Resting Metabolic Rate in Women with Endocrine and Osteoporotic Disorders in Relation to Nutritional Status, Diet and 25(OH)D Concentration
Abstract
:1. Introduction
2. Objectives
3. Material and Method
3.1. Study and Control Group
3.2. Physical Activity
- Insufficient physical activity (less than 600 MET—min/week);
- Sufficient physical activity (between 600 and 1500 MET—min/week);
- Increased physical activity (1500–3000 MET—min/week, but less than 3 days per week of intense exercise);
- High physical activity (above 1500 MET—min/week but at least 3 days per week of intense exercise, or at least 3000 MET—min/week).
3.3. Vitamin D
3.4. Metabolic Rate
3.5. Anthropometry
3.6. Nutrition Assessment
3.7. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McHill, A.; Wright, K., Jr. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes. Rev. 2017, 18, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Brinklov, C.F.; Thorsen, I.K.; Karstoft, K.; Brons, C.; Valentiner, L.; Langberg, H.; Vaag, A.A.; Nielsen, J.S.; Pedersen, B.K.; Ried-Larsen, M. Criterion validity and reliability of a smartphone delivered sub-maximal fitness test for people with type 2 diabetes. BMC Sports Sci. Med. Rehabil. 2016, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, K.R.; Smith-Ryan, A.E.; Blue, M.N.; Mock, M.G.; Trexler, E.T. Influence of segmental body composition and adiposity hormones on resting metabolic rate and substrate utilization in overweight and obese adults. J. Endocrinol. Investig. 2017, 40, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, M.; Kahleova, H.; Alwarith, J.; Burgess, N.N.; Flores, R.A.; Busta, M.L.; Barnard, N.D. The thermic ffect of food: A Review. J. Am. Coll. Nutr. 2019, 38, 547–551. [Google Scholar] [CrossRef]
- Bo, S.; Broglio, F.; Settanni, F.; Parasiliti Caprino, M.; Ianniello, A.; Mengozzi, G.; De Francesco, A.; Fadda, M.; Fedele, D.; Guggino, A.; et al. Effects of meal timing on changes in circulating epinephrine, norepinephrine, and acylated ghrelin concentrations: A pilot study. Nutr. Diabetes 2017, 7, 303. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.; Martens, E.A.P.; Hochstenbach-Waelen, A.; Lemmens, S.G.T.; Westerterp-Plantenga, M.S. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J. Nutr. 2013, 143, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Karstoft, K.; Clark, M.A.; Jakobsen, I.; Muller, I.A.; Pedersen, B.K.; Solomon, T.P.; Ried-Larsen, M. The effects of 2 weeks of interval vs. continuous walking training on glycaemic control and whole-body oxidative stress in individuals with type 2 diabetes: A controlled, randomised, crossover trial. Diabetologia 2017, 60, 508–517. [Google Scholar] [CrossRef]
