Comparison of the Ability of Anthropometric Indices to Predict the Risk of Diabetes Mellitus in South African Males: SANHANES-1
Abstract
:1. Introduction
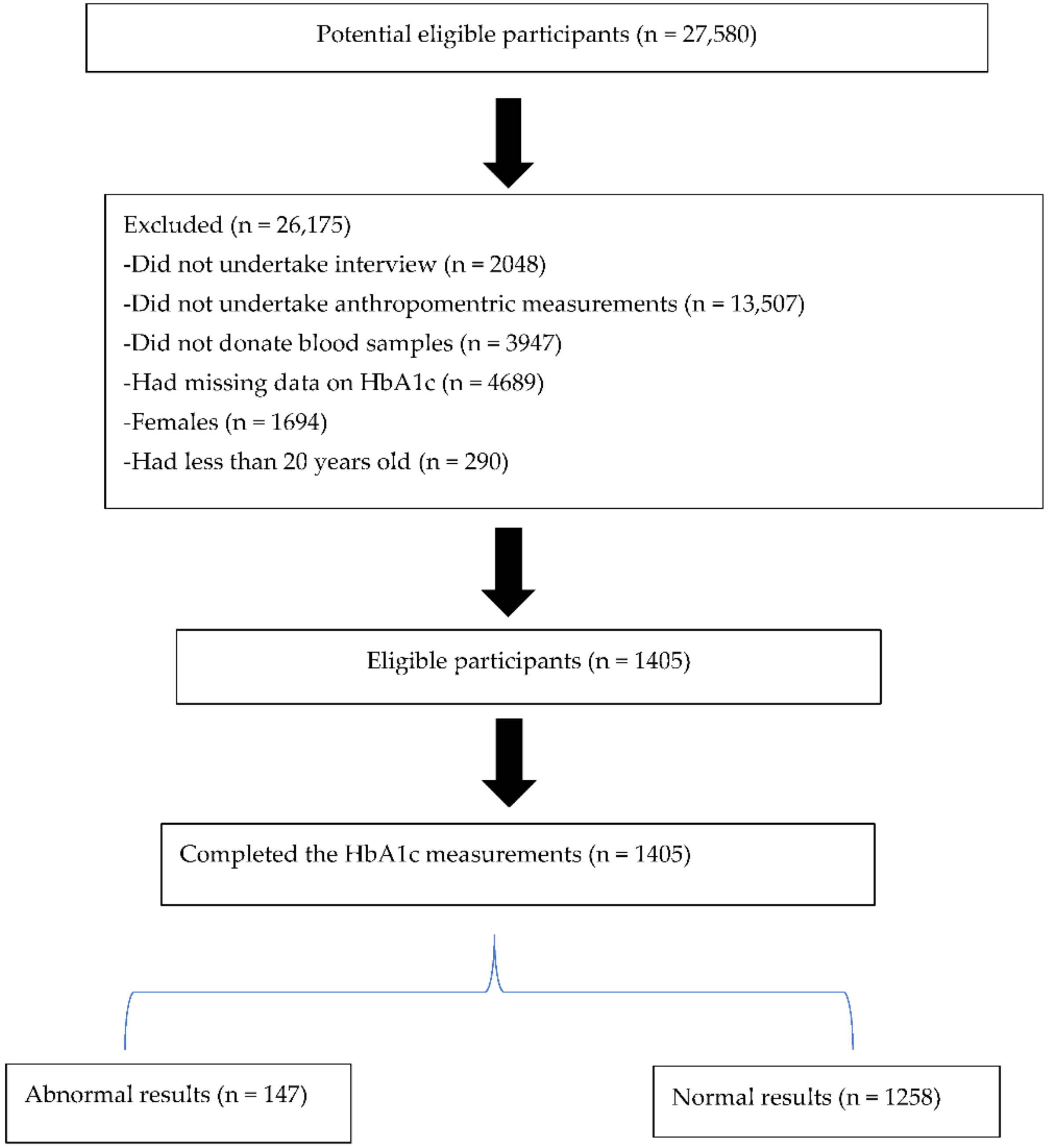
2. Materials and Methods
2.1. Sampling Procedure
2.2. Anthropometric Measurements
2.2.1. Weight
2.2.2. Height
2.2.3. Body Mass Index
2.2.4. Waist Circumference
2.2.5. Hip Circumference
2.2.6. Waist-to-Hip Ratio
2.2.7. Waist-to-Height Ratio
2.3. Biomarkers
Glycated Hemoglobin
2.4. Statistical Analysis
3. Results
Descriptive Analysis
4. Discussion
5. Conclusions
- Based on the current research, 34.6%, 10.5%, 10.4%, 36.6% and 61.0% of South African males aged 20 years and older had BMI ≥ 25 kg/m2, HbA1c ≥ 6.5, WC ≥ 94, WHR > 0.91 and WHtR > 0.5, respectively.
