Land-Use Change Enhanced SOC Mineralization but Did Not Significantly Affect Its Storage in the Surface Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Sampling
2.2. Determination of Soil Physicochemical Properties and Labile SOC Fractions
2.3. Determination of Soil C-Degrading Enzyme Activities
2.4. Soil DNA Extraction and Quantitative PCR
2.5. Determination of Soil Organic C Mineralization Rates
2.6. Statistical Analyses
3. Results
3.1. Soil Physicochemical Properties and Labile SOC Fractions
3.2. SOC Mineralization Rate, Soil Enzyme Activities and Functional Genes Abundance
3.3. Relationship between SOC Mineralization Rate and Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janzen, H.H. Carbon cycling in earth systems—A soil science perspective. Agric. Ecosyst. Environ. 2004, 104, 399–417. [Google Scholar] [CrossRef]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Lal, R. Accelerated Soil erosion as a source of atmospheric CO2. Soil Till. Res. 2019, 188, 35–40. [Google Scholar] [CrossRef]
- Ziegler, S.E.; Billings, S.A.; Lane, C.S.; Li, J.; Fogel, M.L. Warming alters routing of labile and slower-turnover carbon through distinct microbial groups in boreal forest organic soils. Soil Biol. Biochem. 2013, 60, 23–32. [Google Scholar] [CrossRef]
- Ghazoul, J.; Burivalova, Z.; Garcia-Ulloa, J.; King, L.A. Conceptualizing Forest Degradation. Trends Ecol. Evol. 2015, 30, 622–632. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2015: How Are the World’s Forests Changing? Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; pp. 1–56. [Google Scholar]
- Gibbs, H.; Ruesch, A.; Achard, F.; Clayton, M.; Holmgren, P.; Ramankutty, N.; Foley, J. Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl. Acad. Sci. USA 2010, 107, 16732–16737. [Google Scholar] [CrossRef] [Green Version]
- Smith, P. Land use change and soil organic carbon dynamics. Nutr. Cycl. Agroecosys. 2008, 81, 169–178. [Google Scholar] [CrossRef]
- Fan, L.; Han, W. Soil respiration after forest conversion to tea gardens: A chronosequence study. Catena 2020, 190, 104532. [Google Scholar] [CrossRef]
- Lyu, M.; Noormets, A.; Ukonmaanaho, L.; Li, Y.; Yang, Y.; Xie, J. Stability of soil organic carbon during forest conversion is more sensitive in deep soil than in topsoil in subtropical forests. Pedobiologia 2021, 84, 150706. [Google Scholar] [CrossRef]