- Karstoft, K.; Brinkløv, C.F.; Thorsen, I.K.; Nielsen, J.S.; Ried-Larsen, M. Resting metabolic rate does not change in response to different types of training in subjects with type 2 diabetes. Front. Endocrinol. 2017, 8, 132. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe-Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Płudowski, P.; Ducki, C.; Konstantynowicz, J.; Jaworski, M. Vitamin D status in Poland. Pol. Arch. Inter. Med. 2016, 126, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, K.R.; Cruzat, V.; Melder, H.; Jacques, A.; Newsholme, P.; Ducker, K.J. Vitamin D supplementation does not impact resting metabolic rate, body composition and strength in vitamin D sufficient physically active adults. Nutrients 2020, 12, 3111. [Google Scholar] [CrossRef] [PubMed]
- Calton, E.K.; Keane, K.; Soares, M.J. The potential regulatory role of vitamin D in the bioenergetics of inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, M.; Kaczmarczyk, M.; Jastrzębski, Z. Effect of Vitamin D Supplementation on Training Adaptation in Well-Trained Soccer Players. J. Strength Cond. Res. 2016, 30, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Russell, J.; Cobley, J.; Owens, D.; Wilson, G.; Gregson, W.; Fraser, W.; Morton, J. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: Implications for skeletal muscle function. J. Sports Sci. 2013, 31, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Stelmach, M. Physical activity assessment tools in monitoring physical activity: The Global Physical Activity Questionnaire (GPAQ), The International Physical Activity Questionnaire (IPAQ) or accelerometers—Choosing the best tools. Health Prob. Civiliz. 2018, 12, 57–63. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Jarosz, M. Standards of Human Nutrition, 3rd ed.; National Food and Nutrition Institute: Warsaw, Poland, 2012; pp. 154–179. [Google Scholar]
- Bouillon, R.; Carmeliet, G.; Lieben, L.; Watanabe, M.; Perino, A.; Auwerx, J.; Schoonjans, K.; Verstuyf, A. Vitamin D and energy homeostasis-of mice and men. Nat. Rev. Endocrinol. 2014, 10, 79–87. [Google Scholar] [CrossRef]
- Wong, K.E.; Kong, J.; Zhang, W.; Szeto, F.L.; Ye, H.; Deb, D.K.; Brady, M.J.; Li, J.C. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J. Biol. Chem. 2011, 286, 33804–33810. [Google Scholar] [CrossRef] [Green Version]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.J.; Bendahan, D.; Bernard, M.; Martin, J.C.; Giannesini, B.; et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef]
