Pneumocystis Colonization in Dogs Is as in Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Testing
2.2. Definition of PCP
2.3. Statistics
3. Results
3.1. Pneumocystis-Negative Dogs with Lower Airway and/or Lung Disease
3.2. Pneumocystis-Positive Dogs with Lower Airway and Lung Disease with or without PCP
3.3. Comparison Positive/Negative Statistic Data
4. Discussion
- characteristic changes in chest radiographs, consisting of a dense and diffuse interstitial pattern, in association with right sided cardiac enlargement and signs of pulmonary hypertension on radiographs and using spectral Doppler echocardiography
- extensive ground glass densities in pulmonary CT scans
- the presence of cysts and/or trophozoite morphotypes on BALF cytology from stained smears (not always present but definitive when observed)
- P. canis qPCR positivity, with CT less than 26 (our current arbitrary cut-off)
- favourable response to TMS therapy, usually with corticosteroids for the first few days of therapy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
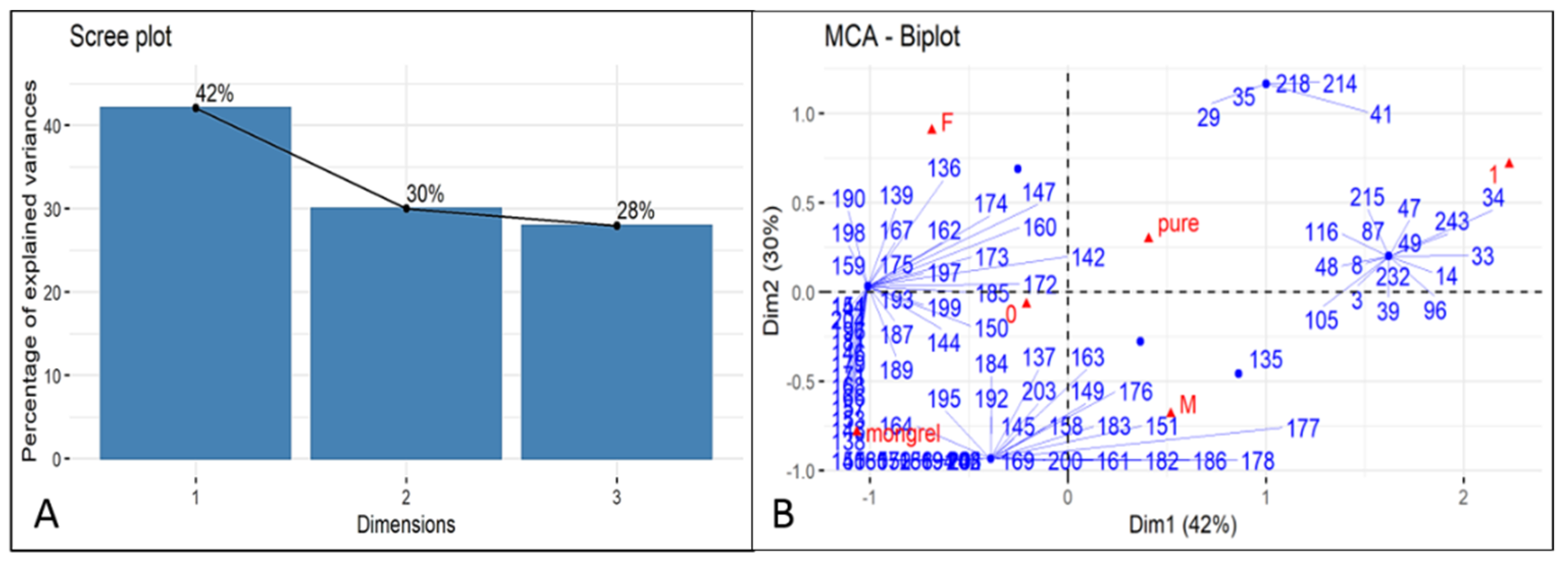
References
- Aliouat-Denis, C.M.; Chabé, M.; Demanche, C.; Aliouat, E.M.; Viscogliosi, E.; Guillot, J.; Delhaes, L.; Dei-Cas, E. Pneumocystis species, co-evolution and pathogenic power. Infect. Genet. Evol. 2008, 8, 708–726. [Google Scholar] [CrossRef]
- Dei-Cas, E. Pneumocystis infections: The iceberg? Med. Mycol. 2000, 38 (Suppl. 1), 23–32. [Google Scholar] [CrossRef][Green Version]
- Durand-Joly, I.; Aliouat, E.M.; Recourt, C.; Guyot, K.; François, N.; Wauquier, M.; Camus, D.; Dei-Cas, E. Pneumocystis carinii f. sp. hominis is not infectious for SCID mice. J. Clin. Microbiol. 2002, 40, 1862–1865. [Google Scholar]
- Frenkel, J.K. Pneumocystis jiroveci n. sp. from man: Morphology, physiology, and immunology in relation to pathology. Natl. Cancer Inst. Monogr. 1976, 43, 13–30. [Google Scholar]
- Cushion, M.; Keely, S.; Stringer, J. Molecular and phenotypic description of Pneumocystis wakefieldiae sp. nov., a new species in rats. Mycologia 2004, 96, 429–438. [Google Scholar] [CrossRef]
- Dei-Cas, E.; Chabé, M.; Moukhlis, R.; Durand-Joly, I.; Aliouat, E.M.; Stringer, J.R.; Cushion, M.; Noë, C.; Sybren De Hoog, G.; Guillot, J.; et al. Pneumocystis oryctolagi sp. nov., an uncultured fungus causing pneumonia in rabbits at weaning: Review of current knowledge, and description of a new taxon on genotypic, phylogenetic and phenotypic bases. FEMS Microbiol. Rev. 2006, 30, 853–871. [Google Scholar] [CrossRef]