- Olson, K.R.; Gennadiyev, A.N.; Zhidkin, A.P.; Markelov, M.V. Impacts of Land-Use Change, Slope, and Erosion on Soil Organic Carbon Retention and Storage. Soil Sci. 2012, 177, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.; Gao, W.; Jin, G. Effects on rhizospheric and heterotrophic respiration of conversion from primary forest to secondary forest and plantations in northeast China. Eur. J. Soil Biol. 2015, 66, 11–18. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Tang, C.; Luo, Y.; Fu, W.; Cai, X.; Li, Y.; Yue, T.; Jiang, P.; Hu, S.; et al. Converting natural evergreen broadleaf forests to intensively managed moso bamboo plantations affects the pool size and stability of soil organic carbon and enzyme activities. Biol. Fertil. Soils 2018, 54, 467–480. [Google Scholar] [CrossRef]
- Han, M.; Zhu, B. Changes in soil greenhouse gas fluxes by land use change from primary forest. Glob. Chang. Biol. 2020, 26, 2656–2667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zou, Y.; Di Gioia, D.; Singh, B.K.; Li, Q. Conversion to agroforestry and monoculture plantation is detrimental to the soil carbon and nitrogen cycles and microbial communities of a rainforest. Soil Biol. Biochem. 2020, 147, 107849. [Google Scholar] [CrossRef]
- Guillaume, T.; Damris, M.; Kuzyakov, Y. Losses of soil carbon by converting tropical forest to plantations: Erosion and decomposition estimated by δ13C. Glob. Chang. Biol. 2015, 21, 3548–3560. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Singh, J. Effect of alkali concentration, volume and absorption area on the measurement of soil respiration in a tropical sward. Pedobiologia 1977, 8, 233–239. [Google Scholar]
- Schütt, M.; Borken, W.; Spott, O.; Stange, C.F.; Matzner, E. Temperature sensitivity of C and N mineralization in temperate forest soils at low temperatures. Soil Biol. Biochem. 2014, 69, 320–327. [Google Scholar] [CrossRef]
- Ding, X.; Qiao, Y.; Filley, T.; Wang, H.; Lü, X.; Zhang, B.; Wang, J. Long-term changes in land use impact the accumulation of microbial residues in the particle-size fractions of a Mollisol. Biol. Fertil. Soils 2017, 53, 281–286. [Google Scholar] [CrossRef]
