Antiviral Drugs in Influenza
Abstract
1. Introduction
2. Influenza Virus
2.1. Structure of Influenza Virus
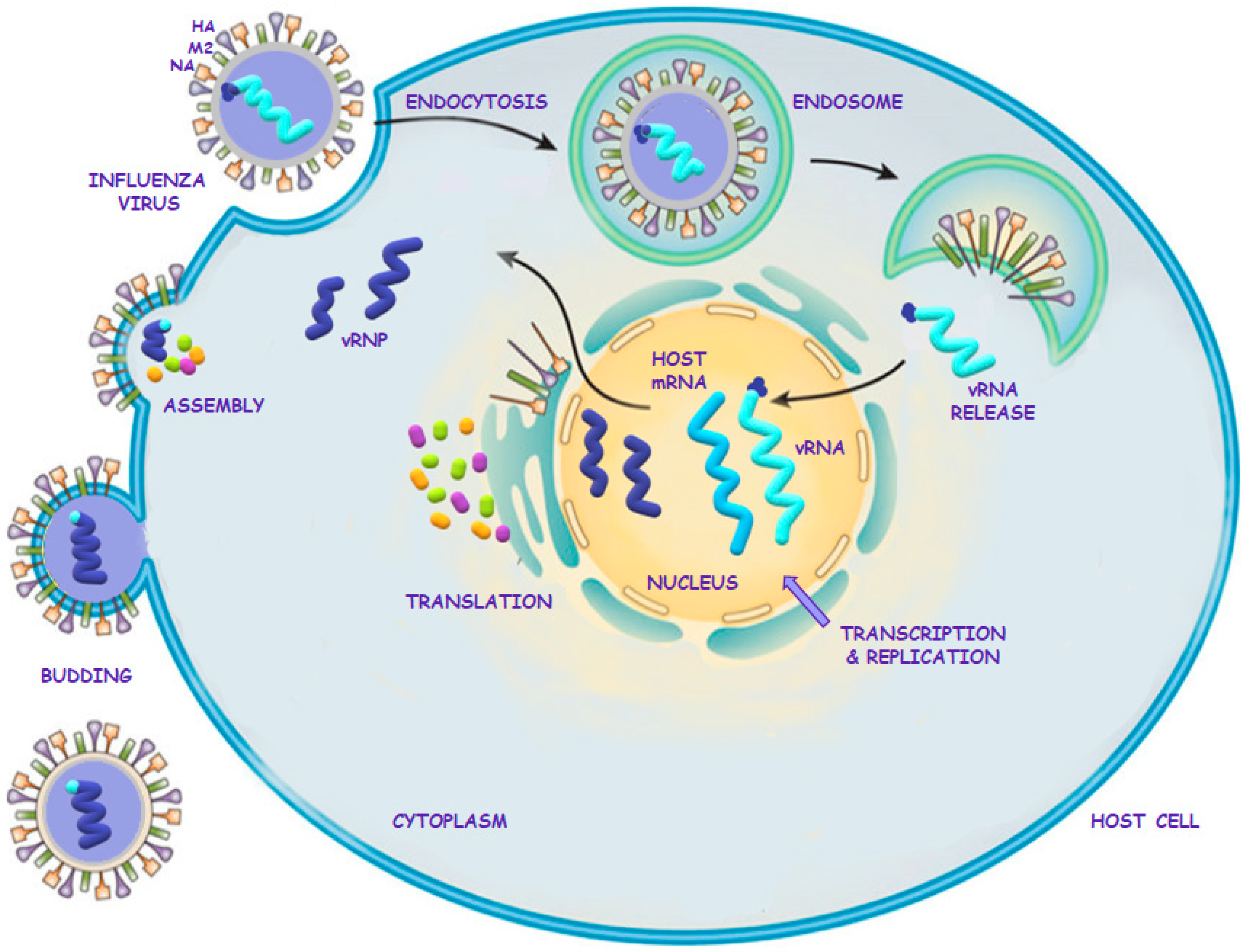
2.2. Influenza Virus Life Cycle
3. Drugs Used for Influenza Treatment and Prophylaxis
3.1. Amantadine
3.2. Neuraminidase Inhibitors (NAIs)
3.2.1. Group Presentation
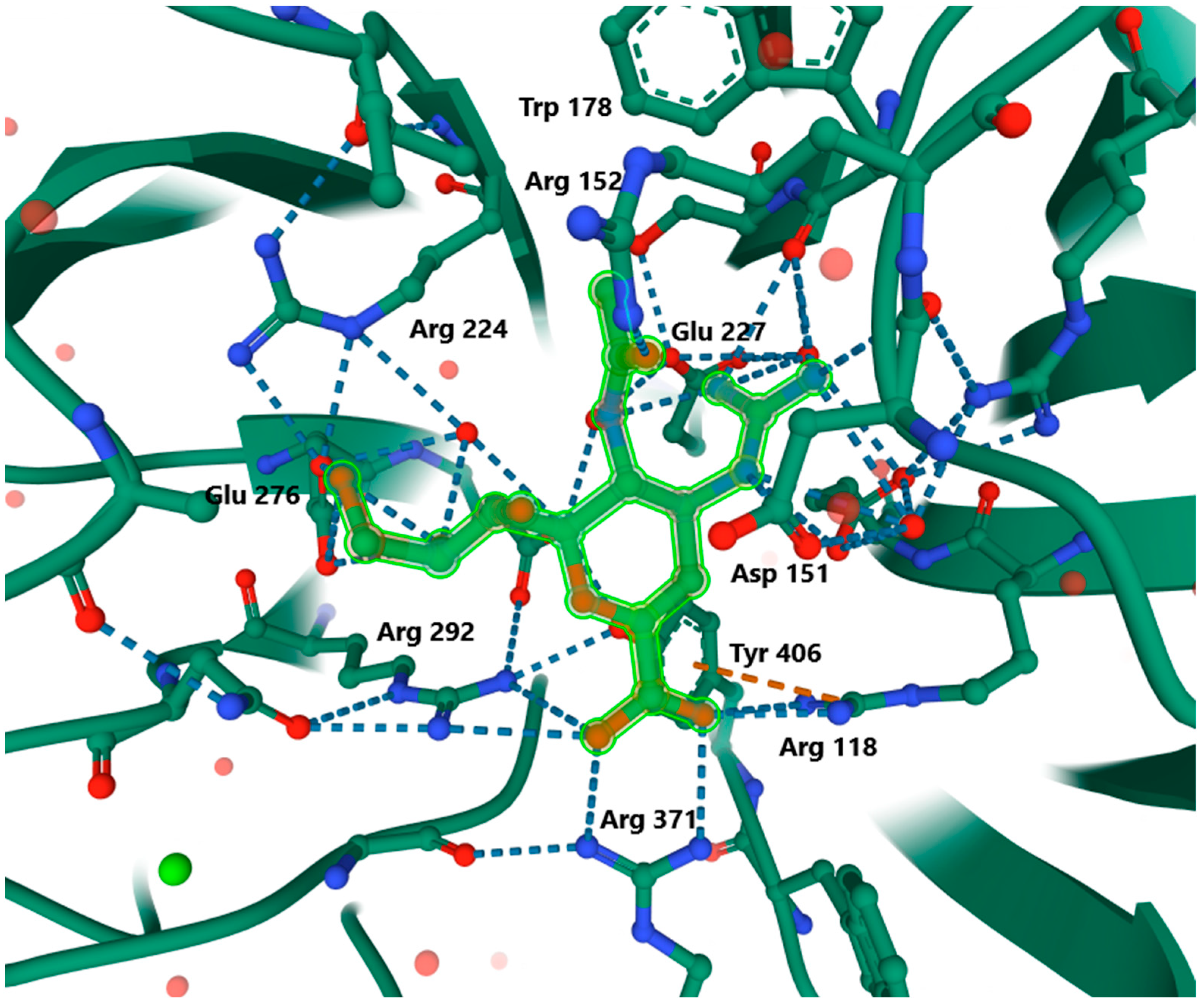
3.2.2. Mode of Action
3.2.3. Zanamivir
Drug Presentation
Pharmacokinetics of Zanamivir for Oral Inhalation
Treatment and Prophylaxis with Zanamivir for Oral Inhalation
Pharmacokinetics of Intravenous Zanamivir
Treatment with Intravenous Zanamivir
3.2.4. Oseltamivir
Drug Presentation
Pharmacokinetics
Treatment and Prophylaxis with Oral Oseltamivir
Oseltamivir as an Over-the-Counter Drug
3.2.5. Peramivir
Drug Presentation
Pharmacokinetics
Treatment with Intravenous Peramivir