- Cissé, O.H.; Ma, L.; Dekker, J.P.; Khil, P.P.; Youn, J.H.; Brenchley, J.M.; Blair, R.; Pahar, B.; Chabé, M.; Van Rompay, K.K.A.; et al. Genomic insights into the host specific adaptation of the Pneumocystis genus. Commun. Biol. 2021, 4, 305. [Google Scholar] [CrossRef]
- Aliouat-Denis, C.M.; Chabé, M.; Delhaes, L.; Dei-Cas, E. Aerially transmitted human fungal pathogens: What can we learn from metagenomics and comparative genomics? Rev. Iberoam. Micol. 2014, 31, 54–61. [Google Scholar] [CrossRef]
- Chabé, M.; Aliouat, E.M.; Durand-Joly, I.; Gantois, N.; Conseil, V.; López, C.; Duriez, T.; Dei-Cas, E.; Vargas, S.L. Exploring transplacental transmission of Pneumocystis oryctolagi in first-time pregnant and multiparous rabbit does. Med. Mycol. 2007, 45, 701–707. [Google Scholar]
- Montes-Cano, M.A.; Chabe, M.; Fontillon-Alberdi, M.; De La Horra, C.; Respaldiza, N.; Medrano, F.J.; Varela, J.M.; Dei-Cas, E.; Calderon, E.J. Vertical transmission of Pneumocystis jirovecii in humans. Emerg. Infect. Dis. 2009, 15, 125–127. [Google Scholar] [CrossRef]
- Vargas, S.L.; Hughes, W.T.; Santolaya, M.E.; Ulloa, A.V.; Ponce, C.A.; Cabrera, C.E.; Cumsille, F.; Gigliotti, F. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin. Infect. Dis. 2001, 32, 855–861. [Google Scholar] [CrossRef]
- Rojas, P.; Friaza, V.; García, E.; de la Horra, C.; Vargas, S.L.; Calderón, E.J.; Pavón, A. Early Acquisition of Pneumocystis jirovecii colonization and potential association with respiratory distress syndrome in preterm newborn infants. Clin. Infect. Dis. 2017, 65, 976–981. [Google Scholar] [CrossRef]
- Weissenbacher-Lang, C.; Fuchs-Baumgartinger, A.; Guija-De-Arespacochaga, A.; Klang, A.; Weissenböck, H.; Künzel, F. Pneumocystosis in dogs: Meta-analysis of 43 published cases including clinical signs, diagnostic procedures, and treatment. J. Vet. Diagn. Investig. 2018, 30, 26–35. [Google Scholar] [CrossRef]
- Best, M.P.; Boyd, S.P.; Danesi, P. Confirmed case of Pneumocystis pneumonia in a Maltese Terrier × Papillon dog being treated with toceranib phosphate. Aust. Vet. J. 2019, 97, 162–165. [Google Scholar] [CrossRef]
- Sakashita, T.; Kaneko, Y.; Izzati, U.Z.; Hirai, T.; Fuke, N.; Torisu, S.; Yamaguchi, R. Disseminated pneumocystosis in a Toy Poodle. J. Comp. Pathol. 2020, 175, 85–89. [Google Scholar] [CrossRef]
- Schiborra, F.; Scudder, C.J.; Littler, R.M.; Lamb, C.R.; McConnell, J.F.; Maddox, T.W. CT findings in Pneumocystis carinii pneumonia in five dogs. Small Anim. Pract. 2018, 59, 508–513. [Google Scholar] [CrossRef]
