The Impact of Functional Bars and Adapted Physical Activity on Quality of Life in Chronic Kidney Disease: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Parameters
2.3. Questionnaires
2.4. Body Composition Assessment
2.5. Ultrasound Evaluation
2.6. Evaluation of Muscle Strength and Physical Performance
2.7. Other Functional Parameters
2.8. Composition of the Bars
2.9. Physical Exercise Protocol
2.10. Statistical Analysis
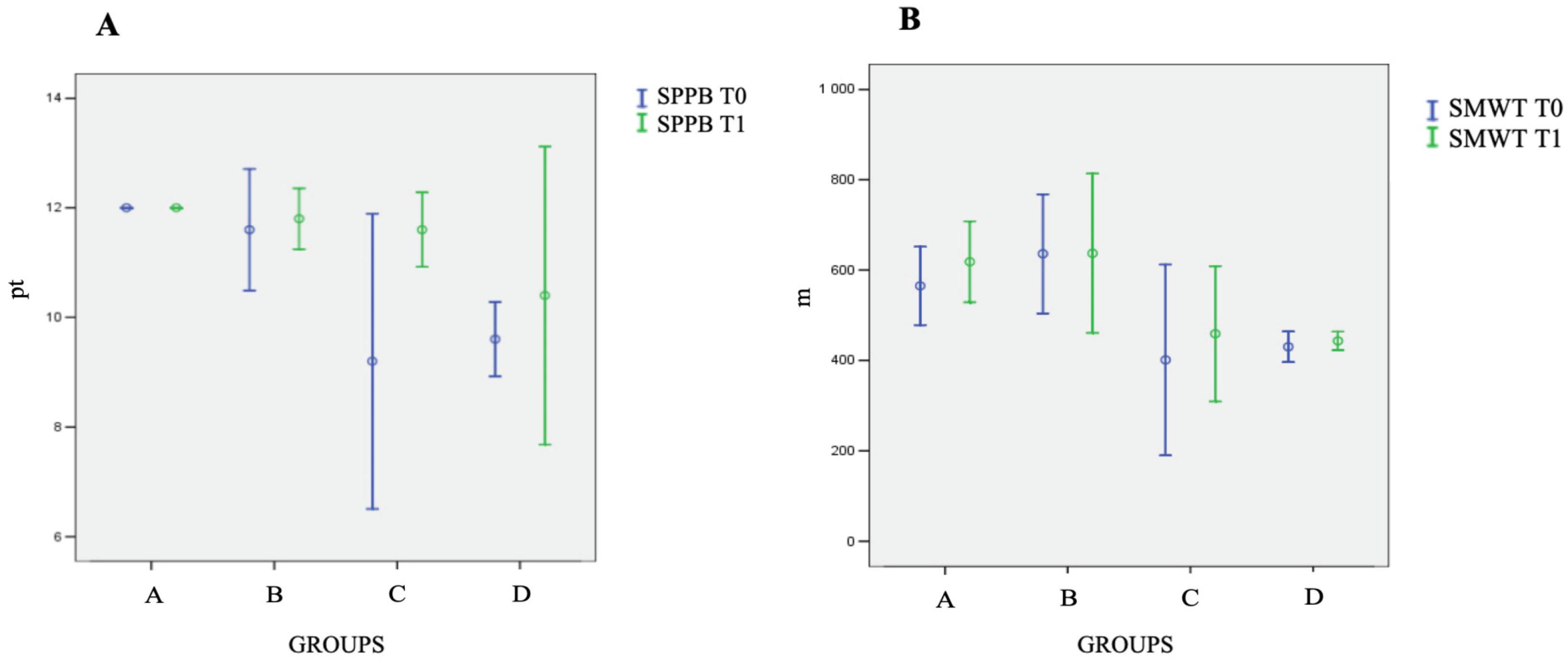
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| % AA | Percentage antiradical activity |
| AH | Arterial hypertension |
| APA | Adapted physical activity |
| BCMI | Body cell mass index |
| BIA | Bioelectrical impedance analysis |
| BMI | Body mass index |
| CDNCDs | Chronic degenerative non-communicable diseases |
| CKD | Chronic kidney disease |
| CRP | C-reactive protein |
| CV | Cardiovascular |
| ECG | Electrocardiogram |
| ECW | Extracellular water |
| EFSA | European food safety authority |
| ESR | Erythrocyte sedimentation rate |
| ESRD | End stage-renal disease |
| EVOO | Extra virgin olive oil |
| EWGSOP2 | European Working Group on Sarcopenia in Older People |
| FFM | Fat free mass |
| FM | Fat mass |
| FORD | Free oxygen radical defense |
| FORT | Free oxygen radical test |
| FOXO1 | Forkhead box protein O1 |
| GAE | Gallic acid |
| HGS | Handgrip strength |
| HPLC-DAD-MS | High-performance liquid chromatography-diode array detector-mass spectrometry |
| ICW | Intracellular water |
| IGF-1 | Insulin-like growth factor-1 |
| IL-6 | Interleukin-6 |
| KD | Kidney disease |
| KDIGO | Kidney disease: improving global outcomes |
| LDL | low-density lipoprotein |
| MD | Mediterranean diet |
| MM | Muscle mass |
| MPCs | Minor polar compounds |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| PA | Physical activity |
| PREDIMED | Prevención con Dieta Mediterránea |
| QOL | Quality of life |
| QRFT | Quadriceps rectus femoris thickness |
| QVIT | Quadriceps vastus intermedius thickness |
| RAAS | Renin-angiotensin-aldosterone system |
| RRT | Renal replacement therapy |
| SCPT | Stair climb power |
| SF-36 | Short form-36 |
| SMWT | Six-minute walk |
| SOCS-3 | Cytokine signalling 3 |
| SPPB | Short physical performance battery |
| TBW | Total body water |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor-α |
| UPP | Ubiquitin-proteasome pathway |
| US | Uremic sarcopenia |
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Gagliardi, I.; Patella, G.; Michael, A.; Serra, R.; Provenzano, M.; Andreucci, M. COVID-19 and the Kidney: From Epidemiology to Clinical Practice. J. Clin. Med. 2020, 9, 2506. [Google Scholar] [CrossRef]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M.; Alberta Kidney Disease, N. Cause of Death in Patients with Reduced Kidney Function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Ballew, S.H.; Matsushita, K. Cardiovascular Risk Prediction in CKD. Semin. Nephrol. 2018, 38, 208–216. [Google Scholar] [CrossRef]
- Roshanravan, B.; Robinson-Cohen, C.; Patel, K.V.; Ayers, E.; Littman, A.J.; de Boer, I.H.; Ikizler, T.A.; Himmelfarb, J.; Katzel, L.I.; Kestenbaum, B.; et al. Association between physical performance and all-cause mortality in CKD. J. Am. Soc. Nephrol. 2013, 24, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Wu, H.L.; Guo, H.R.; Cheng, Y.Y.; Tseng, C.C.; Wang, M.C.; Lin, C.Y.; Sung, J.M. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol. Dial. Transplant. 2011, 26, 3588–3595. [Google Scholar] [CrossRef] [PubMed]
- Kozakai, R.; von Bonsdorff, M.; Sipila, S.; Rantanen, T. Mobility limitation as a predictor of inpatient care in the last year of life among community-living older people. Aging Clin. Exp. Res. 2013, 25, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Moorthi, R.N.; Avin, K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 219–228. [Google Scholar] [CrossRef]