3.2.6. Laninamivir
Drug Presentation
Pharmacokinetics
Treatment and Prophylaxis with Inhaled Laninamivir
3.2.7. Comparison of the Effectiveness of NAIs
3.3. Cap-Dependent Endonuclease Inhibitors—New Group of Anti-Influenza Drugs
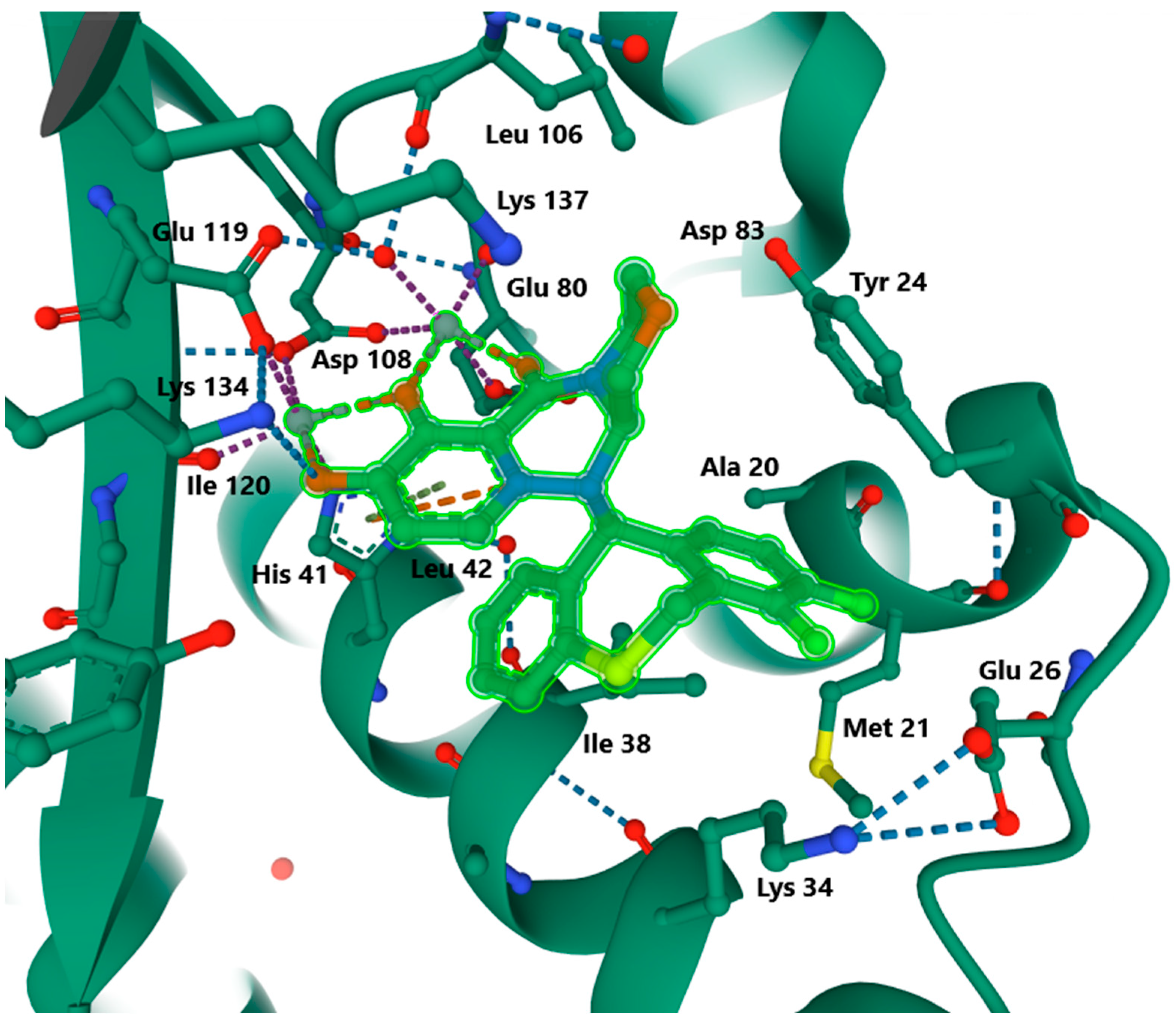
3.3.1. Baloxavir Marboxil
3.3.2. Mode of Action
3.3.3. Pharmacokinetics of Baloxavir
3.3.4. Treatment and Prophylaxis with Oral Baloxavir
3.3.5. Safety of Baloxavir
3.3.6. Effectiveness of Baloxavir
4. Current Studies
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keilman, L.J. Seasonal Influenza (Flu). Nurs. Clin. N. Am. 2019, 54, 227–243. [Google Scholar] [CrossRef] [PubMed]
- WHO. Influenza. Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccines-quality/influenza (accessed on 13 October 2021).
- WHO. Estimate of Influenza Deaths Due to Respiratory Disease. Available online: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/burden-of-disease (accessed on 13 October 2021).
- Putri, W.C.W.S.; Muscatello, D.J.; Stockwell, M.S.; Newall, A.T. Economic burden of seasonal influenza in the United States. Vaccine 2018, 36, 3960–3966. [Google Scholar] [CrossRef] [PubMed]
- Marbus, S.D.; Schweitzer, V.A.; Groeneveld, G.H.; Oosterheert, J.J.; Schneeberger, P.M.; van der Hoek, W.; van Dissel, J.T.; van Gageldonk-Lafeber, A.B.; Mangen, M.-J. Incidence and costs of hospitalized adult influenza patients in The Netherlands: A retrospective observational study. Eur. J. Health Econ. 2020, 21, 775–785. [Google Scholar] [CrossRef] [PubMed]
- WHO. Seasonal Influenza. Available online: https://www.who.int/health-topics/influenza-seasonal#tab=tab_1 (accessed on 13 October 2021).