- Calton, E.K.; Pathak, K.; Soares, M.J.; Alfonso, H.; Keane, K.N.; Newsholme, P.; Cummings, N.K.; Chan, W.; Ping-Delfos, S.; Hamidi, A. Vitamin D status and insulin sensitivity are novel predictors of resting metabolic rate: A cross-sectional analysis in Australian adults. Eur. J. Nutr. 2016, 55, 2075–2080. [Google Scholar] [CrossRef]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyere, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drincic, A.T.; Armas, L.A.; Van Diest, E.E.; Heaney, R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef]
- Muller, M.J.; Enderle, J.; Bosy-Westphal, A. Changes in energy expenditure with weight gain and weight loss in humans. Curr. Obes. Rep. 2016, 5, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, M.J.; Enderle, J.; Pourhassan, M.; Braun, W.; Eggeling, B.; Lagerpusch, M.; Glüer, C.-C.; Kehayias, J.J.; Kiosz, D.; Bosy-Westphal, A. Metabolic adaptation to caloric restriction and subsequent refeeding: The Minnesota Starvation Experiment revisited. Am. J. Clin. Nutr. 2015, 102, 807–819. [Google Scholar] [CrossRef]
- Bo, S.; Fadda, M.; Castiglione, A.; Ciccone, G.; De Francesco, A.; Fedele, D.; Guggino, A.; Parasiliti Caprino, M.; Ferrara, S.; Vezio Boggio, M.; et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int. J. Obes. 2015, 39, 1689–1695. [Google Scholar] [CrossRef] [Green Version]
- Kassis, A.; Godin, J.P.; Moille, S.E.; Nielsen-Moennoz, C.; Groulx, K.; Oguey-Araymon, S.; Praplan, F.; Beaumont, M.; Sauser, J.; Monnard, I.; et al. Effects of protein quantity and type on diet induced thermogenesis in overweight adults: A randomized controlled trial. Clin. Nutr. 2019, 38, 1570–1580. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Feldman, H.A.; Klein, G.L.; Wong, J.M.W.; Bielak, L.; Steltz, S.K.; Luoto, P.K.; Wolfe, R.R.; Wong, W.W.; Ludwig, D.S. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: Randomized trial. BMJ 2018, 363, k4583. [Google Scholar] [CrossRef] [Green Version]
- Ravn, A.M.; Gregersen, N.T.; Christensen, R.; Rasmussen, L.G.; Hels, O.; Belza, A.; Raben, A.; Larsen, T.M.; Toubro, S.; Astrup, A. Thermic effect of a meal and appetite in adults: An individual participant data meta-analysis of meal-test trials. Food Nutr. Res. 2013, 57, 19676. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Study Group n = 223 | Control Group n = 108 | Z, p Mann–Whitney Test |
|---|---|---|---|
| Mean ± SD/n (%) | Mean ± SD/n (%) | ||
| Age [years] | 64.6 ± 12.8 | 61.4 ± 11.3 | 4.2306, 0.4673 |
| Body Mass Index (BMI) [kg/m2] | 27.8 ± 5.5 | 26.1 ± 5.8 | 1.1365, 0.7671 |
| Waist Hip Ratio (WHR) | 0.8 ± 0.1 | 1.0 ± 0.1 | 1.4235, 0.5328 |