- Ci, E.; Al-Kaisi, M.M.; Wang, L.; Ding, C.; Xie, D. Soil Organic Carbon Mineralization as Affected by Cyclical Temperature Fluctuations in a Karst Region of Southwestern China. Pedosphere 2015, 25, 512–523. [Google Scholar] [CrossRef]
- Kéraval, B.; Fontaine, S.; Lallement, A.; Revaillot, S.; Billard, H.; Alvarez, G.; Maestre, F.; Amblard, C.; Lehours, A.-C. Cellular and non-cellular mineralization of organic carbon in soils with contrasted physicochemical properties. Soil Biol. Biochem. 2018, 125, 286–289. [Google Scholar] [CrossRef]
- Küstermann, B.; Munch, J.C.; Hülsbergen, K.J. Effects of soil tillage and fertilization on resource efficiency and greenhouse gas emissions in a long-term field experiment in Southern Germany. Eur. J. Agron. 2013, 49, 61–73. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant. Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, J.; Meng, M.; Guo, X.; Wu, Y.; Liu, X.; Zhao, K.; Ding, L.; Shao, Y.; Fu, W. Forest-type shift and subsequent intensive management affected soil organic carbon and microbial community in southeastern China. Eur. J. For. Res. 2017, 136, 689–697. [Google Scholar] [CrossRef]
- Ramírez, P.B.; Fuentes-Alburquenque, S.; Díez, B.; Vargas, I.; Bonilla, C.A. Soil microbial community responses to labile organic carbon fractions in relation to soil type and land use along a climate gradient. Soil Biol. Biochem. 2020, 141, 107692. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Weintraub, M.N. Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol. Biochem. 2008, 40, 2098–2106. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Gallo, M.E.; Lauber, C.; Waldrop, M.P.; Zak, D.R. Extracellular Enzyme Activities and Soil Organic Matter Dynamics for Northern Hardwood Forests receiving Simulated Nitrogen Deposition. Biogeochemistry 2005, 75, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Edwards, I.P.; Upchurch, R.A.; Zak, D.R. Isolation of fungal cellobiohydrolase I genes from sporocarps and forest soils by PCR. Appl. Environ. Microbiol. 2008, 74, 3481–3489. [Google Scholar] [CrossRef] [Green Version]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; García-Palacios, P.; Cao, J.; Dacal, M.; Zhou, X.; Li, J.; Xia, J.; Niu, S.; Yang, H.; et al. Differential responses of carbon-degrading enzyme activities to warming: Implications for soil respiration. Glob. Change Biol. 2018, 24, 4816–4826. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, J.; Wu, J.; Zhang, D.; Chen, Q.; Li, Q.; Long, C.; Feyissa, A.; Cheng, X. Variations in carbon-decomposition enzyme activities respond differently to land use change in central China. Land Degrad. Dev. 2019, 30, 459–469. [Google Scholar] [CrossRef]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Chen, J.; Sinsabaugh, R.L. Linking microbial functional gene abundance and soil extracellular enzyme activity: Implications for soil carbon dynamics. Glob. Change Biol. 2021, 27, 1322–1325. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Chang, S.X.; Yang, Y.; Fu, S.; Jiang, P.; Luo, Y.; Yang, M.; Chen, Z.; Hu, S.; et al. Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon-degrading microbial activity. Soil Biol. Biochem. 2018, 122, 173–185. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chang, S.X.; Jiang, P.; Zhou, G.; Fu, S.; Yan, E.; Wu, J.; Lin, L. Long-term intensive management effects on soil organic carbon pools and chemical composition in Moso bamboo (Phyllostachys pubescens) forests in subtropical China. For. Ecol. Manag. 2013, 303, 121–130. [Google Scholar] [CrossRef]
- Yan, W.; Mahmood, Q.; Peng, D.; Fu, W.; Chen, T.; Wang, Y.; Li, S.; Chen, J.; Liu, D. The spatial distribution pattern of heavy metals and risk assessment of moso bamboo forest soil around lead–zinc mine in Southeastern China. Soil Till. Res. 2015, 153, 120–130. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, P.; Wang, H.; Zhou, G.; Wu, J.; Yang, F.; Qian, X. Seasonal soil CO2 efflux dynamics after land use change from a natural forest to Moso bamboo plantations in subtropical China. For. Ecol. Manag. 2011, 262, 1131–1137. [Google Scholar] [CrossRef]
- Wang, H.; Jin, J.; Yu, P.; Fu, W.; Morrison, L.; Lin, H.; Meng, M.; Zhou, X.; Lv, Y.; Wu, J. Converting evergreen broad-leaved forests into tea and Moso bamboo plantations affects labile carbon pools and the chemical composition of soil organic carbon. Sci. Total Environ. 2020, 711, 135225. [Google Scholar] [CrossRef]
- Yang, C.; Wang, A.; Zhu, Z.; Lin, S.; Bi, Y.; Du, X. Impact of extensive management system on soil properties and carbon sequestration under an age chronosequence of Moso bamboo plantations in subtropical China. For. Ecol. Manag. 2021, 497, 119535. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources 2014, Update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Technical Reports No. 106; FAO: Rome, Italy, 2015.