- Okine, A.A.K.; Chapman, S.; Hostutler, R.A.; Livingston, R. Diagnosis of Pneumocystis pneumonia in a 2-year-old King Charles Cavalier Spaniel using the polymerase chain reaction. Vet. Clin. Pathol. 2018, 47, 146–149. [Google Scholar] [CrossRef]
- Petini, M.; Furlanello, T.; Danesi, P.; Zoia, A. Nested–polymerase chain reaction detection of Pneumocystis carinii f. sp. canis in a suspected immunocompromised Cavalier King Charles spaniel with multiple infections. SAGE Open Med. Case Rep. 2019, 7, 2050313X1984116. [Google Scholar] [CrossRef]
- Merrill, K.; Coffey, E.; Furrow, E.; Masseau, I.; Rindt, H.; Reinero, C. X-linked CD40 ligand deficiency in a 1-year-old male Shih Tzu with secondary Pneumocystis pneumonia. J. Vet. Intern. Med. 2021, 35, 497–503. [Google Scholar] [CrossRef]
- Alanio, A.; Bretagne, S. Pneumocystis jirovecii detection in asymptomatic patients: What does its natural history tell us? F1000Research 2017, 6, 739. [Google Scholar] [CrossRef]
- Morris, A.; Norris, K.A. Colonization by Pneumocystis jirovecii and its role in disease. Clin. Microbiol. Rev. 2012, 25, 297–317. [Google Scholar] [CrossRef]
- Danesi, P.; Ravagnan, S.; Johnson, L.R.; Furlanello, T.; Milani, A.; Martin, P.; Boyd, S.; Best, M.; Galgut, B.; Irwin, P.; et al. Molecular diagnosis of Pneumocystis pneumonia in dogs. Med. Mycol. 2017, 55, 828–842. [Google Scholar] [CrossRef]
- Danesi, P.; Corrò, M.; Falcaro, C.; Carminato, A.; Furlanello, T.; Cocchi, M.; Krockenberger, M.B.; Meyer, W.; Capelli, G.; Malik, R. Molecular detection of Pneumocystis in the lungs of cats. Med. Mycol. 2019, 57, 813–824. [Google Scholar]
- Ralph, E.; Reppas, G.; Halliday, C.; Krockenberger, M.; Malik, R.; Ralph, E.; Reppas, G.; Halliday, C.; Krockenberger, M.; Malik, R. Pneumocystis canis pneumonia in dogs. Microbiol. Aust. 2015, 36, 79–82. [Google Scholar] [CrossRef][Green Version]
- Hagiwara, Y.; Fujiwara, S.; Takai, H.; Ohno, K.; Masuda, K.; Furuta, T.; Nakayama, H.; Doi, K.; Tsujimoto, H. Pneumocystis carinii pneumonia in a Cavalier King Charles Spaniel. J. Vet. Med. Sci. 2001, 63, 349–351. [Google Scholar] [CrossRef]
- Farrow, B.R.H.; Watson, A.D.J.; Hartley, W.J.; Huxtable, C.R.R. Pneumocystis pneumonia in the dog. J. Comp. Pathol. 1972, 82, 447–453. [Google Scholar] [CrossRef]
- Lobetti, R. Common variable immunodeficiency in miniature dachshunds affected with Pneumonocystis carinii pneumonia. J. Vet. Diagn. Investig. 2000, 12, 39–45. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 29 December 2021).