- After adjusting for age group, South African men with abnormal BMI, WC, WHR and WHtR were 4-, 6-, 5-, and 8-fold more likely to present with higher abnormal levels of HbA1c.
- Further adjusted for age group, race, employment, province, locality, education, triglycerides, LDL-C and total cholesterol, ORs decreased for all indices to 2 for BMI, 5 for WC, 3 for WHR, and 5 for WHtR to pre-sent participants with higher abnormal levels of HbA1c.
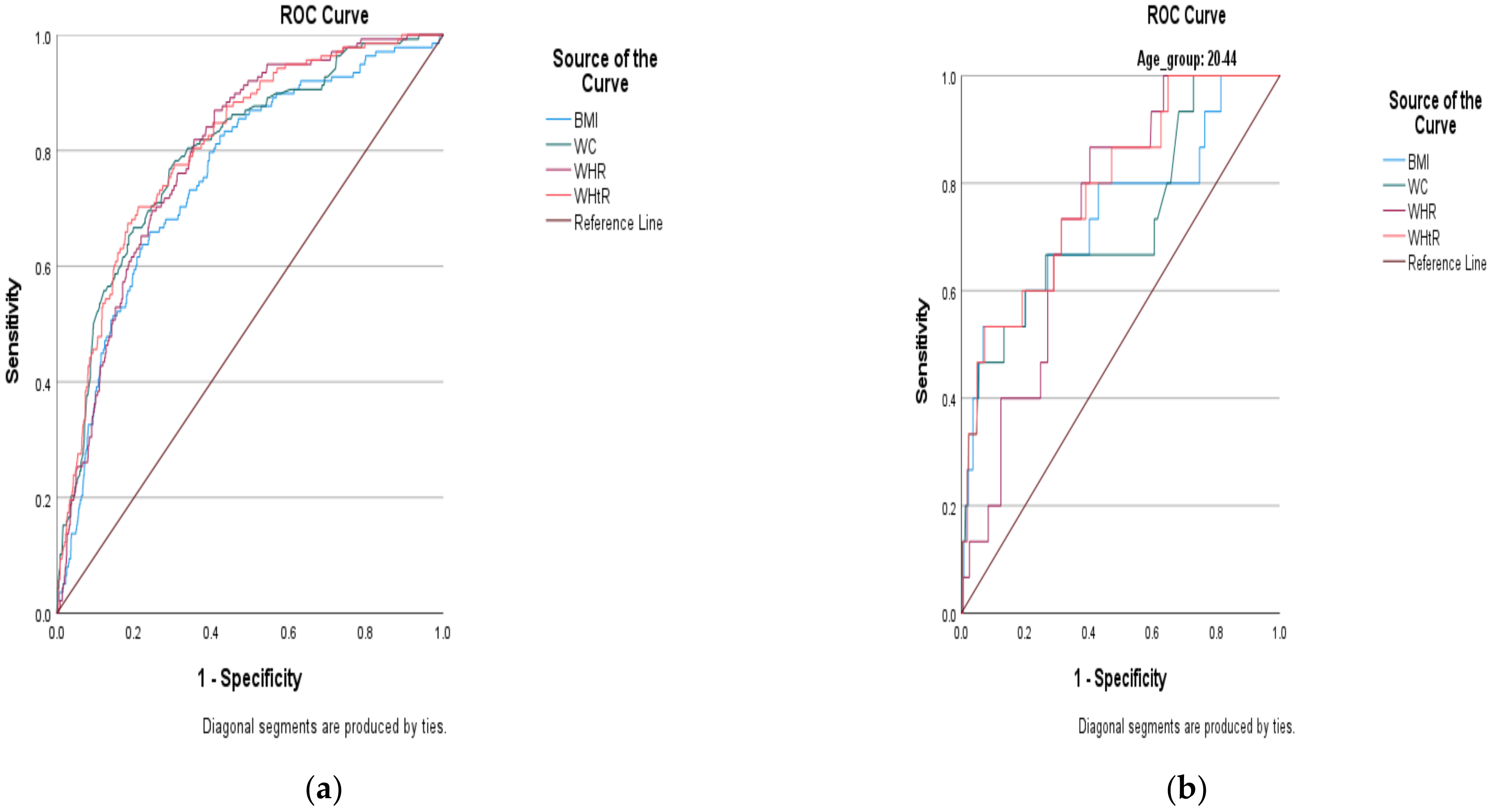
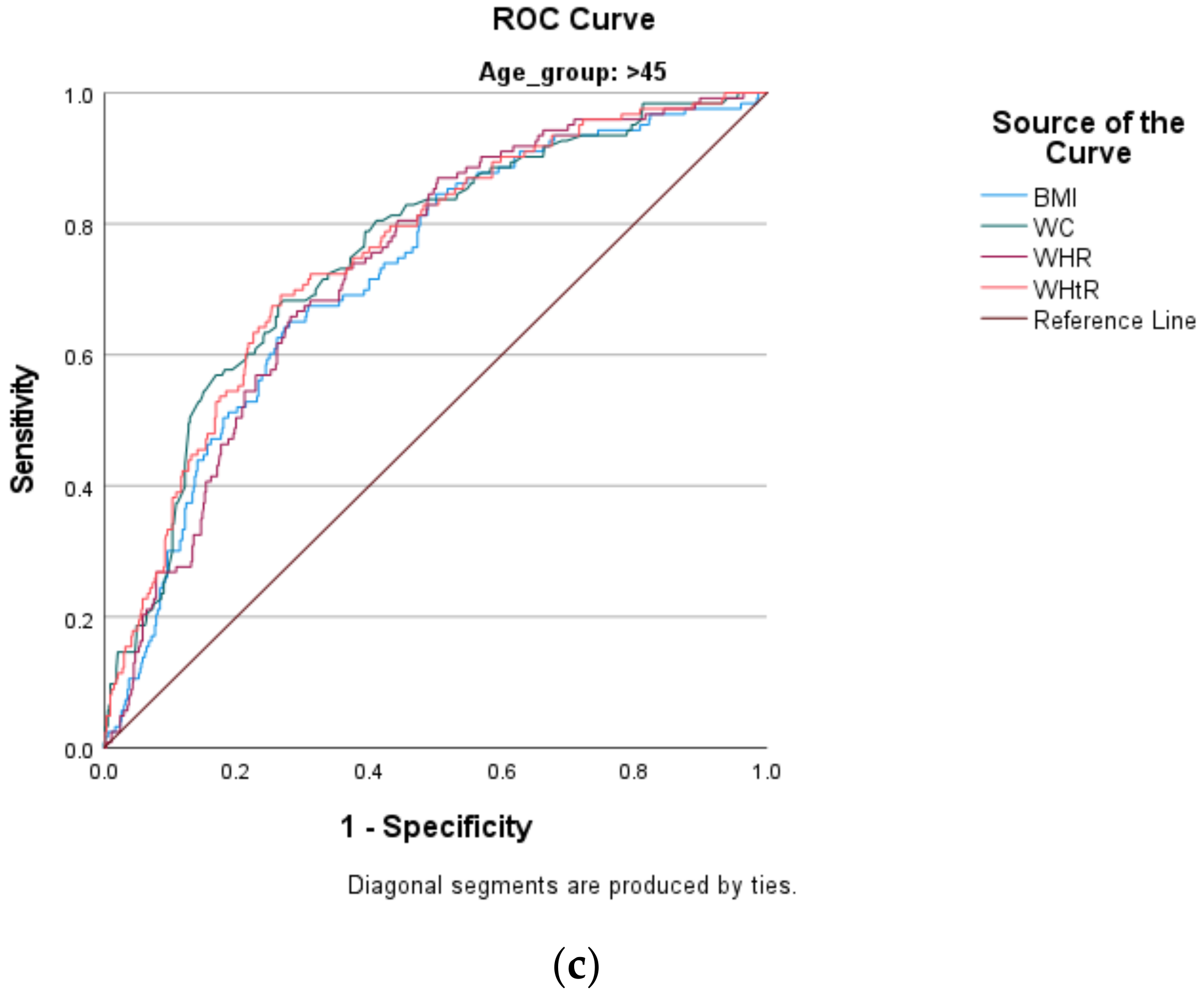
- Based on the area under the curve (AUC) outcomes, WC, WHR and WHtR excellently predicted the risk of DM (with corresponding AUCs of 80.4%, 80.2% and 80.6%, respectively).