- Shirley, M. Baloxavir Marboxil: A Review in Acute Uncomplicated Influenza. Drugs 2020, 80, 1109–1118. [Google Scholar] [CrossRef]
- Dharmapalan, D. Influenza. Indian J. Pediatrics 2020, 87, 828–832. [Google Scholar] [CrossRef]
- McNicholl, I.R.; McNicholl, J.J. Neuraminidase Inhibitors: Zanamivir and Oseltamivir. Ann. Pharmacother. 2001, 35, 57–70. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Palese, P. The biology of Influenza viruses. Vaccine 2008, 265, 49–53. [Google Scholar] [CrossRef]
- FDA—41st Edition—2021—Approved Drug Product List. Prescription Drug Product List. pp. 329–457. Available online: https://thefdalawblog.com/wp-content/uploads/2021/01/Orange-Book-41st-Annual.pdf (accessed on 13 October 2021).
- Gubareva, L.V.; Kaiser, L.; Hayden, F.G. Influenza virus neuraminidase inhibitors. Lancet 2000, 355, 827–835. [Google Scholar] [CrossRef]
- Kim, J.-H.; Resende, R.; Wennekes, T.; Chen, H.-M.; Bance, N.; Buchini, S.; Watts, A.G.; Pilling, P.; Streltsov, V.A.; Petric, M.; et al. Mechanism-Based Covalent Neuraminidase Inhibitors with Broad-Spectrum Influenza Antiviral Activity. Science 2013, 340, 71–75. [Google Scholar] [CrossRef]
- Moscona, A. Neuraminidase Inhibitors for Influenza. N. Engl. J. Med. 2005, 353, 1363–1373. [Google Scholar] [CrossRef]
- Samji, T. Influenza A: Understanding the Viral Life Cycle. Yale J. Biol. Med. 2009, 82, 153–159. [Google Scholar]
- Dreitlein, W.B.; Maratos, J.; Brocavich, J. Zanamivir and oseltamivir: Two new options for the treatment and prevention of influenza. Clin. Ther. 2001, 23, 327–355. [Google Scholar] [CrossRef]
- O’Hanlon, R.; Shaw, M.L. Baloxavir marboxil: The new influenza drug on the market. Curr. Opin. Virol. 2019, 35, 14–18. [Google Scholar] [CrossRef]
- Laborda, P.; Wang, S.-Y.; Voglmeir, J. Influenza Neuraminidase Inhibitors: Synthetic Approaches, Derivatives and Biological Activity. Molecules 2016, 21, 1513. [Google Scholar] [CrossRef]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Hubert, T.J.; Dietrich, D.E.; Emrich, H.M. Possible use of Amantadine in depression. Pharmacopsychiatry 1999, 32, 47–55. [Google Scholar] [CrossRef]
- Tokimatsu, I.; Nasu, N. Anti-influenza A viral drug-amantadine. Nihon Rinsho 2000, 58, 2288–2292. [Google Scholar]
- Yi, M.; Cross, T.A.; Zhou, H.X. A Secondary Gate as a Mechanism for Inhibition of the M2 Proton Channel by Amantadine. J. Phys. Chem. B 2008, 112, 7977–7979. [Google Scholar] [CrossRef]
- Balannik, V.; Wang, J.; Ohigashi, Y.; Jing, X.; Magavern, E.; Lamb, R.A.; Degrado, W.F.; Pinto, L.H. Design and Pharmacological Characterization of Inhibitors of Amantadine-Resistant Mutants of the M2 Ion Channel of Influenza A Virus. Biochemistry 2009, 48, 11872–11882. [Google Scholar] [CrossRef]
- Danielczyk, W. Twenty-five years of amantadine therapy in Parkinson’s disease. J. Neural Transm. Suppl. 1995, 46, 399–405. [Google Scholar]
- Horadam, V.W.; Sharp, J.G.; Smilack, J.D.; McAnalley, B.H.; Garriott, J.C.; Stephens, M.K.; Prati, R.C.; Brater, D.C. Pharmacokinetics of Amantadine Hydrochloride in Subjects with Normal and Impaired Renal Function. Ann. Intern. Med. 1981, 94, 454–458. [Google Scholar] [CrossRef]
- Roin, S.; Winters, S. Amantadine Hydrochloride: Current and News Uses. J. Neurosci. Nurs. 1990, 22, 322–325. [Google Scholar] [CrossRef]
- Dong, G.; Peng, C.; Luo, J.; Wang, C.; Han, L.; Wu, B.; Ji, G.; He, H. Adamantane-resistant influenza a viruses in the world (1902–2013): Frequency and distribution of M2 gene mutations. PLoS ONE 2015, 10, e0119115. [Google Scholar] [CrossRef]
- Jefferson, T.; Demicheli, V.; Di Pietrantonj, C.; Rivetti, D. Amantadine and rimantadine for influenza A in adults. Cochrane Database Syst. Rev. 2006, CD001169. [Google Scholar] [CrossRef]