- Van Ancum, J.M.; Alcazar, J.; Meskers, C.G.M.; Nielsen, B.R.; Suetta, C.; Maier, A.B. Impact of using the updated EWGSOP2 definition in diagnosing sarcopenia: A clinical perspective. Arch. Gerontol. Geriatr. 2020, 90, 104125. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Rovella, V.; Cusumano, A.; Di Daniele, N.; Casasco, M. Beneficial effects of physical activity on uremic sarcopenia. Med. Dello Sport 2018, 71, 370–392. [Google Scholar] [CrossRef]
- Andreoli, A.; Lauro, S.; Di Daniele, N.; Sorge, R.; Celi, M.; Volpe, S.L. Effect of a moderately hypoenergetic Mediterranean diet and exercise program on body cell mass and cardiovascular risk factors in obese women. Eur. J. Clin. Nutr. 2008, 62, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Ottaviani, E.; Guerriero, C.; Di Daniele, F.; Pietroboni Zaitseva, A.; Di Daniele, N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients 2021, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Workeneh, B.T.; Rondon-Berrios, H.; Zhang, L.; Hu, Z.; Ayehu, G.; Ferrando, A.; Kopple, J.D.; Wang, H.; Storer, T.; Fournier, M.; et al. Development of a diagnostic method for detecting increased muscle protein degradation in patients with catabolic conditions. J. Am. Soc. Nephrol. 2006, 17, 3233–3239. [Google Scholar] [CrossRef] [PubMed]
- Cohn, R.D.; van Erp, C.; Habashi, J.P.; Soleimani, A.A.; Klein, E.C.; Lisi, M.T.; Gamradt, M.; ap Rhys, C.M.; Holm, T.M.; Loeys, B.L.; et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 2007, 13, 204–210. [Google Scholar] [CrossRef]
- Yoshida, T.; Galvez, S.; Tiwari, S.; Rezk, B.M.; Semprun-Prieto, L.; Higashi, Y.; Sukhanov, S.; Yablonka-Reuveni, Z.; Delafontaine, P. Angiotensin II inhibits satellite cell proliferation and prevents skeletal muscle regeneration. J. Biol. Chem. 2013, 288, 23823–23832. [Google Scholar] [CrossRef]
- Bocedi, A.; Noce, A.; Marrone, G.; Noce, G.; Cattani, G.; Gambardella, G.; Di Lauro, M.; Di Daniele, N.; Ricci, G. Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution. Nutrients 2019, 11, 1741. [Google Scholar] [CrossRef]
- Noce, A.; Fabrini, R.; Dessi, M.; Bocedi, A.; Santini, S.; Rovella, V.; Pastore, A.; Tesauro, M.; Bernardini, S.; Di Daniele, N.; et al. Erythrocyte glutathione transferase activity: A possible early biomarker for blood toxicity in uremic diabetic patients. Acta Diabetol. 2014, 51, 219–224. [Google Scholar] [CrossRef]
- Noce, A.; Vidiri, M.F.; Marrone, G.; Moriconi, E.; Bocedi, A.; Capria, A.; Rovella, V.; Ricci, G.; De Lorenzo, A.; Di Daniele, N. Is low-protein diet a possible risk factor of malnutrition in chronic kidney disease patients? Cell Death Discov. 2016, 2, 16026. [Google Scholar] [CrossRef]
- Noce, A.; Bocedi, A.; Campo, M.; Marrone, G.; Di Lauro, M.; Cattani, G.; Di Daniele, N.; Romani, A. A Pilot Study of a Natural Food Supplement as New Possible Therapeutic Approach in Chronic Kidney Disease Patients. Pharmaceuticals 2020, 13, 148. [Google Scholar] [CrossRef]
- Romani, A.; Bernini, R.; Noce, A.; Urciuoli, S.; Di Lauro, M.; Pietroboni Zaitseva, A.; Marrone, G.; Di Daniele, N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules 2020, 25, 4757. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Di Lauro, M.; Urciuoli, S.; Pietroboni Zaitseva, A.; Wilson Jones, G.; Di Daniele, N.; Romani, A. Cardiovascular Protection of Nephropathic Male Patients by Oral Food Supplements. Cardiovasc. Ther. 2020, 2020, 1807941. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Urciuoli, S.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Romani, A. Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients. Nutrients 2021, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Pietroboni Zaitseva, A.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef]