- Fauchier, T.; Hasseine, L.; Gari-Toussaint, M.; Casanova, V.; Marty, P.M.; Pomares, C. Detection of Pneumocystis jirovecii by quantitative PCR to differentiate colonization and pneumonia in immunocompromised HIV-positive and HIV-negative patients. J. Clin. Microbiol. 2016, 54, 1487. [Google Scholar] [CrossRef]
- Yang, S.L.; Wen, Y.H.; Wu, Y.S.; Wang, M.C.; Chang, P.Y.; Yang, S.; Lu, J.J. Diagnosis of Pneumocystis pneumonia by real-time PCR in patients with various underlying diseases. J. Microbiol. Immunol. Infect. 2020, 53, 785–790. [Google Scholar] [CrossRef]
- Kureljušić, B.; Weissenbacher-Lang, C.; Nedorost, N.; Stixenberger, D.; Weissenböck, H. Association between Pneumocystis spp. and co-infections with Bordetella bronchiseptica, Mycoplasma hyopneumoniae and Pasteurella multocida in Austrian pigs with pneumonia. Vet. J. 2016, 207, 177–179. [Google Scholar] [CrossRef]
- Morris, A.; Wei, K.; Afshar, K.; Huang, L. Epidemiology and clinical significance of Pneumocystis colonization. J. Infect. Dis. 2008, 197, 10–17. [Google Scholar] [CrossRef]
- Stenner, V.J.; MacKay, B.; King, T.; Barrs, V.R.D.; Irwin, P.; Abraham, L.; Swift, N.; Langer, N.; Bernays, M.; Hampson, E.; et al. Protothecosis in 17 Australian dogs and a review of the canine literature. Med. Mycol. 2007, 45, 249–266. [Google Scholar] [CrossRef]
- Malik, R.; Martin, P.; Wigney, D.; Swan, D.; Sattler, P.S.; Cibilic, D.; Allen, J.; Mitchell, D.H.; Chen, S.C.A.; Hughes, M.S.; et al. Treatment of canine leproid granuloma syndrome: Preliminary findings in seven dogs. Aust. Vet. J. 2001, 79, 30–36. [Google Scholar] [CrossRef]
- Krockenberger, M.B.; Swinney, G.; Martin, P.; Rothwell, T.R.L.; Malik, R. Sequential opportunistic infections in two German Shepherd dogs. Aust. Vet. J. 2011, 89, 9–14. [Google Scholar] [CrossRef]
- Elad, D. Disseminated canine mold infections. Vet. J. 2019, 243, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Watt, P.R.; Robins, G.M.; Galloway, A.M.; O’Boyle, D.A. Disseminated opportunistic fungal disease in dogs: 10 cases (1982–1990). J. Am. Vet. Med. Assoc. 1995, 207, 67–70. [Google Scholar]
- Board, K.F.; Patil, S.; Lebedeva, I.; Capuano, S., 3rd; Trichel, A.M.; Murphey-Corb, M.; Rajakumar, P.A.; Flynn, J.L.; Haidaris, C.G.; Norris, K.A. Experimental Pneumocystis carinii pneumonia in simian immunodeficiency virus-infected rhesus macaques. J. Infect. Dis. 2003, 187, 576–588. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Case No. | Ct | Breed | Age (y) | Sex | Clinical Signs | Radiographs | CT | Cytology BAL | Co-Inf. | Bb (PCR) | My (PCR) | Other (Culture) | Diagnosis | Medical Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | Akita Inu | 1 | ME | Fever, weakness, weight loss, tachypnoea | Right mid lobe consolidation with air bronchogram | ‘Ground glass’ opacity of the cranial lobes. Moderate enlargement of the cranial mediastinal and sternal lymph nodes. | Pyo granulomatous septic inflammation | Yes | −ve | −ve | Escherichia coli, Staphylococcus epidermis | Pyo-granulomatous pneumonia | Amoxiclav and doxycycline | Alive after 7 months |
| 2 | 30 | American Stafford-shire | 5 | MN | Chronic cough | ND | Moderate ‘ground glass’ opacity of the peri-bronchovascular area. Slight enlargement of the cranial mediastinal and sternal lymph nodes. | Neutrophilic inflammation | Yes | −ve | −ve | Leishmania | Tracheo-bronchitis | Miltefosine and allopurinol | Alive after 11 months |