- ○
- This means that these indices could be used independently to predict the risk of DM.
- Body mass index (BMI) shows acceptable ability to predict the risk of DM (i.e., AUC of 75.6%).
- ○
- This means that BMI could still be used independently to predict the risk of DM.
- ○
- However, we recommend pairing it with another strong index (especially a high-performing index such as WHtR) that considers central adiposity to supplement its ability to predict the risk of DM.
- Based on confidence interval (CI) levels that do not overlap, the AUC for BMI was not significantly differ-ent from those of WC, WHR and WHtR.
- Because age is a confounder when using anthropometric indices to predict the risk of DM, we recommend the use of lower cut-off points than those pre-specified by the WHO and the IDF, in order to improve the predictability of DM in all age groups of adult South African men.
- ○
- The following cut-off points are for younger men and are presented in Table 5 (i.e., ≥24.23 kg/m2, ≥87.45 cm, >0.88 and 0.48 for BMI, WC, WHR, and WHtR, respectively)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Department of Health (NDoH); Statistics South Africa (Stats SA); South African Medical Research Council (SAMRC); ICF. South Africa Demographic and Health Survey 2016; National Department of Health: Pretoria, South Africa, 2019.
- Shisana, O.; Labadarios, D.; Rehle, T.; Simbayi, L.; Zuma, K.; Dhansay, A.; Reddy, P.; Parker, W.; Hoosain, E.; Naidoo, P.; et al. The South African National Health and Nutrition Examination Survey, 2012: SANHANES-1: The Health and Nutritional Status of the Nation; HSRC Press: Cape Town, South Africa, 2014; Available online: http://www.hsrc.ac.za/en/research-outputs/view/6493 (accessed on 13 February 2022).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.J.; Lagerpusch, M.; Enderle, J.; Schautz, B.; Heller, M.; Bosy-Westphal, A. Beyond the body mass index: Tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes. Rev. 2012, 13, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Hong, T.J.; Hong, J.Y.; Choi, J.H.; Kim, B.W.; Ahn, J.; Park, J.S.; Oh, J.H.; Choi, J.H.; Lee, H.C.; et al. Waist–hip ratio and 1-year clinical outcome in patients with non-ST-elevation myocardial infarctions. Coron. Artery Dis. 2016, 27, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.; Knuiman, M.; Davis, W.A.; Davis, T.M.; Bruce, D.G. A comparison of obesity indices in relation to mortality in type 2 diabetes: The Fremantle diabetes study. Diabetologia 2020, 63, 528–536. [Google Scholar] [CrossRef]
- Prakash, K.; Chandran, D.S.; Khadgawat, R.; Jaryal, A.K.; Deepak, K.K. Waist Circumference Rather than Body Mass Index is Better Indicator of Insulin Resistance in Type 2 Diabetes Mellitus in North Indian Population. Indian J. Physiol. Pharmacol. 2016, 60, 52–56. [Google Scholar]
- Thomas, R.; Ambookan, P.V.; Jose, J.; Unnikrishnan, U.G. The accuracy of anthropometric measurements of general and central obesity for the prediction of impaired glucose tolerance among the adult population of South India. J. Fam. Med. Prim. Care 2020, 9, 3416. [Google Scholar] [CrossRef]
- Oboh, H.A.; Adedeji Adebowale, A. Correlation of waist-hip-ratio and waist-height-ratio to cardiovascular risks factors in a Nigerian population. Nig. Q. J. Med. 2011, 21, 16–24. [Google Scholar]
- Zyriax, B.C.; Schoeffauer, M.; Klipstein-Grobusch, K.; Boeing, H.; Windler, E. Differential association of anthropometric parameters with coronary risk in women–data of the CORA study. Obes. Fact. 2011, 4, 358–364. [Google Scholar] [CrossRef]
- Stokes, A.; Berry, K.M.; Mchiza, Z.; Parker, W.A.; Labadarios, D.; Chola, L.; Hongoro, C.; Zuma, K.; Brennan, A.T.; Rockers, P.C.; et al. Prevalence and unmet need for diabetes care across the care continuum in a national sample of South African adults: Evidence from the SANHANES-1, 2011–2012. PLoS ONE 2017, 12, e0184264. [Google Scholar] [CrossRef] [Green Version]
- IDF Diabetes Atlas, 2018–2019, 9th ed. Available online: https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf (accessed on 26 February 2021).