- Grazioli, E.; Romani, A.; Marrone, G.; Di Lauro, M.; Cerulli, C.; Urciuoli, S.; Murri, A.; Guerriero, C.; Tranchita, E.; Tesauro, M.; et al. Impact of Physical Activity and Natural Bioactive Compounds on Endothelial Dysfunction in Chronic Kidney Disease. Life 2021, 11, 841. [Google Scholar] [CrossRef]
- Heiwe, S.; Jacobson, S.H. Exercise training in adults with CKD: A systematic review and meta-analysis. Am. J. Kidney Dis. 2014, 64, 383–393. [Google Scholar] [CrossRef]
- Cheema, B.S.; Chan, D.; Fahey, P.; Atlantis, E. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health-related quality of life in patients with chronic kidney disease: A systematic review and meta-analysis. Sports Med. 2014, 44, 1125–1138. [Google Scholar] [CrossRef]
- Milam, R.H. Exercise Guidelines for Chronic Kidney Disease Patients. J. Ren. Nutr. 2016, 26, e23–e25. [Google Scholar] [CrossRef][Green Version]
- Kirkman, D.L.; Ramick, M.G.; Muth, B.J.; Stock, J.M.; Pohlig, R.T.; Townsend, R.R.; Edwards, D.G. Effects of aerobic exercise on vascular function in nondialysis chronic kidney disease: A randomized controlled trial. Am. J. Physiol. Ren. Physiol. 2019, 316, F898–F905. [Google Scholar] [CrossRef]
- Kumar, T.G.S.; Soundararajan, P.; Maiya, A.G.; Ravi, A. Effects of graded exercise training on functional capacity, muscle strength, and fatigue after renal transplantation: A randomized controlled trial. Saudi J. Kidney Dis. Transpl. 2020, 31, 100–108. [Google Scholar] [CrossRef]
- Noce, A.; Ferrannini, M.; Fabrini, R.; Bocedi, A.; Dessi, M.; Galli, F.; Federici, G.; Palumbo, R.; Di Daniele, N.; Ricci, G. Erythrocyte glutathione transferase: A new biomarker for hemodialysis adequacy, overcoming the Kt/V(urea) dogma? Cell Death Dis. 2012, 3, e377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bocedi, A.; Noce, A.; Rovella, V.; Marrone, G.; Cattani, G.; Iappelli, M.; De Paolis, P.; Iaria, G.; Sforza, D.; Gallu, M.; et al. Erythrocyte glutathione transferase in kidney transplantation: A probe for kidney detoxification efficiency. Cell Death Dis. 2018, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.I.; Maiolo, C.; Iacopino, L.; Pepe, M.; Di Daniele, N.; De Lorenzo, A. The impact of body-weight components on forced spirometry in healthy italians. Lung 2002, 180, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2001, 37, S66–S70. [Google Scholar] [CrossRef] [PubMed]
- Gaman, M.A.; Epingeac, M.E.; Diaconu, C.C.; Gaman, A.M. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World J. Diabetes 2020, 11, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Pavlatou, M.G.; Papastamataki, M.; Apostolakou, F.; Papassotiriou, I.; Tentolouris, N. FORT and FORD: Two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism 2009, 58, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Garcia-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schroder, H.; Aros, F.; Gomez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Di Daniele, N.; Di Renzo, L.; Noce, A.; Iacopino, L.; Ferraro, P.M.; Rizzo, M.; Sarlo, F.; Domino, E.; De Lorenzo, A. Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J. Nephrol. 2014, 27, 529–536. [Google Scholar] [CrossRef]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4, 2050312116671725. [Google Scholar] [CrossRef]
- Baecke, J.A.; Burema, J.; Frijters, J.E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 1982, 36, 936–942. [Google Scholar] [CrossRef]