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 3—Chemical Methods; American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEN value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.R.; Mo, J.M.; Wang, J.X.; Makeschin, F.; Wolff, M. Soil organic carbon stock and chemical composition in four plantations of indigenous tree species in subtropical China. Ecol. Res. 2010, 25, 1071–1079. [Google Scholar] [CrossRef]
- German, D.P.; Weintraub, M.N.; Grandy, A.S.; Lauber, C.L.; Rinkes, Z.L.; Allison, S.D. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 2011, 43, 1387–1397. [Google Scholar] [CrossRef]
- Zheng, T.; Liang, C.; Xie, H.; Zhao, J.; Yan, E.; Zhou, X.; Bao, X. Rhizosphere effects on soil microbial community structure and enzyme activity in a successional subtropical forest. FEMS Microbiol. Ecol. 2019, 95, fiz043. [Google Scholar] [CrossRef]
- Luis, P.; Walther, G.; Kellner, H.; Martin, F.; Buscot, F. Diversity of laccase genes from basidiomycetes in a forest soil. Soil Biol. Biochem. 2004, 36, 1025–1036. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, F.; He, T.; Wang, S. Responses of labile soil organic carbon and enzyme activity in mineral soils to forest conversion in the subtropics. Ann. Forest Sci. 2013, 70, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, Y.; Hu, S.; Chang, S.X. Plant mixture effects on carbon-degrading enzymes promote soil organic carbon accumulation. Soil Biol. Biochem. 2021, 163, 108457. [Google Scholar] [CrossRef]
- Frankenberger, W.T.; Johanson, J.B. Effect of pH on enzyme stability in soils. Soil Biol. Biochem. 1982, 14, 433–437. [Google Scholar] [CrossRef]
- Pocknee, S.; Sumner, M.E. Cation and nitrogen contents of organic matter determine its soil liming potential. Soil Sci. Soc. Am. J. 1997, 61, 86–92. [Google Scholar] [CrossRef]
- Yan, F.; Schubert, S. Soil pH changes after application of plant shoot materials of faba bean and wheat. Plant Soil 2000, 220, 279–287. [Google Scholar] [CrossRef]