- Bailey, S.L.; Ayles, H.; Beyers, N.; Godfrey-Faussett, P.; Muyoyeta, M.; du Toit, E.; Yudkin, J.S.; Floyd, S. Diabetes mellitus in Zambia and the Western Cape province of South Africa: Prevalence, risk factors, diagnosis and management. Diabetes Res. Clin. Pract. 2016, 118, 1–11. [Google Scholar] [CrossRef] [Green Version]
- South Africa Demographic and Health Survey 1998. Available online: https://www.dhsprogram.com/pubs/pdf/FR131/FR131.pdf (accessed on 26 February 2021).
- Shukla, A.; Kumar, K.; Singh, A. Association between obesity and selected morbidities: A study of BRICS countries. PLoS ONE 2014, 9, e94433. [Google Scholar] [CrossRef] [Green Version]
- Maimela, E.; Alberts, M.; Modjadji, S.E.; Choma, S.S.; Dikotope, S.A.; Ntuli, T.S.; Van Geertruyden, J.P. The prevalence and determinants of chronic non-communicable disease risk factors amongst adults in the Dikgale health demographic and surveillance system (HDSS) site, Limpopo Province of South Africa. PLoS ONE 2016, 11, e0147926. [Google Scholar] [CrossRef] [Green Version]
- Joubert, J.; Norman, R.; Bradshaw, D.; Goedecke, J.H.; Steyn, N.P.; Puoane, T. Estimating the burden of disease attributable to excess body weight in South Africa in 2000. S. Afr. Med. J. 2007, 97, 683–690. [Google Scholar]
- Patel, C.J.; Bhattacharya, J.; Butte, A.J. An environment-wide association study (EWAS) on type 2 diabetes mellitus. PLoS ONE 2010, 5, e10746. [Google Scholar] [CrossRef]
- Department of Health/South Africa; Medical Research Council/South Africa; ORC Macro. South Africa Demographic and Health Survey 2003. Department of Health/South Africa: Pretoria, South Africa, 2017. Available online: http://dhsprogram.com/pubs/pdf/FR206/FR206.pdf (accessed on 11 March 2021).
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Bradshaw, D.; Steyn, K.; Norman, R.; Laubscher, R. Poverty and non-communicable diseases in South Africa. Scand. J. Public Health 2009, 37, 176–186. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body mass index: Obesity, BMI, and health: A critical review. Nutr. Today 2015, 50, 117. [Google Scholar] [CrossRef] [Green Version]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Tchernof, A.; Després, J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Zalesin, K.C.; Franklin, B.A.; Miller, W.M.; Peterson, E.D.; McCullough, P.A. Impact of obesity on cardiovascular disease. Endocrinol. Metab. Clin. N. Am. 2008, 37, 663–684. [Google Scholar] [CrossRef]
- Chen, B.D.; Yang, Y.N.; Ma, Y.T.; Pan, S.; He, C.H.; Liu, F.; Ma, X.; Fu, Z.Y.; Li, X.M.; Xie, X.; et al. Waist-to-height ratio and triglycerides/high-density lipoprotein cholesterol were the optimal predictors of metabolic syndrome in Uighur men and women in Xinjiang, China. Metab. Syndr. Relat. Disord. 2015, 13, 214–220. [Google Scholar] [CrossRef]
- Chen, B.D.; He, C.H.; Ma, Y.T.; Yang, Y.N.; Liu, F.; Pan, S.; Ma, X.; Li, X.M.; Fu, Z.Y.; Xie, X.; et al. Best anthropometric and atherogenic predictors of metabolic syndrome in the Chinese Han population in Xinjiang: The Cardiovascular Risk Survey. Ann. Nutr. Metab. 2014, 65, 280–288. [Google Scholar] [CrossRef]
- Chiang, J.K.; Koo, M. Lipid accumulation product: A simple and accurate index for predicting metabolic syndrome in Taiwanese people aged 50 and over. BMC Cardiovasc. Disord. 2012, 12, 78. [Google Scholar] [CrossRef] [Green Version]
- Shisana, O.; Rehle, T.; Simbayi, L.; Zuma, K.; Jooste, S.; Wyk, P.V.; Mbelle, N.; Van Zyl, J.; Parker, W.; Zungu, N.P.; et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2008: A Turning Tide Among Teenagers? HSRC Press: Cape Town, South Africa, 2009. [Google Scholar]
- WHO. World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of the WHO Consultation of Obesity. Available online: https://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ (accessed on 13 February 2021).