| 3 | 30 | Basset hound | <1 | ME | Chronic cough | ND | ND | Mixed inflammation | Yes | +ve | −ve | ND | ND | ND | ND |
| 4 | 29 | Border collie | <1 | FE | Chronic cough | ND | ND | Mixed inflammation | No | −ve | −ve | ND | ND | ND | ND |
| 5 | 27 | Boxer | <1 | ME | Chronic cough | ND | ND | Neutrophilic inflammation | Yes | +ve | +ve | ND | ND | ND | ND |
| 6 | 32 | Boxer | <1 | ME | Acute cough and respiratory dyspnoea | Diffuse broncho-interstitial-alveolar pattern | ND | Mixed inflammation | Yes | +ve | +ve | ND | Pneumonia | Doxycycline, TMS suggested but patient LTFU | LTFU |
| 7 | 34 | Boxer | <1 | FE | Nasal discharge and chronic cough. | Moderate bronchial pattern | Multiple lung area of ‘ground glass’ opacity. Severe enlargement of the tracheobronchial lymph nodes. | Neutrophilic inflammation | Yes | +ve | +ve | Pseudomonas aeruginosa | Pneumonia | Amoxiclav and marbofloxacin | LTFU |
| 8 | 25 | Bracco Italiano | <1 | ME | Joints pain, neck rigidity, chronic cough and fever. | ND | Diffuse ‘ground glass’ opacity of the left caudal lung lobe. Marked enlargement of the tracheobronchial lymph nodes. | Neutrophilic inflammation | No | −ve | −ve | ND | SRMA | Doxycycline, prednisolone, and cyclosporine (for SRMA) | Alive after 29 months |
| 9 | 31 | Chihuahua | <1 | FE | Chronic cough | ND | Diffuse bronchial wall thickness | Septic inflammation | Yes | +ve | −ve | Pseudomonas putida | Septic Pneumonia and PCP | Doxycycline, marbofloxacin and TMS | Improved after one months, then LTFU |
| 10 | 32 | Chow Chow | <1 | ME | Fever, weakness, and chronic cough | Diffuse bronchial-interstitial pattern | Diffuse bronchial wall thickness and diffuse ‘ground glass’ opacity of the lung. Enlargement of the sternal and tracheobronchial lymph nodes. | Neutrophilic inflammation | Yes | −ve | +ve | Klebsiella pneumoniae, S. pseudointermedius | Septic pneumonia and pulmonary pneumocystosis | TMS | Improved after 45 days |
| 11 | 22 | CKCS | <1 | M | ND | ND | ND | Neutrophilic inflammation | Yes | +ve | +ve | ND | ND | ND | ND |
| 12 | 34 | CKCS | <1 | ME | Chronic cough | ND | ND | Mixed inflammation | Yes | +ve | −ve | ND | Pneumonia | ND | ND |
| 13 | 25 | German Shepherd | 6 | ME | PU-PD and tachypnoea | ND | Disseminated ‘ground glass’ opacity. Enlargement and moderate enhancement of the tracheobronchial lymph nodes. | Mixed inflammation | Yes | −ve | −ve | Acinetobacter berenziniae (by BAL culture) | Pneumonia | Ceftriaxone | LTFU |
| 14 | 28 | Golden Retriever | 11 | ME | Acute cough | Diffuse oesophageal dilation, diffuse broncho-interstitial pattern | Diffuse oesophageal dilation, diffuse bilateral ‘ground glass’ opacity andperi bronchovascular thickening of various lung lobes. | Septic inflammation | Yes | −ve | +ve | Enterobacter kobei | Aspiration pneumonia due to mega-oesophagus secondary to polyneuropathy | Ceftriaxone | Dead after 18 days |
| 15 | 32 | Italian Grey-hound | 8 | ME | Cough and enforced respiratory sounds | Broncho-interstitial pattern of the right lung | Increased lung opacity of the peri-bronchovascular area of the cranial lung lobes. Slight enlargement of the tracheo-bronchial lymph nodes | Neutrophilic inflammation | Yes | −ve | +ve | NA | Pneumonia | Amoxyclav, doxycycline and TMS for amoxiclav | Improved |