- Danysz, W.; Parsons, C.G.; Kornhuber, J.; Schmidt, W.J.; Quack, G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents—Preclinical studies. Neurosci. Biobehav. Rev. 1997, 21, 455–468. [Google Scholar] [CrossRef]
- Müller, T.; Kuhn, W.; Möhr, J.-D. Evaluating ADS5102 (amantadine) for the treatment of Parkinson’s disease patients with dyskinesia. Expert Opin. Pharmacother. 2019, 20, 1181–1187. [Google Scholar] [CrossRef]
- Hauser, R.A.; Pahwa, R.; Wargi, W.A.; Souza-Prien, C.J.; McClure, N.; Johnson, R.; Nguyen, J.T.; Patni, R.; Went, G.T. Pharmacokinetics of ADS-5102 (Amantadine) Extended Release Capsules Administered Once Daily at Bedtime for the Treatment of Dyskinesia. Clin Pharmacokinet. 2019, 58, 77–88. [Google Scholar] [CrossRef]
- Loggini, A.; Tangonan, R.; Ammar, F.E.; Mansour, A.; Goldenberg, F.D.; Kramer, C.L.; Lazaridis, C. The role of amantadine in cognitive recovery early after traumatic brain injury: A systematic review. Clin. Neurol. Neurosurg. 2020, 194, 1–6. [Google Scholar] [CrossRef]
- Carabenciov, I.D.; Bureau, B.L.; Cutrer, M.; Savica, R. Amantadine Use for Postconcussion Syndrome. Mayo Clin. Proc. 2019, 94, 275–277. [Google Scholar] [CrossRef]
- Nourbakhsh, B.; Revirajan, N.; Morris, B.; Cordano, C.; Creasman, J.; Manguinao, M.; Krysko, K.; Rutatangwa, A.; Auvray, C.; Aljarallah, S.; et al. Safety and efficacy of amantadine, modafinil, and methylphenidate for fatigue in multiple sclerosis: A randomised, placebo-controlled, crossover, double-blind trial. Lancet Neurol. 2020, 20, 38–48. [Google Scholar] [CrossRef]
- Rejdak, K.; Grieb, P. Adamantanes might be protective from COVID-19 in patients with neurological diseases: Multiple sclerosis, parkinsonism and cognitive impairment. Mult. Scler. Relat. Disorders Elsevier 2020, 42, 102163. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Abreu, G.E.; Aranda-Martinez, J.D.; Araújo, R.; Hernandez-Aguilar, M.E.; Herrera-Covarrubias, D.; Rojas-Durán, F. Observational study of people infected with SARS-CoV-2, treated with amantadine. Pharmacol. Rep. 2020, 72, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Castaldo, N.; Carnelutti, A. Neuraminidase inhibitors as a strategy for influenza treatment: Pros, cons and future perspectives. Expert Opin. Pharmacother. 2019, 1465–6566, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lackenby, A.; Besselaar, T.G.; Daniels, R.S.; Fry, A.; Gregory, V.; Gubareva, L.V.; Huang, W.; Hurt, A.C.; Leang, S.-K.; Lee, R.T.C.; et al. Global update on the susceptibility of human influenza viruses to neuraminidase inhibitors and status of novel antivirals, 2016–2017. Antivir. Res. 2018, 157, 38–46. [Google Scholar] [CrossRef]
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar]
- Rech, M.A. Oseltamivir for All: Should It Become an Over-the-Counter Medication for Influenza Treatment? Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 182–185. [Google Scholar] [CrossRef]
- Hayden, F.G.; Osterhaus, A.D.M.E.; Treanor, J.J.; Fleming, D.M.; Aoki, F.Y.; Nicholson, K.G.; Bohnen, A.M.; Hirst, H.M.; Kenee, O.; Wightman, K. Efficacy and Safety of the Neuraminidase Inhibitor Zanamivir in the Treatment of Influenzavirus Infections. N. Engl. J. Med. 1997, 337, 874–880. [Google Scholar] [CrossRef]
- Kim, C.U.; Lew, W.; Williams, M.A.; Liu, H.; Zhang, L.; Swaminathan, S.; Bischofberger, N.; Chen, M.S.; Mendel, D.B.; Tai, C.Y.; et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: Design, synthesis and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 1997, 119, 681–690. [Google Scholar] [CrossRef]
- EMA. Dectova, Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/dectova-epar-product-information_en.pdf (accessed on 13 October 2021).