- Lohman, T.; Roche, A.F.; Martorell, R. Anthropometric Standardization Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Jones, P.R.; Hunt, M.J.; Brown, T.P.; Norgan, N.G. Waist-hip circumference ratio and its relation to age and overweight in British men. Hum. Nutr. Clin. Nutr. 1986, 40, 239–247. [Google Scholar] [PubMed]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation: Geneva. 2008. Available online: https://www.who.int/publications/i/item/9789241501491 (accessed on 13 February 2021).
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Clark, J.R. Positional assessment and physical fitness characteristics of male professional soccer players in South Africa: Sport science. J. Phys. Health Educ. Recreat. Dance 2007, 13, 453–464. [Google Scholar]
- Wells, J.C. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef]
- Yang, J.; Wang, F.; Wang, J.; Han, X.; Hu, H.; Yu, C.; Yuan, J.; Yao, P.; Miao, X.; Wei, S.; et al. Using different anthropometric indices to assess prediction ability of type 2 diabetes in elderly population: A 5 year prospective study. BMC Geriatr. 2018, 18, 218. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, L.; Li, J.; Sun, L.; Qin, W.; Ding, G.; Zhu, J.; Zhang, J.; Yu, Z.; Xie, S. Association of anthropometric indices of obesity with hypertension in Chinese elderly: An analysis of age and gender differences. Int. J. Environ. Res. Public Health 2018, 15, 801. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.H.; Ho, C.C.; Yang, C.F.; Huang, Y.C.; Lai, C.H.; Liaw, Y.P. Waist-to-hip ratio is a better anthropometric index than body mass index for predicting the risk of type 2 diabetes in Taiwanese population. Nutr. Res. 2010, 30, 585–593. [Google Scholar] [CrossRef]
- Mutyambizi, C.; Chola, L.; Groot, W.; Pavlova, M.; Labadarios, D.; Hongoro, C. The extent and determinants of diabetes and cardiovascular disease comorbidity in South Africa—Results from the South African National Health and Nutrition Examination Survey (SANHANES-1). BMC Public Health 2017, 17, 745. [Google Scholar] [CrossRef] [Green Version]
- Lo, K.; Wong, M.; Khalechelvam, P.; Tam, W. Waist-to-height ratio, body mass index and waist circumference for screening paediatric cardio-metabolic risk factors: A meta-analysis. Obes. Rev. 2016, 17, 1258–1275. [Google Scholar] [CrossRef]
- Wang, Y.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am. J. Clin. Nutr. 2005, 81, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Dhana, K.; Ikram, M.; Hofman, A.; Franco, O.; Kavousi, M. Anthropometric measures in cardiovascular disease prediction: Comparison of laboratory-based versus non-laboratory-based model. Heart 2015, 101, 377–383. [Google Scholar] [CrossRef]
- Ahmad, N.; Adam, S.I.; Nawi, A.M.; Hassan, M.R.; Ghazi, H.F. Abdominal obesity indicators: Waist circumference or waist-to-hip ratio in Malaysian adults population. Int. J. Prev. Med. 2016, 7, 82. [Google Scholar]
- Hadaegh, F.; Zabetian, A.; Sarbakhsh, P.; Khalili, D.; James, W.; Azizi, F. Appropriate cutoff values of anthropometric variables to predict cardiovascular outcomes: 7.6 years follow-up in an Iranian population. Int. J. Obes. 2009, 33, 1437–1445. [Google Scholar] [CrossRef] [Green Version]