| Waist circumference [cm] | 88.8 ± 12.9 | 87.2 ± 7.2 | 1.1463, 0.5659 |
| Regular vitamin D supplements user [n (%)] | 173 (77.6) | 31 (28.7) | 4.5638, <0.0001 |
| Physical activity | |||
| Insufficient | 38 (17.1) | 17 (15.7) | 0.8735, 0.5374 |
| Sufficient | 120 (53.8) | 55 (50.9) | 0.3467, 0.3859 |
| Increased | 44 (19.7) | 20 (18.5) | 0.7436, 0.4783 |
| High | 21 (9.4) | 16 (14.8) | 0.8834, 0.7739 |
| Body composition | |||
| Body mass [kg] | 71.5 ± 14.7 | 73.5 ± 7.9 | 0.9453, 0.6233 |
| Fat free mass [kg] | 46.1 ± 6.2 | 49.1 ± 5.2 | 2.9964, <0.0001 |
| Fat mass [kg] | 32.4 ± 13.5 | 28.4 ± 8.3 | 3.5762, <0.0001 |
| Fat mass [%] | 39.7 ± 8.7 | 36.2 ± 4.7 | 4.0276, <0.0001 |
| Metabolic Rate | |||
| Resting Metabolic Rate [kcal/d] | 1332.7 ± 309.9 | 1557.8 ± 187.3 | 7.0590, <0.0001 |
| Basal Metabolic Rate [kcal/d] | 1453.2 ± 245.6 | 1574.0 ± 134.9 | 5.0123, <0.0001 |
| Total Metabolic Rate [kcal/d] | 2409.7 ± 254.7 | 2427.5 ± 288.7 | 0.3632, 0.0762 |
| Slow [n (%)] | 92 (41.3) | 12 (11.1) | 3.9432, <0.0001 |
| Normal [n (%)] | 83 (37.2) | 14 (13.0) | 2.9964, <0.0001 |
| High [n (%)] | 48 (21.5) | 82 (75.9) | 4.0275, <0.0001 |
| Dietary intake | |||
| Energy [kcal/day] | 1415.7 ± 447.4 | 2127.2 ± 1182.3 | 4.2349, <0.0001 |
| Proteins [g/day] | 57.9 ± 19.5 | 99.6 ± 45.8 | 2.9836, <0.0001 |
| Proteins [% total energy intake] | 16.7 ± 4.5 | 19.4 ± 3.5 | 2.7906, <0.0001 |
| Carbohydrates [g/day] | 189.0 ± 61.0 | 268.8 ± 139.8 | 6.4326, <0.0001 |
| Carbohydrates [% total energy intake] | 50.3 ± 8.8 | 48.1 ± 8.8 | 0.7683, 0.7639 |
| Fats [g/day] | 53.4 ± 24.3 | 79.3 ± 57.7 | 3.9465, <0.0001 |
| Fats [% total energy intake] | 32.6 ± 8.1 | 32.1 ± 7.2 | 0.8032, 0.4327 |
| MUFA [g/day] | 21.2 ± 10.6 | 30.8 ± 28.9 | 5.0348, <0.0001 |
| PUFA [g/day] | 8.1 ± 4.2 | 10.3 ± 7.1 | 3.9674, <0.0001 |
| SFA [g/day] | 20.2 ± 11.3 | 34.4 ± 28.4 | 4.4876, <0.0001 |
| Fiber [g/day] | 15.7 ± 5.9 | 24.3 ± 11.2 | 4.0824, <0.0001 |
| Vitamin D (without supplements) [µg/day] | 3.7 ±2.3 | 7.4 ± 5.6 | 2.9987, <0.0001 |
| Total vitamin D (including supplements) [µg/day] | 38.2 ± 15.0 | 21.1 ± 13.6 | 3.9075, 0.0028 |
| Serum concentration | |||
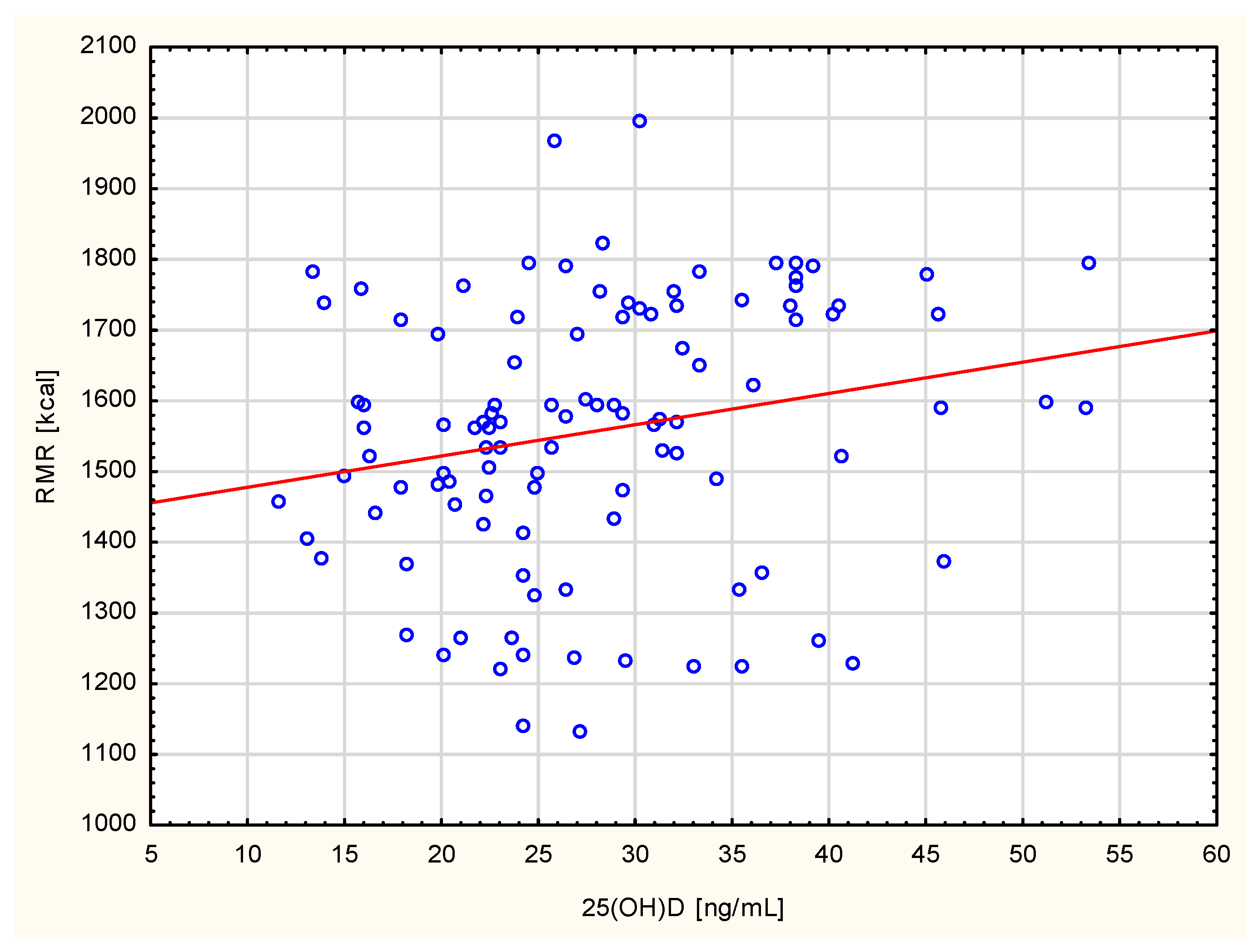
| 25(OH)D Total [ng/mL] | 25.9 ± 11.8 | 28.1 ± 9.1 | 2.1808, 0.0029 |
| 25(OH)D deficiency [n (%)] | 149 (66.8) | 68 (62.9) | 0.8725, 0.8043 |
| Characteristics | 25(OH)D < 30 ng/mL n = 217 | 25(OH)D ≥ 30 ng/mL n = 114 | Z, p Mann–Whitney Test |
|---|---|---|---|
| Mean ± SD/n (%) | Mean ± SD/n (%) | ||
| Age [years] | 65.2 ± 10.2 | 61.9 ± 9.8 | 3.2904, 0.7325 |
| Body Mass Index (BMI) [kg/m2] | 26.9 ± 4.9 | 26.3 ± 2.9 | 1.1763, 0.7781 |
