd, M.E.; Morris, J.; Webb, A.K. Individualised unsupervised exercise training in adults with cystic fibrosis: A 1 year randomised controlled trial. Thorax 2004, 59, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Santana Sosa, E.; Groeneveld, I.F.; Gonzalez-Saiz, L.; López-Mojares, L.M.; Villa-Asensi, J.R.; Barrio Gonzalez, M.I.; Fleck, S.J.; Pérez, M.; Lucia, A. Intrahospital weight and aerobic training in children with cystic fibrosis: A randomized controlled trial. Med. Sci. Sports Exerc. 2012, 44, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Santana-Sosa, E.; Gonzalez-Saiz, L.; Groeneveld, I.F.; Villa-Asensi, J.R.; Barrio Gómez de Aguero, M.I.; Fleck, S.J.; López-Mojares, L.M.; Pérez, M.; Lucia, A. Benefits of combining inspiratory muscle with ’whole muscle’ training in children with cystic fibrosis: A randomised controlled trial. Br. J. Sports Med. 2014, 48, 1513–1517. [Google Scholar] [CrossRef]
- Beaudoin, N.; Bouvet, G.F.; Coriati, A.; Rabasa-Lhoret, R.; Berthiaume, Y. Combined Exercise Training Improves Glycemic Control in Adult with Cystic Fibrosis. Med. Sci. Sports Exerc. 2017, 49, 231–237. [Google Scholar] [CrossRef]
- Selvadurai, H.; Blimkie, C.; Meyers, N.; Mellis, C.; Cooper, P.; Van Asperen, P. Randomized controlled study of in-hospital exercise training programs in children with cystic fibrosis. Pediatr. Pulmonol. 2002, 33, 194–200. [Google Scholar] [CrossRef]
- Orenstein, D.M.; Hovell, M.F.; Mulvihill, M.; Keating, K.K.; Hofstetter, C.R.; Kelsey, S.; Morris, K.; Nixon, P.A. Strength vs. Aerobic Training in Children with Cystic Fibrosis: A Randomized Controlled Trial. Chest 2004, 126, 1204–1214. [Google Scholar] [CrossRef] [Green Version]
| Age (yr) | 15.80 ± 2.20 |
| Height (cm) | 163.97 ± 9.31 |
| Height Z-score | −0.21 ± 0.68 |
| Weight (kg) | 60.05 ± 15.12 |
| Weight Z-score | −0.04 ± 1.58 |
| Body Mass Index (kg/m2) | 22.55 ± 6.52 |
| Body Mass index Z-score | −0.09 ± 1.70 |
| Fasting Glucose (mg/dL) | 100.80 ± 11.30 |
| 2-h Glucose (mg/dL) | 156.22 ± 45.41 |
| Forced Expiratory Volume (FEV1%) | 109.13 ± 17.64 |
| Forced Vital Capacity (FVC1%) | 109.25 ± 14.34 |
| Participant | Completed Sessions | Missed Sessions | Completed Percentage |
|---|---|---|---|
| 001 | 31 | 5 | 86.1 |
| 002 | 30 | 6 | 83.3 |
| 003 | 30 | 6 | 83.3 |
| 004 | 28 | 8 | 77.8 |
| 005 | 29 | 7 | 80.6 |
| 006 | 32 | 4 | 88.9 |
| 007 | 31 | 5 | 86.1 |
| 008 | 19 | 17 | 52.8 |
| 009 | 29 | 7 | 80.6 |
| 010 | 25 | 11 | 69.4 |
| M ± SD | 28.4 ± 3.8 | 7.6 ± 3.8 | 78.9 ± 10.7 |
| N | Pre-Intervention | Post-Intervention | Mean Difference | ES | p-Value | |
|---|---|---|---|---|---|---|
| Fasting Glucose (mg/dL) | 10 | 100.8 ± 11.3 | 95.6 ± 5.3 | −5.2 | 0.6 | 0.11 |
| 2-h Glucose (mg/dL) | 9 | 156.2 ± 45.4 | 158.9 ± 40.4 | 2.7 | 0.1 | 0.84 |
| Fasting Insulin (U/mL) | 10 | 9.7 ± 5.5 | 11.3 ± 8.7 | 1.6 | 0.2 | 0.36 |