- Zavorsky, G.S.; Wilson, B. Sex, girth, waists and hips (what matters for gas exchange in extreme obesity?). Respir. Physiol. Neurobiol. 2010, 170, 120–122. [Google Scholar] [CrossRef]
- Ho, S.Y.; Lam, T.H.; Janus, E.D. Waist to stature ratio is more strongly associated with cardiovascular risk factors than other simple anthropometric indices. Ann. Epidemiol. 2003, 13, 683–691. [Google Scholar] [CrossRef]
- Mutyambizi, C.; Booysen, F.; Stokes, A.; Pavlova, M.; Groot, W. Lifestyle and socio-economic inequalities in diabetes prevalence in South Africa: A decomposition analysis. PLoS ONE 2019, 14, e0211208. [Google Scholar] [CrossRef]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- Goh, L.G.; Dhaliwal, S.S.; Welborn, T.A.; Lee, A.H.; Della, P.R. Anthropometric measurements of general and central obesity and the prediction of cardiovascular disease risk in women: A cross-sectional study. BMJ Open 2014, 4, e004138. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. 2000. Available online: https://books.google.co.za/books?hl=en&lr=&id=AvnqOsqv9doC&oi=fnd&pg=PA1&dq=1World+Health+Organization+(WHO)+.+Obesity:+Preventing+and+Managing+the+Global+Epidemic.+WHO+Technical+Report+Series+No.+894.+Geneva:+WHO,+2000.&ots=6WE5crZV9M&sig=yG4z3_KoJWoSORmzbw9b6rcYK4&redir_esc=y#v=onepage&q&f=false (accessed on 16 March 2021).
- Mchiza, Z.J.; Parker, W.A.; Hossin, M.Z.; Heshmati, A.; Labadarios, D.; Falkstedt, D.; Koupil, I. Social and psychological predictors of body mass index among south africans 15 years and older: SANHANES-1. Int. J. Environ. Res. Public Health 2019, 16, 3919. [Google Scholar] [CrossRef] [Green Version]
| n (%) | |
|---|---|
| Age Group (Years) | |
| 20–44 | 695 (49.5) |
| >45 | 710 (50.5) |
| Race *** | |
| Black * | 888 (63.2) |
| Non-Black ** | 510 (36.3) |
| Locality | |
| Urban formal | 744 (53.0) |
| Urban informal | 147 (10.5) |
| Rural formal (farms) | 260 (18.5) |
| Rural informal (tribal) | 254 (18.1) |
| Body mass index (kg/m2) | |
| Normal BMI, 18.5–24.9 | 878 (65.4) |
| Overweight/obesity, >25 | 465 (34.6) |
| Diabetes mellitus (%) | |
| Normal, HbA1c < 6.5 | 1258 (89.5) |
| Abnormal, HbA1c ≥ 6.5 | 147 (10.5) |
| Waist circumference (cm) | |
| Normal, WC < 94 | 1203 (89.2) |
| Abnormal, WC ≥ 94 | 146 (10.4) |
| Waist-to-hip ratio | |
| Normal, WHR < 0.91 | 850 (63.4) |
| Abnormal, WHR > 0.91 | 491 (36.6) |
| Waist-to-height ratio | |
| Normal, WHtR < 0.5 | 470 (35.4) |
| Abnormal, WHtR > 0.5 | 857 (61.0) |
| Anthropometric Indices and HbA1c | Mean ± SD |
|---|---|
| Weight (kg) | 67.3 ± 16.4 |
| Height (cm) | 167.9 ± 8.2 |
| Body mass index (kg/m2) | 24.1 ± 5.9 |
| Waist circumference (cm) | 83.0 ± 14.2 |
| Hip circumference (cm) | 93.9 ± 11.8 |
| Waist-to-hip ratio | 0.9 ± 0.1 |
| Waist-to-height ratio | 0.5 ± 0.1 |
| HbA1c (%) | 5.9 ± 1.0 |
| Unadjusted | Adjusted OR Model 1 | Adjusted OR Model 2 | Adjusted OR Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude OR | 95% CI | p-Value | AOR | 95% CI | p-Value | AOR | 95%CI | p-Value | AOR | 95% CI | p-Value | |