- Katzen, J.; Kohn, R.; Houk, J.L.; Ison, M.G. Early oseltamivir after hospital admission is associated with shortened hospitalization: A five-year analysis of oseltamivir timing and clinical outcomes. Clin. Infect. Dis. 2018, 69, 52–58. [Google Scholar] [CrossRef]
- Lee, N.; Choi, K.W.; Chan, P.K.; Hui, D.S.C.; Lui, G.C.Y.; Wong, B.C.K.; Wong, R.Y.K.; Sin, W.Y.; Hui, W.M.; Ngai, K.L.K.; et al. Outcomes of adults hospitalised with severe influenza. BMJ J. Thorax. 2010, 65, 510–515. [Google Scholar] [CrossRef]
- Louie, J.K.; Yang, S.; Acosta, M.; Yen, C.; Samuel, M.C.; Schechter, R.; Guevara, H.; Uyeki, T.M. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin. Infect. Dis. 2012, 55, 1198–1204. [Google Scholar] [CrossRef]
- Doll, M.K.; Winters, N.; Boikos, C.; Kraicer-Melamed, H.; Gore, G.; Quach, C. Safety and effectiveness of neuraminidase inhibitors for influenza treatment, prophylaxis, and outbreak control: A systematic review of systematic reviews and/or meta-analyses. J. Antimicrob. Chemother. 2017, 72, 2990–3007. [Google Scholar] [CrossRef]
- Muthuri, S.G.; Venkatesan, S.; Myles, P.R.; Leonardi-Bee, J.; Al Khuwaitir, T.S.A.; Al Mamun, A.; Anovadiya, A.P.; Azziz-Baumgartner, E.; Báez, C.; Bassetti, M.; et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: A meta-analysis of individual participant data. Lancet Respir. Med. 2014, 2, 395–404. [Google Scholar] [CrossRef]
- Chow, E.J.; Doyle, J.D.; Uyeki, T.M. Influenza virus-related critical illness: Prevention, diagnosis, treatment. Crit. Care 2019, 23, 214. [Google Scholar] [CrossRef]
- Hiba, V.; Chowers, M.; Levi-Vinograd, I.; Rubinovitch, B.; Leibovici, L.; Paul, M. Benefit of early treatment with oseltamivir in hospitalized patients with documented 2009 influenza A (H1N1): Retrospective cohort study. J. Antimicrob. Chemother. 2011, 66, 1150–1155. [Google Scholar] [CrossRef]
- Hsu, J.; Santesso, N.; Mustafa, R.; Brozek, J.; Chen, Y.L.; Hopkins, J.P.; Cheung, A.; Hovhannisyan, G.; Ivanova, L.; Flottorp, S.A.; et al. Antivirals for treatment of influenza: A systematic review and meta-analysis of observational studies. Ann. Intern. Med. 2012, 156, 512–524. [Google Scholar] [CrossRef]
- Muthuri, S.G.; Myles, P.R.; Venkatesan, S.; Leonardi-Bee, J.; Nguyen-Van-Tam, J.S. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009–2010 influenza a(H1N1) pandemic: A systematic review and meta-analysis in hospitalized patients. J. Infect. Dis. 2013, 207, 553–563. [Google Scholar] [CrossRef]
- Heneghan, C.J.; Onakpoya, I.; Jones, M.A.; Doshi, P.; Del Mar, C.B.; Hama, R.; Thompson, M.J.; Spencer, E.A.; Mahtani, K.R.; Nunan, D.; et al. Neuraminidase inhibitors for influenza: A systematic review and meta-analysis of regulatory and mortality data. Health Technol. Assess. 2016, 42, 5–96. [Google Scholar] [CrossRef]
- Kumar, S.; Goicoechea, S.; Kumar, S.; Pearce, C.M.; Durvasula, R.; Kempaiah, P.; Poonam, B.R. Oseltamivir analogs with potent anti-influenza virus activity. Drug Discov. Today. 2020, 25, 1389–1402. [Google Scholar] [CrossRef]
- Shtyrya, Y.A.; Mochalova, L.V.; Bovin, N.V. Influenza virus neuraminidase: Structure and function. Acta Naturae. 2009, 1, 26–32. [Google Scholar] [CrossRef]
- Gubareva, L.V.; Webster, R.G.; Hayden, F.G. Comparison of the Activities of Zanamivir, Oseltamivir, and RWJ-270201 against Clinical Isolates of Influenza Virus and Neuraminidase Inhibitor-Resistant Variants. Antimicrob. Agents Chemother. 2021, 45, 3403–3408. [Google Scholar] [CrossRef]
- Yin, X.; Jiang, N.; Shi, W.; Chi, X.; Liu, S.; Chen, J.-L.; Wang, S. Development and Effects of Influenza Antiviral Drugs. Molecules 2021, 26, 810. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. Oseltamivir for influenza infection in children: Risks and benefits. Expert Rev. Respir. Med. 2015, 10, 79–87. [Google Scholar] [CrossRef]
- Sur, M.; Lopez, M.J.; Baker, M.B. Oseltamivir. 2020. Available online: https://www-1ncbi-1nlm-1nih-1gov-100001atj0c66.han3.wum.edu.pl/books/NBK539909/ (accessed on 13 October 2021).
- EMA. List of Nationally Authorised Medicinal Products. 2017. Available online: https://www.ema.europa.eu/en/documents/psusa/zanamivir-list-nationally-authorised-medicinal-products-psusa/00003141/201701_en.pdf (accessed on 13 October 2021).