- Yan, F.; Schubert, S.; Mengel, K. Soil pH increase due to biological decarboxylation of organic anions. Soil Biol. Biochem. 1996, 28, 17–24. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Pathan, S.I.; Ceccherini, M.T.; Hansen, M.A.; Giagnoni, L.; Ascher, J.; Arenella, M.; Sørensen, S.J.; Pietramellara, G.; Nannipieri, P.; Renella, G. Maize lines with different nitrogen use efficiency select bacterial communities with different β-glucosidase-encoding genes and glucosidase activity in the rhizosphere. Biol. Fertil. Soils 2015, 51, 995–1004. [Google Scholar] [CrossRef]
- Baraniya, D.; Puglisi, E.; Ceccherini, M.T.; Pietramellara, G.; Giagnoni, L.; Arenella, M.; Nannipieri, P.; Renella, G. Protease encoding microbial communities and protease activity of the rhizosphere and bulk soils of two maize lines with different N uptake efficiency. Soil Biol. Biochem. 2016, 96, 176–179. [Google Scholar] [CrossRef]
- Cañizares, R.; Moreno, B.; Benitez, E. Biochemical characterization with detection and expression of bacterial β-glucosidase encoding genes of a Mediterranean soil under different long-term management practices. Biol. Fertil. Soils 2012, 48, 651–663. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chang, S.X.; Jiang, P.; Zhou, G.; Shen, Z.; Wu, J.; Lin, L.; Wang, Z.; Shen, M. Converting native shrub forests to Chinese chestnut plantations and subsequent intensive management affected soil C and N pools. For. Ecol. Manag. 2014, 312, 161–169. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Y.S.; Xie, J.; Guo, J.; Gao, R.; Qian, W. Conversion of a natural broad-leafed evergreen forest into pure plantation forests in a subtropical area: Effects on carbon storage. Ann. Forest Sci. 2005, 62, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Lal, R.; Kimble, J.M.; Follett, R.F.; Stewart, B.A. Soil Processes and the Carbon Cycle; CRC Press: Boca Raton, FL, USA, 1997; Volume 11. [Google Scholar]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilsson, L.O.; Gundersen, P. Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
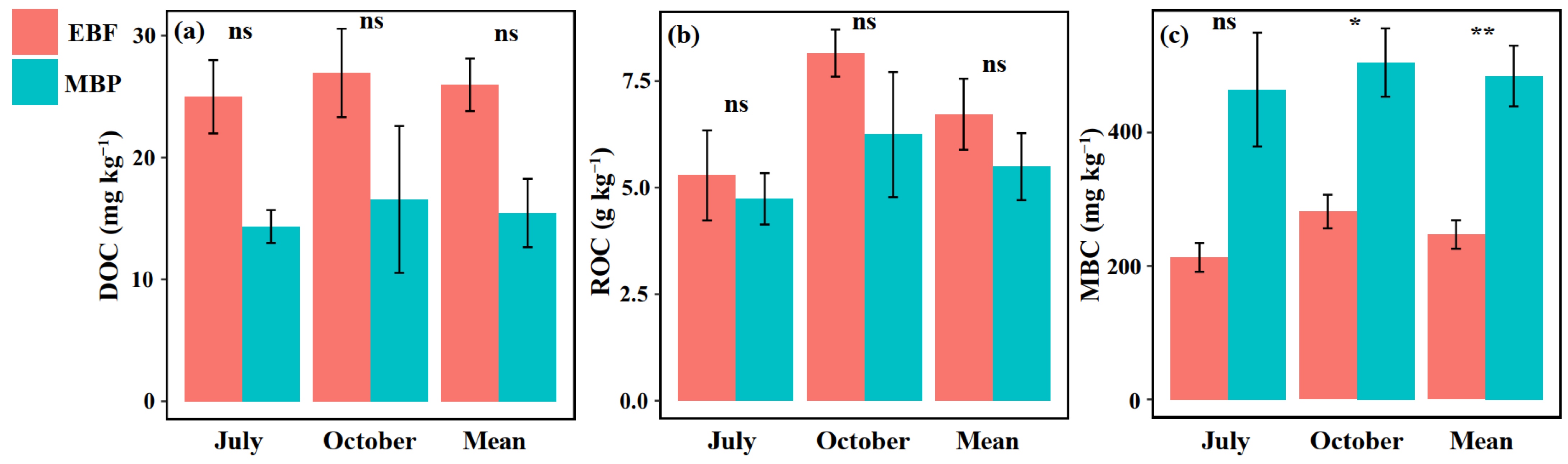

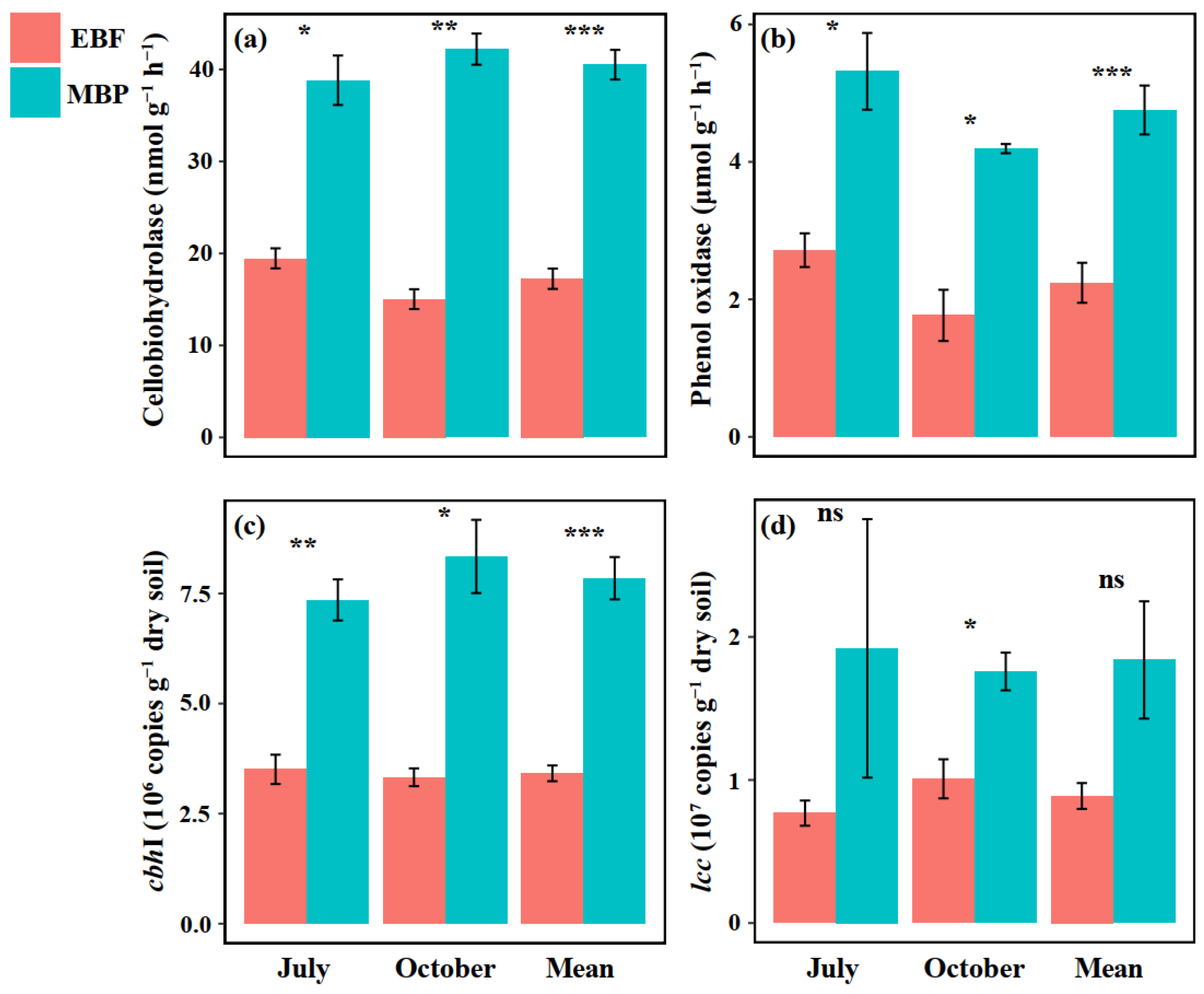
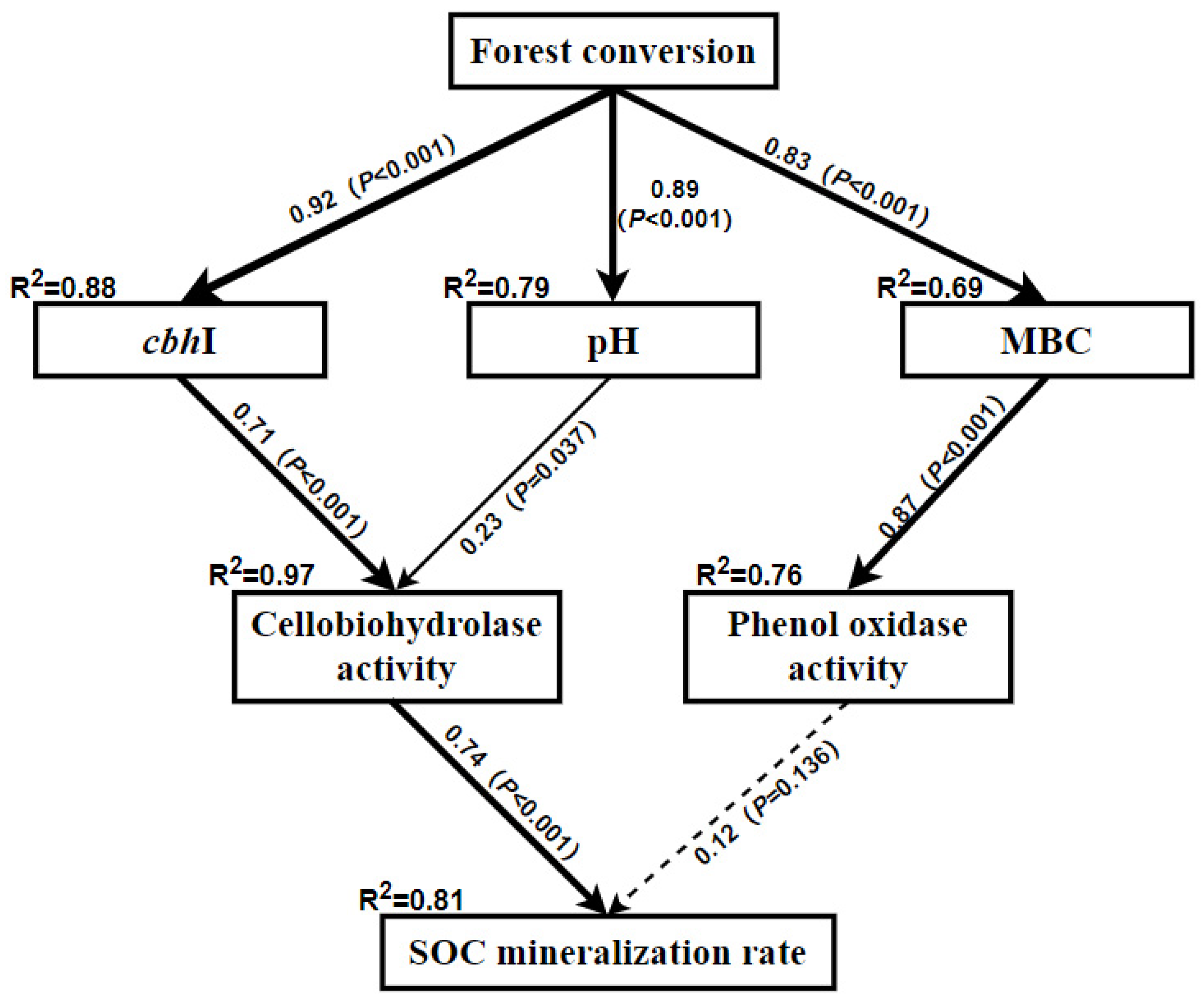
| Soil Property | Sampling Time | EBF | MBP | p-Value (Paired t-Tests) | |
|---|---|---|---|---|---|
| Bulk density (g cm−3) | July | 1.52 ± 0.06 | 1.23 ± 0.11 | 0.227 | |
| Clay (%) | July | 32.46 ± 1.30 | 31.89 ± 0.17 | 0.668 | |