| Waist Hip Ratio (WHR) | 0.8 ± 0.3 | 0.9 ± 0.9 | 1.6233, 0.6371 |
| Waist circumference [cm] | 87.9 ± 11.2 | 86.2 ± 6.9 | 1.1393, 0.4938 |
| Regular vitamin D supplements user [n (%)] | 94 (43.3) | 110 (96.5) | 2.5032, <0.0001 |
| Physical activity | |||
| Insufficient | 42 (19.4) | 13 (11.4) | 0.2756, 0.6314 |
| Sufficient | 115 (53.0) | 60 (52.6) | 0.2961, 0.7851 |
| Increased | 43 (19.8) | 21 (18.4) | 0.5826, 0.4764 |
| High | 17 (7.8) | 20 (17.6) | 0.7463, 0.3753 |
| Metabolic Rate | |||
| Resting Metabolic Rate [kcal/d] | 1284.9 ± 279.1 | 1538.5 ± 217.8 | 3.7352, p < 0.0001 |
| Basal Metabolic Rate [kcal/d] | 1351.2 ± 179.8 | 1602.0 ± 171.6 | 1.7821, <0.0001 |
| Total Metabolic Rate [kcal/d] | 25,024.2 ± 174.8 | 2479.5 ± 308.1 | 0.3037, 0.1772 |
| Slow [n (%)] | 89 (41.0) | 15 (13.2) | 2.7472, <0.0001 |
| Normal [n (%)] | 86 (39.6) | 11 (9.6) | 1.9071, <0.0001 |
| High [n (%)] | 42 (19.4) | 88 (77.2) | 3.1234, <0.0001 |
| Dietary intake | |||
| Energy [kcal/day] | 1493.7 ± 507.8 | 2221.8 ± 982.4 | 3.9843, <0.0001 |
| Proteins [g/day] | 55.8 ± 15.9 | 90.6 ± 49.1 | 1.9037, <0.0001 |
| Proteins [% total energy intake] | 15.9 ± 6.1 | 20.7 ± 3.1 | 2.2972, <0.0001 |
| Carbohydrates [g/day] | 177.9 ± 60.5 | 251.8 ± 143.1 | 3.7896, <0.0001 |
| Carbohydrates [% total energy intake] | 53.3 ± 6.8 | 49.7 ± 7.5 | 0.7323, 0.6739 |
| Fats [g/day] | 57.4 ± 21.5 | 81.3 ± 49.7 | 1.0467, <0.0001 |
| Fats [% total energy intake] | 30.7 ± 9.1 | 34.1 ± 8.1 | 0.2092, 0.1357 |
| MUFA [g/day] | 20.9 ± 9.9 | 32.1 ± 25.9 | 4.1287, <0.0001 |
| PUFA [g/day] | 8.7 ± 4.4 | 12.1 ± 5.7 | 3.0631, <0.0001 |
| SFA [g/day] | 24.2 ± 6.3 | 37.4 ± 19.7 | 4.8671, <0.0001 |
| Fiber [g/day] | 13.9 ± 6.2 | 26.3 ± 9.7 | 3.8021, <0.0001 |
| Vitamin D (without supplements) [µg/day] | 3.1 ± 2.9 | 8.2 ± 4.8 | 1.9042, <0.0001 |
| Total vitamin D (including supplements) [µg/day] | 36.7 ± 16.3 | 20.3 ± 14.1 | 3.0472, 0.0017 |
| Characteristics | Slow and Normal Metabolic Rate n = 201 | High Metabolic Rate n = 130 | Z, p Mann–Whitney Test |
|---|---|---|---|
| Mean ± SD/n (%) | Mean ± SD/n (%) | ||
| Age [years] | 67.4 ± 11.1 | 59.9 ± 11.4 | 1.2307, 0.0025 |
| Body Mass Index (BMI) [kg/m2] | 28.3 ± 6.2 | 24.9 ± 4.7 | 1.1763, 0.0018 |
| Waist Hip Ratio (WHR) | 1.1 ± 0.9 | 0.9 ± 0.7 | 1.0537, 0.7872 |
| Waist circumference [cm] | 89.7 ± 10.7 | 85.2 ± 7.4 | 1.8378, 0.6431 |
| Regular vitamin D supplements user [n (%)] | 92 (45.8) | 112 (86.2) | 2.5032, <0.0001 |
| Physical activity | |||
| Insufficient | 45 (22.4) | 10 (7.7) | 1.3432, 0.0034 |
| Sufficient | 114 (56.7) | 61 (46.9) | 0.9468, 0.0051 |
| Increased | 31 (15.4) | 33 (25.4) | 0.9456, 0.0013 |