| BMI | 5.061 | 3.474–7.374 | <0.001 | 4.142 | 2.814–6.097 | <0.001 | 3.687 | 2.260–6.016 | <0.001 | 2.445 | 1.213–4.929 | 0.012 |
| WC | 7.133 | 4.779–10.647 | <0.001 | 6.285 | 4.136–9.550 | <0.001 | 6.533 | 3.746–11.394 | <0.001 | 4.950 | 2.243–10.926 | <0.001 |
| WHR | 7.064 | 4.698–10.623 | <0.001 | 4.800 | 3.141–7.334 | <0.001 | 4.836 | 2.881–8.118 | <0.001 | 2.926 | 1.503–5.697 | 0.002 |
| WHtR | 12.151 | 5.632–26.215 | <0.001 | 8.108 | 3.721–17.667 | <0.001 | 8.406 | 3.235–21.840 | <0.001 | 4.590 | 1.603–13.141 | 0.005 |
| Anthropometric Index | AUC | p-Value | 95% CI | Cut-Off Point | Sensitivity | 1-Specificity |
|---|---|---|---|---|---|---|
| BMI kg/m2 | 0.756 | <0.001 | 0.714–0.798 | 24.64 | 0.703 | 0.319 |
| WC cm | 0.804 | <0.001 | 0.754–0.833 | 88.95 | 0.710 | 0.262 |
| WHR | 0.802 | <0.001 | 0.757–0.827 | 0.921 | 0.703 | 0.257 |
| WHtR | 0.806 | <0.001 | 0.769–0.842 | 0.543 | 0.703 | 0.211 |
| Age 20–44 Years (n = 695) | Age > 45 years (n = 710) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthropometric Index | AUC | 95% CI | p-Value | Cut-Off Point | Sensitivity | 1-Specificity | AUC | 95% CI | p-Value | Cut-Off Point | Sensitivity | 1-Specificity |
| BMI kg/m2 | 0.742 | 0.593–0.890 | 0.001 | 24.23 | 0.667 | 0.271 | 0.724 | 0.676–0.772 | <0.001 | 24.65 | 0.707 | 0.400 |
| WC cm | 0.729 | 0.583–0.874 | 0.002 | 87.45 | 0.600 | 0.201 | 0.754 | 0.707–0.801 | <0.001 | 89.75 | 0.715 | 0.330 |
| WHR | 0.740 | 0.645–0.836 | 0.001 | 0.875 | 0.733 | 0.314 | 0.733 | 0.688–0.779 | <0.001 | 0.926 | 0.707 | 0.358 |
| WHtR | 0.788 | 0.674–0.903 | <0.001 | 0.483 | 0.733 | 0.314 | 0.753 | 0.707–0.800 | <0.001 | 0.544 | 0.707 | 0.300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekgala, M.D.; Sewpaul, R.; Opperman, M.; Mchiza, Z.J. Comparison of the Ability of Anthropometric Indices to Predict the Risk of Diabetes Mellitus in South African Males: SANHANES-1. Int. J. Environ. Res. Public Health 2022, 19, 3224. https://doi.org/10.3390/ijerph19063224
Sekgala MD, Sewpaul R, Opperman M, Mchiza ZJ. Comparison of the Ability of Anthropometric Indices to Predict the Risk of Diabetes Mellitus in South African Males: SANHANES-1. International Journal of Environmental Research and Public Health. 2022; 19(6):3224. https://doi.org/10.3390/ijerph19063224
Chicago/Turabian StyleSekgala, Machoene D., Ronel Sewpaul, Maretha Opperman, and Zandile J. Mchiza. 2022. "Comparison of the Ability of Anthropometric Indices to Predict the Risk of Diabetes Mellitus in South African Males: SANHANES-1" International Journal of Environmental Research and Public Health 19, no. 6: 3224. https://doi.org/10.3390/ijerph19063224
APA StyleSekgala, M. D., Sewpaul, R., Opperman, M., & Mchiza, Z. J. (2022). Comparison of the Ability of Anthropometric Indices to Predict the Risk of Diabetes Mellitus in South African Males: SANHANES-1. International Journal of Environmental Research and Public Health, 19(6), 3224. https://doi.org/10.3390/ijerph19063224