| 2-h Insulin (U/mL) | 9 | 85.0 ± 42.6 | 120.0 ± 91.3 | 35.0 | 0.5 | 0.10 |
| Fasting C-peptide (ng/mL) | 10 | 2.0 ± 0.7 | 2.1 ± 1.2 | 0.1 | 0.2 | 0.54 |
| 2-h C-peptide (ng/mL) | 9 | 9.6 ± 3.0 | 11.7 ± 4.9 | 2.1 * | 0.5 | 0.04 |
| Matsuda Index | 9 | 4.1 ± 2.5 | 4.0 ± 3.7 | −0.1 | 0.03 | 0.93 |
| HOMA-IR | 9 | 2.4 ± 1.3 | 2.8 ± 2.1 | 0.4 | 0.3 | 0.35 |
| OGIS | 8 | 363.6 ± 42.0 | 362.4 ± 75.9 | −1.2 | 0.02 | 0.96 |
| Insulin/Glucose-iAUC | 9 | 0.6 ± 0.5 | 0.6 ± 0.4 | −0.1 | 0.2 | 0.59 |
| N | Pre-Intervention | Post-Intervention | Mean Difference | ES | p-Value | |
|---|---|---|---|---|---|---|
| %Fat | 10 | 29.7 ± 14.7 | 28.4 ± 15.3 | −1.3 * | 0.1 | 0.03 |
| FM | 10 | 19.3 ± 14.1 | 19.0 ± 15.0 | −0.3 | 0.02 | 0.60 |
| FFM | 10 | 40.8 ± 7.2 | 42.3 ± 7.2 | 1.5 * | 0.2 | 0.01 |
| LBM | 10 | 39.6 ± 7.1 | 40.6 ± 7.0 | 1.0 | 0.1 | 0.09 |
| FMI | 10 | 6.8 ± 5.5 | 6.0 ± 6.2 | −0.8 | 0.1 | 0.23 |
| FFMI | 10 | 14.1 ± 1.8 | 14.5 ± 1.9 | 0.4 * | 0.2 | 0.01 |
| LBMI | 10 | 13.4 ± 1.8 | 12.5 ± 5.0 | −0.9 | 0.3 | 0.52 |
| FMI-Z-score | 10 | −0.7 ± 1.6 | −0.9 ± 1.9 | −0.2 | 0.1 | 0.13 |
| LBMI-Z-score | 10 | −0.8 ± 1.2 | −0.9 ± 1.2 | −0.1 | 0.04 | 0.88 |
| Isometric | Pre-Intervention | Post-Intervention | Mean Difference | ES | p-Value |
|---|---|---|---|---|---|
| Peak Torque (N·m) | 117.7 ± 37.8 | 124.8 ± 32.1 | 7.1 ± 13.3 | 0.2 | 0.13 |
| Peak Torque (N·m/kg) | 2.0 ± 0.7 | 2.1 ± 0.7 | 0.1 ± 0.2 | 0.1 | 0.27 |
| Time to Peak Torque (s) | 3.0 ± 1.0 | 2.8 ± 1.0 | −0.3 ± 0.9 | 0.3 | 0.40 |
| Isokinetic 90°/s | |||||
| Peak Torque (N·m) | 9.9 ± 35.1 | 95.3 ± 41.9 | 5.4 ± 24.0 | 0.1 | 0.50 |
| Peak Torque (N·m/kg) | 1.6 ± 0.6 | 1.6 ± 0.8 | 0.1 ± 0.5 | 0.1 | 0.64 |
| Work Done (J) | 85.7 ± 42.5 | 98.4 ± 41.7 | 12.7 ± 23.1 | 0.3 | 0.12 |
| Average Power (W) | 73.4 ± 38.6 | 89.5 ± 39.8 | 16.2 ± 17.7 * | 0.4 | 0.02 |
| Time to Peak Torque (s) | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.01 ± 0.2 | 0.2 | 0.91 |
| Isokinetic 180°/s | |||||
| Peak Torque (N·m) | 70.4 ± 39.9 | 71.8 ± 32.9 | 1.3 ± 25.0 | 0.04 | 0.87 |
| Peak Torque (N·m/kg) | 1.2 ± 0.7 | 1.2 ± 0.6 | −0.01 ± 0.5 | 0.02 | 0.94 |
| Work Done (J) | 67.1 ± 46.2 | 76.2 ± 39.1 | 9.9 ± 24.3 | 0.2 | 0.23 |
| Average Power (W) | 98.0 ± 74.6 | 113.6 ± 63.6 | 21.0 ± 47.0 | 0.2 | 0.19 |
| Time to Peak Torque (s) | 0.3 ± 0.1 | 0.3 ± 0.1 | −0.04 ± 0.2 | 0.00 | 0.54 |
| Isokinetic 270°/s | |||||
| Peak Torque (N·m) | 62.1 ± 38.9 | 63.4 ± 29.5 | 1.6 ± 17.5 | 0.04 | 0.78 |
| Peak Torque (N·m/kg) | 1.1 ± 0.70 | 1.1 ± 0.6 | −0.02 ± 0.3 | 0.03 | 0.88 |
| Work Done (J) | 52.0 ± 38.6 | 61.9 ± 35.7 | 6.9 ± 15.0 | 0.3 | 0.18 |
| Average Power (W) | 103.2 ± 86.2 | 124.1 ± 75.6 | 18.2 ± 43.7 | 0.4 | 0.22 |
| Time to Peak Torque (s) | 0.3 ± 0.2 | 0.2 ± 0.1 | −0.03 ± 0.2 | 0.3 | 0.56 |
| Isokinetic 360°/s | |||||