- FDA. Highlights of Prescribing Information. Relenza. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021036s025lbl.pdf (accessed on 13 October 2021).
- Australia’s National Science Agency. Relenza—The First Effective Flu-Fighter. Available online: https://www.csiro.au/en/research/health-medical/vaccines/relenza (accessed on 13 October 2021).
- EMA. Dectova, Authorisation Details. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/dectova (accessed on 13 October 2021).
- EMC. Relenza. Available online: https://www.medicines.org.uk/emc/product/3809/smpc (accessed on 13 October 2021).
- EMC. Dectova. Available online: https://www.medicines.org.uk/emc/product/10193/smpc (accessed on 13 October 2021).
- EMA. Dectova, Assessment Report. Available online: https://www.ema.europa.eu/en/documents/assessment-report/dectova-epar-public-assessment-report_en.pdf (accessed on 13 October 2021).
- EMA; Tamiflu. Summary of Product Characteristic. Available online: https://www.ema.europa.eu/en/documents/product-information/tamiflu-epar-product-information_en.pdf (accessed on 13 October 2021).
- FDA; Tamiflu. Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021087s068,021246s051lbl.pdf (accessed on 13 October 2021).
- EMC. Tamiflu. Available online: https://www.medicines.org.uk/emc/medicine/20294#gref (accessed on 13 October 2021).
- Bardsley-Elliot, A.; Noble, S. Oseltamivir. Drugs 1999, 58, 851–860. [Google Scholar] [CrossRef]
- McLaughlin, M.M.; Skoglund, E.W.; Ison, M.G. Peramivir: An intravenous neuraminidase inhibitor. Expert Opin. Pharmacother. 2015, 16, 1889–9000. [Google Scholar] [CrossRef]
- Scott, L.J. Peramivir: A Review in Uncomplicated Influenza. Drugs 2018, 78, 1363–1370. [Google Scholar] [CrossRef]
- FDA. Rapivab, Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206426lbl.pdf (accessed on 13 October 2021).
- Shetty, A.K.; Peek, L.A. Peramivir for the treatment of influenza. Expert Rev. Anti-Infect. Ther. 2012, 10, 123–143. [Google Scholar] [CrossRef]
- Kashiwagi, S.; Yoshida, S.; Yamaguchi, H.; Niwa, S.; Mitsui, N.; Tanigawa, M.; Yamaguchi, F. Safety of the long-acting neuraminidase inhibitor laninamivir octanoate hydrate in post-marketing surveillance. Int. J. Antimicrob. Agents 2012, 40, 381–388. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Efficacy and Safety Study of Laninamivir Octanoate TwinCaps® Dry Powder Inhaler in Adults with Influenza (Igloo). Available online: https://clinicaltrials.gov/ct2/show/results/NCT01793883?view=results (accessed on 7 February 2022).
- Cass, L.M.R.; Efthymiopoulos, C.; Bye, A. Pharmacokinetics of Zanamivir After Intravenous, Oral, Inhaled or Intranasal Administration to Healthy Volunteers. Clin Pharmacokinet. 1999, 36, 1–11. [Google Scholar] [CrossRef]
- Weller, S.; Jones, L.S.; Lou, Y.; Peppercorn, A.; Ng-Cashin, J. Pharmacokinetics of Zanamivir following Intravenous Administration to Subjects with and without Renal Impairment. Antimicrob. Agents Chemother. 2013, 57, 2967–2971. [Google Scholar] [CrossRef] [PubMed][Green Version]
- MacConnachie, A.M. Zanamivir (Relenza®)—A new treatment for influenza. Intensive Crit. Care Nurs. 1999, 15, 369–370. [Google Scholar] [CrossRef]
- Marty, F.M.; Man, C.Y.; Horst, C.; Francois, B.; Garot, D.; Máňez, R.; Thamlikitkul, V.; Lorente, J.A.; Álvarez-Lerma, F.; Brealey, D.; et al. Safety and Pharmacokinetics of Intravenous Zanamivir Treatment in Hospitalized Adults with Influenza: An Open-label, Multicenter, Single-Arm, Phase II Study. J. Infect. Dis. 2014, 209, 542–550. [Google Scholar] [CrossRef] [PubMed]
- EMA. Dectova (zanamivir) An overview of Dectova and Why It Is Authorised in the EU. Available online: https://www.ema.europa.eu/en/documents/overview/dectova-epar-medicine-overview_en.pdf (accessed on 13 October 2021).