- Bellizzi, V.; Scalfi, L.; Terracciano, V.; De Nicola, L.; Minutolo, R.; Marra, M.; Guida, B.; Cianciaruso, B.; Conte, G.; Di Iorio, B.R. Early changes in bioelectrical estimates of body composition in chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Candi, E.; Tesauro, M.; Cardillo, C.; Lena, A.M.; Schinzari, F.; Rodia, G.; Sica, G.; Gentileschi, P.; Rovella, V.; Annicchiarico-Petruzzelli, M.; et al. Metabolic profiling of visceral adipose tissue from obese subjects with or without metabolic syndrome. Biochem. J. 2018, 475, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Sahathevan, S.; Khor, B.H.; Singh, B.K.S.; Sabatino, A.; Fiaccadori, E.; Daud, Z.A.M.; Ali, M.S.; Narayanan, S.S.; Tallman, D.; Chinna, K.; et al. Association of Ultrasound-Derived Metrics of the Quadriceps Muscle with Protein Energy Wasting in Hemodialysis Patients: A Multicenter Cross-Sectional Study. Nutrients 2020, 12, 3597. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Broers, N.J.H.; van der Sande, F.M.; Hemmelder, M.H.; Fiaccadori, E.; Kooman, J.P. Estimation of Muscle Mass in the Integrated Assessment of Patients on Hemodialysis. Front. Nutr. 2021, 8, 697523. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Regolisti, G.; Delsante, M.; Di Motta, T.; Cantarelli, C.; Pioli, S.; Grassi, G.; Batini, V.; Gregorini, M.; Fiaccadori, E. Noninvasive evaluation of muscle mass by ultrasonography of quadriceps femoris muscle in End-Stage Renal Disease patients on hemodialysis. Clin. Nutr. 2019, 38, 1232–1239. [Google Scholar] [CrossRef]
- Battaglia, Y.; Ullo, I.; Massarenti, S.; Esposito, P.; Prencipe, M.; Ciancio, G.; Provenzano, M.; Fiorini, F.; Andreucci, M.; Storari, A.; et al. Ultrasonography of Quadriceps Femoris Muscle and Subcutaneous Fat Tissue and Body Composition by BIVA in Chronic Dialysis Patients. Nutrients 2020, 12, 1388. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Bozzoli, L.; Fani, F.; Antoniotti, R.; Maggiore, U.; Fiaccadori, E. Reliability of bedside ultrasound for measurement of quadriceps muscle thickness in critically ill patients with acute kidney injury. Clin. Nutr. 2017, 36, 1710–1715. [Google Scholar] [CrossRef]
- Treacy, D.; Hassett, L. The Short Physical Performance Battery. J. Physiother. 2018, 64, 61. [Google Scholar] [CrossRef]
- Agarwala, P.; Salzman, S.H. Six-Minute Walk Test: Clinical Role, Technique, Coding, and Reimbursement. Chest 2020, 157, 603–611. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols from Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020, 7, 120. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Peppicelli, S.; Bianchini, F.; Andreucci, E.; Urciuoli, S.; Romani, A.; Tortora, K.; Caderni, G.; Nediani, C.; Calorini, L. Cancer Glycolytic Dependence as a New Target of Olive Leaf Extract. Cancers 2020, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Chioccioli, S.; Monaco, N.; Peppicelli, S.; Andreucci, E.; Urciuoli, S.; Romani, A.; Luceri, C.; Tortora, K.; Calorini, L.; et al. Oleuropein-Rich Leaf Extract as a Broad Inhibitor of Tumour and Macrophage iNOS in an Apc Mutant Rat Model. Antioxidants 2021, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, P.; Campo, M.; Romani, A. Hydrolyzable Tannins from Sweet Chestnut Fractions Obtained by a Sustainable and Eco-friendly Industrial Process. Nat. Prod. Commun. 2015, 11, 409–415. [Google Scholar] [CrossRef]
- Ichikawa, T.; Miyaaki, H.; Miuma, S.; Motoyoshi, Y.; Yamashima, M.; Yamamichi, S.; Koike, M.; Honda, T.; Yajima, H.; Uehara, R.; et al. Calculated body muscle mass as a useful screening marker for low skeletal muscle mass and sarcopenia in chronic liver disease. Hepatol. Res. 2020, 50, 704–714. [Google Scholar] [CrossRef]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transplant. 2015, 30, 1718–1725. [Google Scholar] [CrossRef]
- Kelly, P.; Kahlmeier, S.; Gotschi, T.; Orsini, N.; Richards, J.; Roberts, N.; Scarborough, P.; Foster, C. Systematic review and meta-analysis of reduction in all-cause mortality from walking and cycling and shape of dose response relationship. Int. J. Behav.Nutr. Phys. Act. 2014, 11, 132. [Google Scholar] [CrossRef]