| Peak Torque (N·m) | 58.9 ± 31.1 | 60.5 ± 24.6 | 1.6 ± 17.5 | 0.1 | 0.78 |
| Peak Torque (N·m/kg) | 1.0 ± 0.6 | 1.0 ± 0.4 | −0.02 ± 0.3 | 0.02 | 0.88 |
| Work Done (J) | 46.0 ± 33.0 | 52.9 ± 27.4 | 6.9 ± 15.0 | 0.2 | 0.18 |
| Average Power (W) | 106.0 ± 100.0 | 124.2 ± 74.6 | 18.2 ± 43.7 | 0.2 | 0.22 |
| Time to Peak Torque (s) | 0.3 ± 0.2 | 0.3 ± 0.1 | −0.03 ± 0.2 | 0.2 | 0.56 |
| N | Pre-Intervention | Post-Intervention | Mean Difference | ES | p-Value | |
|---|---|---|---|---|---|---|
| V̇O2peak (L/min) | 10 | 1.7 ± 0.5 | 1.9 ± 0.6 | 0.1 ± 0.1 * | 0.2 | 0.01 |
| V̇O2peak (mL/kg/min) | 10 | 30.0 ± 10.0 | 31.7 ± 11.2 | 1.7 ± 3.1 | 0.2 | 0.11 |
| V̇CO2peak (L/min) | 10 | 2.1 ± 0.7 | 2.2 ± 0.7 | 0.1 ± 0.1 * | 0.2 | 0.01 |
| V̇CO2peak (mL/kg/min) | 10 | 35.9 ± 12.8 | 37.1 ± 13.6 | 1.2 ± 2.0 | 0.1 | 0.11 |
| RER | 10 | 1.2 ± 0.1 | 1.2 ± 0.1 | −0.03 ± 0.1 | 0.3 | 0.29 |
| V̇E (L/min) | 10 | 51.8 ± 11.3 | 57.1 ± 16.8 | 5.3 ± 6.8 * | 0.4 | 0.04 |
| MWR (W) | 10 | 137.0 ± 49.0 | 135.0 ± 47.9 | −2.0 ± 20.4 | 0.04 | 0.76 |
| MWR (W/kg) | 10 | 2.4 ± 0.9 | 2.3 ± 0.9 | −0.1 ± 0.4 | 0.1 | 0.59 |
| HRmax (bpm) | 10 | 179.5 ± 12.8 | 180.5 ± 16.9 | 1.0 ± 5.3 | 0.1 | 0.57 |
| Age-Predicted HRmax (%) | 10 | 87.9 ± 5.9 | 88.5 ± 8.0 | 0.6 ± 2.7 | 0.1 | 0.51 |
| RPE | 9 | 18.9 ± 1.3 | 17.7 ± 1.3 | −1.4 ± 2.0 | 0.9 | 0.06 |
| HRR @ 5-min (bpm) | 8 | 121.3 ± 13.9 | 119.5 ± 12.7 | −2.9 ± 6.7 | 0.1 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes, C.J.; Racette, S.B.; Symonds, L.; Arbeláez, A.M.; Cao, C.; Granados, A. Feasibility and Efficacy of Telehealth-Based Resistance Exercise Training in Adolescents with Cystic Fibrosis and Glucose Intolerance. Int. J. Environ. Res. Public Health 2022, 19, 3297. https://doi.org/10.3390/ijerph19063297
Holmes CJ, Racette SB, Symonds L, Arbeláez AM, Cao C, Granados A. Feasibility and Efficacy of Telehealth-Based Resistance Exercise Training in Adolescents with Cystic Fibrosis and Glucose Intolerance. International Journal of Environmental Research and Public Health. 2022; 19(6):3297. https://doi.org/10.3390/ijerph19063297
Chicago/Turabian StyleHolmes, Clifton J., Susan B. Racette, Leslie Symonds, Ana Maria Arbeláez, Chao Cao, and Andrea Granados. 2022. "Feasibility and Efficacy of Telehealth-Based Resistance Exercise Training in Adolescents with Cystic Fibrosis and Glucose Intolerance" International Journal of Environmental Research and Public Health 19, no. 6: 3297. https://doi.org/10.3390/ijerph19063297
APA StyleHolmes, C. J., Racette, S. B., Symonds, L., Arbeláez, A. M., Cao, C., & Granados, A. (2022). Feasibility and Efficacy of Telehealth-Based Resistance Exercise Training in Adolescents with Cystic Fibrosis and Glucose Intolerance. International Journal of Environmental Research and Public Health, 19(6), 3297. https://doi.org/10.3390/ijerph19063297