| High | 11 (5.5) | 26 (20.0) | 1.6874, 0.0041 |
| Dietary intake | |||
| Energy [kcal/day] | 1523.7 ± 587.1 | 2171.8 ± 782.7 | 1.9563, <0.0001 |
| Proteins [g/day] | 56.8 ± 15.8 | 87.6 ± 51.1 | 1.9037, <0.0001 |
| Proteins [% total energy intake] | 17.1 ± 6.9 | 22.4 ± 3.7 | 2.0962, <0.0001 |
| Carbohydrates [g/day] | 197.9 ± 56.5 | 231.8 ± 178.1 | 2.7094, <0.0001 |
| Carbohydrates [% total energy intake] | 54.1 ± 7.2 | 50.7 ± 6.5 | 0.9373, 0.0023 |
| Fats [g/day] | 58.8 ± 23.4 | 80.7 ± 47.4 | 1.9404, <0.0001 |
| Fats [% total energy intake] | 32.5 ± 8.7 | 33.6 ± 7.1 | 0.4592, 0.4953 |
| MUFA [g/day] | 19.3 ± 7.8 | 31.1 ± 19.7 | 3.3982, <0.0001 |
| PUFA [g/day] | 6.9 ± 5.6 | 14.1 ± 3.5 | 2.9934, <0.0001 |
| SFA [g/day] | 27.2 ± 7.3 | 35.4 ± 18.9 | 2.7073, <0.0001 |
| Fiber [g/day] | 11.9 ± 4.2 | 28.3 ± 8.7 | 4.2028, <0.0001 |
| Vitamin D (without supplements) [µg/day] | 3.4 ± 2.7 | 8.9 ± 5.1 | 1.4541, <0.0001 |
| Total vitamin D (including supplements) [µg/day] | 34.5 ± 11.9 | 26.3 ± 11.8 | 2.6734, 0.0076 |
| Serum concentration | |||
| 25(OH)D Total [ng/mL] | 20.5 ± 3.1 | 28.2 ± 3.8 | 5.0276, <0.0001 |
| 25(OH)D deficiency [n (%)] | 151 (75.1) | 66 (50.8) | 1.2736, <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godala, M.; Sewerynek, E.; Maślach, D.; Krzyżak, M.; Gaszyńska, E. Resting Metabolic Rate in Women with Endocrine and Osteoporotic Disorders in Relation to Nutritional Status, Diet and 25(OH)D Concentration. Int. J. Environ. Res. Public Health 2022, 19, 3118. https://doi.org/10.3390/ijerph19053118
Godala M, Sewerynek E, Maślach D, Krzyżak M, Gaszyńska E. Resting Metabolic Rate in Women with Endocrine and Osteoporotic Disorders in Relation to Nutritional Status, Diet and 25(OH)D Concentration. International Journal of Environmental Research and Public Health. 2022; 19(5):3118. https://doi.org/10.3390/ijerph19053118
Chicago/Turabian StyleGodala, Małgorzata, Ewa Sewerynek, Dominik Maślach, Michalina Krzyżak, and Ewelina Gaszyńska. 2022. "Resting Metabolic Rate in Women with Endocrine and Osteoporotic Disorders in Relation to Nutritional Status, Diet and 25(OH)D Concentration" International Journal of Environmental Research and Public Health 19, no. 5: 3118. https://doi.org/10.3390/ijerph19053118
APA StyleGodala, M., Sewerynek, E., Maślach, D., Krzyżak, M., & Gaszyńska, E. (2022). Resting Metabolic Rate in Women with Endocrine and Osteoporotic Disorders in Relation to Nutritional Status, Diet and 25(OH)D Concentration. International Journal of Environmental Research and Public Health, 19(5), 3118. https://doi.org/10.3390/ijerph19053118