- Orkaby, A.R.; Forman, D.E. Physical activity and CVD in older adults: An expert’s perspective. Expert Rev. Cardiovasc. Ther. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Albarrati, A.M.; Alghamdi, M.S.M.; Nazer, R.I.; Alkorashy, M.M.; Alshowier, N.; Gale, N. Effectiveness of Low to Moderate Physical Exercise Training on the Level of Low-Density Lipoproteins: A Systematic Review. Biomed Res. Int. 2018, 2018, 5982980. [Google Scholar] [CrossRef]
- Mann, S.; Beedie, C.; Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef]
- Duni, A.; Dounousi, E.; Pavlakou, P.; Eleftheriadis, T.; Liakopoulos, V. Hypertension in Chronic Kidney Disease: Novel Insights. Curr. Hypertens. Rev. 2020, 16, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Zheng, K.; Zhang, H.; Feng, J.; Wang, L.; Zhou, H. Physical Exercise and Patients with Chronic Renal Failure: A Meta-Analysis. Biomed Res. Int. 2017, 2017, 7191826. [Google Scholar] [CrossRef] [PubMed]
- Uusitupa, M. Hypertension in diabetic patients—Use of exercise in treatment. Ann. Med. 1991, 23, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Pedralli, M.L.; Marschner, R.A.; Kollet, D.P.; Neto, S.G.; Eibel, B.; Tanaka, H.; Lehnen, A.M. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: A randomized clinical trial Exercise, endothelium and blood pressure. Sci. Rep. 2020, 10, 7628. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lassalle-Claux, G.; Touaibia, M.; Rupasinghe, H.P. Antihypertensive effect of caffeic acid and its analogs through dual renin-angiotensin-aldosterone system inhibition. Eur. J. Pharmacol. 2014, 730, 125–132. [Google Scholar] [CrossRef]
- Bekpinar, S.; Karaca, E.; Yamakoglu, S.; Alp-Yildirim, F.I.; Olgac, V.; Uydes-Dogan, B.S.; Cibali, E.; Gultepe, S.; Uysal, M. Resveratrol ameliorates the cyclosporine-induced vascular and renal impairments: Possible impact of the modulation of renin-angiotensin system. Can. J. Physiol. Pharmacol. 2019, 97, 1115–1123. [Google Scholar] [CrossRef]
- Jang, I.A.; Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrients 2018, 10, 1741. [Google Scholar] [CrossRef]
- Ajibola, C.F.; Eleyinmi, A.F.; Aluko, R.E. Kinetics of the inhibition of renin and angiotensin i converting enzyme by polar and non-polar polyphenolic extracts of Vernonia amygdalina and Gongronema latifolium leaves. Plant Foods Hum. Nutr. 2011, 66, 320–327. [Google Scholar] [CrossRef]
- Nunes-Silva, A.; Rocha, G.C.; Magalhaes, D.M.; Vaz, L.N.; de Faria, M.H.S.; Simoes, E.S.A.C. Physical Exercise and ACE2-Angiotensin-(1-7)-Mas Receptor Axis of the Renin Angiotensin System. Protein Pept. Lett. 2017, 24, 809–816. [Google Scholar] [CrossRef]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef]
- Takahashi, S.; Satou, A.; Shimoda, H.; Hata, K. Inhibition of renin-angiotensin system related enzymes (renin, angiotensin converting enzyme, chymase, and angiotensin converting enzyme 2) by water shield extracts. J. Biol. Macromol. 2017, 17, 3–13. [Google Scholar] [CrossRef][Green Version]
- Bansal, N.; Zelnick, L.R.; Himmelfarb, J.; Chertow, G.M. Bioelectrical Impedance Analysis Measures and Clinical Outcomes in CKD. Am. J. Kidney Dis. 2018, 72, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wu, M.; Wu, H.; Ye, H.; Peng, Y.; Yi, C.; Yu, X.; Yang, X. Lower Phase Angle Measured by Bioelectrical Impedance Analysis Is a Marker for Increased Mortality in Incident Continuous Ambulatory Peritoneal Dialysis Patients. J. Ren. Nutr. 2020, 30, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.; Bakir, A.; Koseoglu, Y.K.; Velidedeoglu, M.; Trabulus, S.; Seyahi, N. Association of Nutritional Assessment by Phase Angle with Mortality in Kidney Transplant Patients in an 8-Year Follow-Up. Prog. Transplant. 2019, 29, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef]