| Texture | Silt (%) | 44.44 ± 0.43 | 45.06 ± 2.09 | 0.783 | |
| Sand (%) | 23.10 ± 1.44 | 23.05 ± 1.92 | 0.988 | ||
| Moisture content (%) | July | 22.45 ± 2.67 | 24.18 ± 4.34 | 0.604 | |
| October | 12.59 ± 0.83 | 14.57 ± 0.20 | 0.183 | ||
| Mean | 17.52 ± 2.54 | 19.37 ± 2.90 | 0.225 | ||
| pH (H2O) | July | 4.33 ± 0.03 | 4.64 ± 0.07 | 0.023 | |
| October | 4.17 ± 0.04 | 4.55 ± 0.03 | 0.003 | ||
| Mean | 4.25 ± 0.04 | 4.60 ± 0.04 | <0.001 | ||
| Soil organic carbon (SOC) (mg kg−1) | July | 21.12 ± 1.39 | 23.61 ± 2.01 | 0.209 | |
| October | 20.64 ± 7.01 | 22.84 ± 4.76 | 0.445 | ||
| Mean | 20.88 ± 3.20 | 23.22 ± 2.32 | 0.110 | ||
| Total nitrogen (mg kg−1) | July | 1.04 ± 0.04 | 1.67 ± 0.26 | 0.151 | |
| October | 1.69 ± 0.13 | 1.93 ± 0.32 | 0.603 | ||
| Mean | 1.37 ± 0.16 | 1.80 ± 0.19 | 0.119 | ||
| C/N | July | 20.45 ± 2.14 | 14.74 ± 2.13 | 0.135 | |
| October | 12.93 ± 5.30 | 11.86 ± 1.34 | 0.838 | ||
| Mean | 16.69 ± 3.06 | 13.30 ± 1.30 | 0.240 | ||
| SOC storage (Mg ha−1) | July | 64.31 ± 6.27 | 58.27 ± 8.34 | 0.634 | |
| October | 64.38 ± 4.62 | 54.00 ± 5.66 | 0.639 | ||
| Mean | 64.34 ± 3.59 | 56.13 ± 4.61 | 0.442 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zheng, X.; Cai, Y.; Chang, S.X. Land-Use Change Enhanced SOC Mineralization but Did Not Significantly Affect Its Storage in the Surface Layer. Int. J. Environ. Res. Public Health 2022, 19, 3020. https://doi.org/10.3390/ijerph19053020
Zhang H, Zheng X, Cai Y, Chang SX. Land-Use Change Enhanced SOC Mineralization but Did Not Significantly Affect Its Storage in the Surface Layer. International Journal of Environmental Research and Public Health. 2022; 19(5):3020. https://doi.org/10.3390/ijerph19053020
Chicago/Turabian StyleZhang, Haikuo, Xuli Zheng, Yanjiang Cai, and Scott X. Chang. 2022. "Land-Use Change Enhanced SOC Mineralization but Did Not Significantly Affect Its Storage in the Surface Layer" International Journal of Environmental Research and Public Health 19, no. 5: 3020. https://doi.org/10.3390/ijerph19053020
APA StyleZhang, H., Zheng, X., Cai, Y., & Chang, S. X. (2022). Land-Use Change Enhanced SOC Mineralization but Did Not Significantly Affect Its Storage in the Surface Layer. International Journal of Environmental Research and Public Health, 19(5), 3020. https://doi.org/10.3390/ijerph19053020