| 16 | 30 | Labrador Retriever | <1 | ME | Chronic cough | Bronchial pattern | ND | Septic inflammation | No | −ve | −ve | ND | ND | ND | ND |
| 17 | 30 | Mongrel | 5 | ME | ND | ND | ND | No | −ve | −ve | ND | ND | ND | ND | |
| 18 | 34 | Pomeranian dog | <1 | FN | Chronic cough | Normal | ND | Mixed inflammation | Yes | +ve | +ve | ND | Eosinophilic bronchitis | Fluticasone and doxycycline | Improved after 3 months, then LTFU |
| 19 | 28 | Pomeranian dog | 11 | MN | Chronic cough | ND | ND | Neutrophilic inflammation | No | −ve | −ve | ND | ND | ND | ND |
| 20 | 30 | Rottweiler | <1 | FE | Cough | ND | ND | Neutrophilic inflammation | Yes | −ve | −ve | Rhodococcus hoagii | ND | ND | ND |
| 21 | 31 | Toy poodle | <1 | ME | Chronic cough, acute vomiting and diarrhoea | ND | Normal lung and pseudoaneurysmal dilatation of the right auricle. | Normal | Yes | +ve | +ve | ND | Tracheo-bronchitis | NA | LYFU |
| 22 | 27 | Yorkshire Terrier | 1 | ME | Chronic cough | ND | ND | Septic inflammation | No | −ve | −ve | ND | ND | ND | ND |
| Classification | Specimens (No. of Cases) | Organisms | CT | Reference |
|---|---|---|---|---|
| Dogs with confirmed PCP | FFPE sections (3) | Numerous | <26 | [22] |
| DQ smears (2) | Numerous | <26 | [22] | |
| BALF (3) | Numerous | <26 | [22] | |
| BALF (1) | Numerous | <26 | [14] | |
| DQ smears (2) | Occasional | 27–34 | [22] | |
| Dogs with confirmed or suspected PCP | DQ (1) | None | 27–34 | [22] |
| BALF (5) | None | 27–34 | [22] | |
| Lung tissue (1) | None | 27–34 | [22] | |
| DQ (3) | None | ≥35 | [22] | |
| BALF (62) | None | ≥35 | [22] | |
| Dogs not suspected of having PCP | Lung tissue (10) | Not done | ≥35 | [22] |
| Dogs with LRT disease | BALF (19) | None | 27–34 | This study |
| Dogs colonised by P. canis with another cause of RT disease or with PCP | BALF (3) | None or Not done | <26 | This study |
| Dogs with LRT disease but no colonisation by P. canis | BALF (233) | None or Not done | ≥35 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danesi, P.; Petini, M.; Falcaro, C.; Bertola, M.; Mazzotta, E.; Furlanello, T.; Krockenberger, M.; Malik, R. Pneumocystis Colonization in Dogs Is as in Humans. Int. J. Environ. Res. Public Health 2022, 19, 3192. https://doi.org/10.3390/ijerph19063192
Danesi P, Petini M, Falcaro C, Bertola M, Mazzotta E, Furlanello T, Krockenberger M, Malik R. Pneumocystis Colonization in Dogs Is as in Humans. International Journal of Environmental Research and Public Health. 2022; 19(6):3192. https://doi.org/10.3390/ijerph19063192
Chicago/Turabian StyleDanesi, Patrizia, Matteo Petini, Christian Falcaro, Michela Bertola, Elisa Mazzotta, Tommaso Furlanello, Mark Krockenberger, and Richard Malik. 2022. "Pneumocystis Colonization in Dogs Is as in Humans" International Journal of Environmental Research and Public Health 19, no. 6: 3192. https://doi.org/10.3390/ijerph19063192
APA StyleDanesi, P., Petini, M., Falcaro, C., Bertola, M., Mazzotta, E., Furlanello, T., Krockenberger, M., & Malik, R. (2022). Pneumocystis Colonization in Dogs Is as in Humans. International Journal of Environmental Research and Public Health, 19(6), 3192. https://doi.org/10.3390/ijerph19063192