- Covas, M.I.; Nyyssonen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Baumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Commission Regulation. Commission Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health Text with EEA Relevance. In Force: This Act Has Been Changed; Special Edition in Croatian; 2012; Chapter 13, Volume 43, pp. 281–320; Current Consolidated Version: 17 May 2021; Available online: https://eur-lex.europa.eu/eli/reg/2012/432/oj (accessed on 4 January 2022).
- Garcia-Redondo, A.B.; Aguado, A.; Briones, A.M.; Salaices, M. NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharmacol. Res. 2016, 114, 110–120. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Calabriso, N.; Santarpino, G.; Verri, T.; De Caterina, R. Effects of Olive Oil on Blood Pressure: Epidemiological, Clinical, and Mechanistic Evidence. Nutrients 2020, 12, 1548. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Noce, A.; Canale, M.P.; Capria, A.; Rovella, V.; Tesauro, M.; Splendiani, G.; Annicchiarico-Petruzzelli, M.; Manzuoli, M.; Simonetti, G.; Di Daniele, N. Coronary artery calcifications predict long term cardiovascular events in non diabetic Caucasian hemodialysis patients. Aging 2015, 7, 269–279. [Google Scholar] [CrossRef]
- Basilicata, M.; Di Lauro, M.; Campolattano, V.; Marrone, G.; Celotto, R.; Mitterhofer, A.P.; Bollero, P.; Di Daniele, N.; Noce, A. Natural Bioactive Compounds in the Management of Oral Diseases in Nephropathic Patients. Int. J. Environ. Res. Public Health 2022, 19, 1665. [Google Scholar] [CrossRef] [PubMed]

| Name | Ingredients | Weight (g) |
| Bar 1 | dates *, thompson grapes *, cashews nuts *, raw cocoa butter *, extra virgin olive oil * 5.7% almonds *, plums * 5%, kiwi powder * 3%, micronized grape pomace * 2.8%, carob flour * 2.5%, acerola powder * 2%, cabbage powder * 2%, beet powder * 2%, micronized grape seeds * 1.4%, açai powder * 1%, blueberry powder * 1%, rhubarb powder * 0.5%. | 32 |
| Bar 2 | dates *, cashews nuts *, thompson grapes *, raw cocoa butter *, figs * 9.6%, extra virgin olive oil * 5.7%, apple * 3%, micronized grape pomace * 2.8%, carob flour * 2.5%, fennel powder * 2%, cabbage powder * 2%, spinach powder * 2%, barley grass powder * 2%, micronized grape seeds * 1.4%, kiwi powder * 1.4%, olive leaf powder 0.1% *, natural lemon flavor. | 32 |
| Group | N | Mean Value ± SD | |
|---|---|---|---|
| Age (years) | A | 6 | 62.2 ± 5.6 |
| B | 5 | 57.2 ± 3.4 | |
| C | 5 | 65.6 ± 3.6 | |
| D | 5 | 65.8 ± 1.1 | |
| Sex (F/M) | A | 6 | 3/3 |
| B | 5 | 3/2 | |
| C | 5 | 2/3 | |
| D | 5 | 3/2 | |
| Weight (kg) | A | 6 | 80.5 ± 13.3 |
| B | 5 | 76.8 ± 10.9 | |
| C | 5 | 77.4 ± 10.3 | |
| D | 5 | 70.1 ± 6.0 | |
| Height (cm) | A | 6 | 168.3 ± 11.7 |
| B | 5 | 173.0 ± 9.5 | |
| C | 5 | 164.0 ± 15.3 | |
| D | 5 | 160.4 ± 3.3 | |
| BMI (kg/m2) | A | 6 | 28.3 ± 4.5 |
| B | 5 | 25.5 ± 1.8 | |
| C | 5 | 28.9 ± 3.0 | |
| D | 5 | 27.3 ± 3.3 |
| Laboratory Parameters | Group | T0 | T1 |
|---|---|---|---|
| Hemoglobin * (g/dL) | A | 13.5 (12.3–17.9) | 13.2 (12.2–18.0) |
| B | 14.7 (12.7–15.4) | 14.9 (12.6–15.5) | |
| C | 13.1 (10.9–14.2) | 12.65 (10.8–14.0) | |
| D | 11.1 (11.2–12.1) | 11.8 (11.6–12-0) | |
| Creatinine * (mg/dL) | A | 1.32 (0.85–2.21) | 1.34 (1.02–2.63) |
| B | 1.43 (1.16–1.78) | 1.37 (1.02–2.06) | |
| C | 1.31 (1.15–1.99) | 1.39 (1.17–1.75) | |
| D | 0.96 (0.95–1.32) | 1.23 (1.09–1.31) | |
| Glomerular Filtration Rate * (mL/min) | A | 52.6 (30.0–92.8) | 48.1 (24.3–62.7) |
| B | 50.7 (32.0–60.1) | 45.2 (28.8–78.4) | |
| C | 51.0 (30.0–59.5) | 50.15 (29.0–55.0) | |
| D | 54.8 (42.2–82.8) | 45.8 (42.6–52.9) | |
| Azotemia * (mg/dL) | A | 50 (37–55) | 54 (48–63) |
| B | 50 (32–60) | 45 (27–78) | |
| C | 54 (43–66) | 47 (44–78) | |
| D | 56 (56–80) | 79 (76–88) | |
| Sodium * (mg/dL) | A | 142 (132–143) | 140.5 (139–143) |
| B | 141 (139–141) | 140 (139–142) | |
| C | 141 (138–143) | 140.5 (138–143) | |
| D | 141 (140–141) | 143 (141–145) | |
| Potassium * (mg/dL) | A | 4.5 (3.8–4.7) | 4.4 (3.4–5.0) |
| B | 4.4 (3.6–4.8) | 4.2 (3.8–4.6) | |
| C | 4.3 (3.9–5.1) | 4.3 (3.7–4.9) | |
| D | 4.9 (4.7–5.2) | 4.8 (4.7–5.7) | |
| Calcium * (mg/dL) | A | 9.2 (8.9–10.0) | 9.2 (8.7–9.6) |
| B | 9.6 (9.0–10.3) | 9.9 (8.9–9.9) | |
| C | 9.1 (9.1–9.4) | 9.1 (8.9–9.7) | |
| D | 9.4 (9.2–9.7) | 10.2 (9.0–10.4) | |
| Phosphorus * (mg/dL) | A | 3.35 (2.6–4.2) | 3.1 (2.9–3.7) |
| B | 3.2 (3.2–4.0) | 3.5 (2.3–3.9) | |
| C | 3.2 (2.6–3.9) | 3.2 (2.3–3.9) | |
| D | 3.9 (3.7–4.1) | 4.2 (3.9–4.7) | |
| Total cholesterol * (mg/dL) | A | 196 (188–240) | 170 (153–215) |
| B | 245(230–333) | 200(195–234) | |
| C | 163 (143–192) | 163.5 (140–186) | |
| D | 215 (212–218) | 220 (210–222) | |
| HDL cholesterol * (mg/dL) | A | 48.5 (35–73) | 45 (32–66) |
| B | 58 (52–75) | 52 (47–73) | |
| C | 48 (27–67) | 46 (27–58) | |
| D | 62 (56–63) | 61 (54–64) | |
| LDL cholesterol * (mg/dL) | A | 123 (82–175) | 115 (97–129) |
| B | 152 (118–243) | 134 (127–218) | |
| C | 82 (67–120) | 104 (93–118) | |
| D | 107 (105–122) | 130 (129–131) | |
| Triglycerides * (mg/dL) | A | 111 (56–275) | 92 (48–306) |
| B | 147 (92–222) | 126 (106–159) | |
| C | 115 (45–182) | 120 (87–221) | |
| D | 124 (121–127) | 118 (110–126) | |
| Uricemia * (mg/dL) | A | 6.1 (5.5–7.3) | 6.1 (6.4–7.5) |
| B | 6.6 (5.9–8.6) | 6.1 (3.9–7.3) | |
| C | 5.1 (4.3–7.5) | 5.8 (4.4–6.9) | |
| D | 7.2 (5.2–7.7) | 9.0 (4.5–9.2) | |
| microalbuminuria on a spot morning urine * (mg/g creatinine) | A | 14 (4–314) | 20 (0–312) |
| B | 8 (0–240) | 11 (0–163) | |
| C | 57 (0–185) | 29 (0–80) | |
| D | 12 (10–148) | 12(10–92) |
| Blood Pressure Parameters | Group | T0 | T1 |
|---|---|---|---|
| Systolic blood pressure * (mmHg) | A | 132 (124–175) | 128 (116–154) |
| B | 138 (126–143) | 132 (112–170) | |
| C | 127 (122–159) | 128 (122–134) | |
| D | 140 (110–145) | 144 (105–148) | |
| Diastolic blood pressure * (mmHg) | A | 89 (77–118) | 72 (65–95) |
| B | 89 (82–100) | 80 (64–85) | |
| C | 77 (70–91) | 77(72–84) | |
| D | 72 (69–80) | 70 (71–75) |
| Inflammatory and OS Biomarkers | Group | T0 | T1 |
|---|---|---|---|
| FORT * (U) | A | 204 (160–358) | 276 (160–600) |
| B | 217 (160–442) | 160 (160–413) | |
| C | 229 (171–392) | 385 (160–600) | |
| D | 338 (246–340) | 358 (233–346) | |
| FORD * (mmol/L) | A | 1.61 (0.87–2.26) | 1.17 (0.52–1.72) |
| B | 1.76 (1.01–2.10) | 1.25 (1.09–2.23) | |
| C | 1.08 (0.7–1.3) | 1.1 (0.87–1.29) | |
| D | 0.62 (0.63–1.21) | 1.2 (0.73–1.3) | |
| CRP * (mg/L) | A | 4.25 (0.4–9.5) | 2.2 (0.4–7.3) |
| B | 1.7 (1.2–5.1) | 1.9 (0.8–5.6) | |
| C | 1.7 (0.4–5.0) | 1.45 (0.6–3.2) | |
| D | 5.4 (0.5–5.8) | 4.4 (0.5–4.6) | |
| ESR * (mm/h) | A | 25 (5–58) | 23 (7–44) |
| B | 32(12–61) | 18 (6–63) | |
| C | 19 (2–32) | 19 (3–30) | |
| D | 92 (43–96) | 85 (48–88) |
| Parameters Useful to Detect Uremic Sarcopenia | Group | T0 | T1 |
|---|---|---|---|
| Weight * (kg) | A | 74.1 (68.6–98.6) | 74 (70.1–99.4) |
| B | 71.8 (65–92.5) | 71.5 (64.8–92.5) | |
| C | 74.7 (57.2–88.6) | 76.9 (62.3–87) | |
| D | 71.5 (63.6–74.5) | 73.5 (62.5–75) | |
| BMI * (kg/m2) | A | 28.2 (22.5–35.3) | 28.7 (22.9–35.5) |
| B | 24.6 (23.6–27.8) | 24.6 (23.5–27.9) | |
| C | 29 (22.1–33.3) | 29 (24.8–33.4) | |
| D | 28.7 (23.6–29.7) | 27.8 (23.2–29.8) | |
| Resistance * (Ω) | A | 494.5 (376–580) | 467 (377–582) |
| B | 569 (525–649) | 484 (442–552) | |
| C | 565 (457–663) | 535 (371–557) | |
| D | 490 (488–740) | 494 (494–694) | |
| Reactance * (Ω) | A | 38 (31–45) | 45 (37–62) |
| B | 51 (40–58) | 60 (56–68) | |
| C | 44 (35–49) | 39 (38–45) | |
| D | 46 (44–48) | 48 (43–47) | |
| Phase angle * (°) | A | 4.7 (3.8–5.0) | 5.9 (5–6.5) |
| B | 5.5 (5.1–5.8) | 6.1 (5.6–6.6) | |
| C | 4.2 (3.8–5.1) | 5.1 (4.5–6) | |
| D | 5.1 (3.7–5.2) | 5.3 (3.8–5.4) | |
| TBW * (%) | A | 39.7 (32.4–54.2) | 40.2 (33.9–55.6) |
| B | 47.9 (46.7–57.9) | 53 (47.3–61.5) | |
| C | 48.5 (43.3–60.2) | 56.7 (42.8–68.3) | |
| D | 44.9 (45.6–48.9) | 47.3 (47.9–48.3) | |
| ECW * (%) | A | 51.6 (49.1–58.5) | 46.5 (52.5–43.6) |
| B | 48 (46.7–50.3) | 45.4 (42.9–46.1) | |
| C | 55.6 (47.5–59) | 53.1 (45.7–56.7) | |
| D | 50.9 (49.9–59.3) | 49.4 (49–58.7) | |
| FM * (%) | A | 34.6 (23.1–38.7) | 28.3 (18.8–30.2) |
| B | 36.0 (24.1–37.0) | 25.6 (18.4–37.8) | |
| C | 34.1 (18.4–43) | 35.3 (19.1–41.9) | |
| D | 35.8 (33.8–38.1) | 33.5 (34.5–35.1) | |
| FFM * (%) | A | 64.6 (61.9–65.6) | 74.7 (68.3–81.2) |
| B | 65.7 (62.5–74.3) | 77.6 (66.2–82.6) | |
| C | 65.9 (57–81.6) | 64.7 (58.1–80.9) | |
| D | 65.2 (61.9–66.2) | 64.5 (63.9–65.5) | |
| BCM * (%) | A | 47.3 (40.2–50.1) | 51.3 (46.5–56.1) |
| B | 54 (51.9–56.8) | 51.3 (48.8–52.6) | |
| C | 43.2 (39.7–51.7) | 45.9 (42.1–53.8) | |
| D | 46.2 (39.3–49.2) | 49.1 (40–50.1) | |
| QRFT left * (cm) | A | 1.03 (0.89–1.63) | 1.12 (0.88–1.98) |
| B | 1.52 (1.40–1.8) | 1.87 (1.26–2.19) | |
| C | 1.23 (0.73–1.8) | 1.25 (0.7–1.85) | |
| D | 1.27 (1.34–1.87) | 1.34 (1.2–1.84) | |
| QRFT right * (cm) | A | 1.05 (0.77–1.74) | 1.13 (0.94–1.8) |
| B | 1.24 (1.05–1.84) | 1.50 (1.17–1.90) | |
| C | 1.18 (0.87–1.44) | 1.22 (0.7–1.68) | |
| D | 1.65 (1.15–1.7) | 1.72 (1.15–1.92) | |
| QVIT left * (cm) | A | 0.75 (0.57–1.38) | 1.06 (0.72–1.52) |
| B | 1.60 (1.02–1.90) | 1.78 (1.33–2.38) | |
| C | 0.86 (0.76–1.4) | 1.01 (0.73–1.54) | |
| D | 1.36 (0.84–1.46) | 1.43 (1.00–1.53) | |
| QVIT right * (cm) | A | 0.76 (0.62–1.37) | 1.14 (0.85–1.46) |
| B | 1.37 (0.91–2.11) | 1.53 (0.98–1.73) | |
| C | 0.87 (0.66–1.64) | 1.06 (0.78–1.46) | |
| D | 1.57 (0.87–1.67) | 1.47 (0.82–1.67) | |
| Handgrip left * (kg) | A | 33.1 (15.2–56.9) | 35.2 (16.7–54.0) |
| B | 42.4 (18.9–55.7) | 41.3 (24.3–58.4) | |
| C | 25.4 (14–57.5) | 34.3 (18.3–54.3) | |
| D | 26.5 (18.9–27.5) | 23.8 (19.1–24.8) | |
| Handgrip right * (kg) | A | 39.7 (18.5–56.0) | 38.6 (10.2–62.3) |
| B | 47.9 (26.1–62.3) | 44.7 (25.3–62.3) | |
| C | 24.4 (15.6–47.8) | 31.3 (17.2–47.5) | |
| D | 23.6 (23.6–23.9) | 22.7 (21.7–25.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grazioli, E.; Tranchita, E.; Marrone, G.; Urciuoli, S.; Di Lauro, M.; Cerulli, C.; Piacentini, N.; Murri, A.; Celotto, R.; Romani, A.; et al. The Impact of Functional Bars and Adapted Physical Activity on Quality of Life in Chronic Kidney Disease: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 3281. https://doi.org/10.3390/ijerph19063281
Grazioli E, Tranchita E, Marrone G, Urciuoli S, Di Lauro M, Cerulli C, Piacentini N, Murri A, Celotto R, Romani A, et al. The Impact of Functional Bars and Adapted Physical Activity on Quality of Life in Chronic Kidney Disease: A Pilot Study. International Journal of Environmental Research and Public Health. 2022; 19(6):3281. https://doi.org/10.3390/ijerph19063281
Chicago/Turabian StyleGrazioli, Elisa, Eliana Tranchita, Giulia Marrone, Silvia Urciuoli, Manuela Di Lauro, Claudia Cerulli, Nicolò Piacentini, Arianna Murri, Roberto Celotto, Annalisa Romani, and et al. 2022. "The Impact of Functional Bars and Adapted Physical Activity on Quality of Life in Chronic Kidney Disease: A Pilot Study" International Journal of Environmental Research and Public Health 19, no. 6: 3281. https://doi.org/10.3390/ijerph19063281
APA StyleGrazioli, E., Tranchita, E., Marrone, G., Urciuoli, S., Di Lauro, M., Cerulli, C., Piacentini, N., Murri, A., Celotto, R., Romani, A., Parisi, A., Di Daniele, N., & Noce, A. (2022). The Impact of Functional Bars and Adapted Physical Activity on Quality of Life in Chronic Kidney Disease: A Pilot Study. International Journal of Environmental Research and Public Health, 19(6), 3281. https://doi.org/10.3390/ijerph19063281