- Slain, D. Intravenous Zanamivir: A Viable Option for Critically Ill Patients with Influenza. Ann. Pharmacother. 2020, 55, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Torti, C.; Mazzitelli, M.; Longhini, F.; Garofalo, E.; Bruni, A.; Giancotti, A.; Barreca, G.S.; Quirino, A.; Liberto, M.C.; Serapide, F.; et al. Clinical outcomes of patients treated with intravenous zanamivir for severe influenza A(H1N1)pdm09 infection: A case report series. BMC Infect. Dis. 2019, 19, 858. [Google Scholar] [CrossRef]
- Marty, F.M.; Vidal-Puigserver, J.; Clark, C.; Gupta, S.K.; Merino, E.; Garot, D.; Chapman, M.J.; Jacobs, F.; Rodriguez-Noriega, E.; Husa, P.; et al. Intravenous zanamivir or oral oseltamivir for hospitalised patients with influenza: An international, randomised, double-blind, double-dummy, phase 3 trial. Lancet Respir. Med. 2017, 5, 135–146. [Google Scholar] [CrossRef]
- Wijaya, L.; Chua, Y.Y.; Cui, L.; Chan, K.; Tan, B.H. Intravenous zanamivir in critically ill patients due to pandemic 2009 (H I N I) influenza A virus. Singap. Med. 2011, 52, 481. [Google Scholar]
- Bradley, J.S.; Blumer, J.L.; Romero, J.R.; Michaels, M.G.; Munoz, F.M.; Kimberlin, D.W.; Pahud, B.; DeBiasi, R.L.; Yamamoto, G.; Roberts, G.; et al. Intravenous Zanamivir in Hospitalized Patients with Influenza. Pediatrics 2017, 140, 2016–2727. [Google Scholar] [CrossRef]
- Louie, J.K.; Lampiris, H. Treating Influenza with Neuraminidase Inhibitors. JAMA Intern. Med. 2015, 175, 1–9. [Google Scholar] [CrossRef]
- Burnham, A.J.; Baranovich, T.; Govorkova, E.A. Neuraminidase inhibitors for influenza B virus infection: Efficacy and resistance. Antivir. Res. 2013, 100, 520–534. [Google Scholar] [CrossRef]
- Davies, B.E. Pharmacokinetics of oseltamivir: An oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J. Antimicrob. Chemother. 2010, 65, ii5–ii10. [Google Scholar] [CrossRef]
- Ariano, R.E.; Sitar, D.S.; Zelenitsky, S.A.; Zarychanski, R.; Pisipati, A.; Ahern, S.; Kanji, A.; Rello, J.; Kumar, A. Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza. Can. Med. Assoc. J. 2010, 182, 357–363. [Google Scholar] [CrossRef]
- Eyler, R.F.; Heung, M.; Pleva, M.; Sowinski, K.M.; Park, P.K.; Napolitano, L.M.; Mueller, B.A. Pharmacokinetics of Oseltamivir and Oseltamivir Carboxylate in Critically Ill Patients Receiving Continuous Venovenous Hemodialysis and/or Extracorporeal Membrane Oxygenation. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2012, 32, 1061–1069. [Google Scholar] [CrossRef]
- WHO Guidelines for Pharmacological Management of Pandemic Influenza A(H1N1) 2009 and other Influenza Viruses. Available online: https://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf (accessed on 13 October 2021).
- He, G.; Massarella, J.; Ward, P. Clinical Pharmacokinetics of the Prodrug Oseltamivir and its Active Metabolite Ro 64-0802. Clin. Pharmacokinet. 1999, 37, 471–484. [Google Scholar] [CrossRef]
- Cies, J.J.; Moore, W.S.; Enache, A.; Chopra, A. Peramivir for Influenza A and B Viral Infections: A Pharmacokinetic Case Series. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 1060–1065. [Google Scholar] [CrossRef]
- EMA. Ebilfumin. Authorisation Details. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ebilfumin#authorisation-details-section (accessed on 13 October 2021).
- Okamoto, E. Is oseltamivir (Tamiflu®) safe? Re-examining the Tamiflu “ado” from Japan. Expert Rev. Pharm. Outcomes Res. 2010, 10, 17–24. [Google Scholar] [CrossRef]
- FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process (accessed on 13 October 2021).
- Drugs.com. Tamiflu Alternatives Compared. Available online: https://www.drugs.com/compare/tamiflu (accessed on 13 October 2021).
- EMA. Alpivab, Withdrawal Marketing Authorisation in the European Union. Available online: https://www.ema.europa.eu/en/documents/public-statement/public-statement-alpivab-withdrawal-marketing-authorisation-european-union_en.pdf (accessed on 13 October 2021).
- Saisho, Y.; Ishibashi, T.; Fukuyama, H.; Fukase, H.; Shimada, J. Pharmacokinetics and safety of intravenous peramivir, neuraminidase inhibitor of influenza virus, in healthy Japanese subjects. Antivir. Ther. 2016, 22, 313–323. [Google Scholar] [CrossRef]
- Elsevier; Peramivir. Published 4 February 2021. Available online: https://elsevier.health/en-US/preview/peramivir (accessed on 7 February 2022).
- DaiichiSankyo. Approval for the Use of Inavir® to Prevent Influenza. Available online: https://www.daiichisankyo.com/files/news/pressrelease/pdf/006052/20131220_484_E.pdf (accessed on 13 October 2021).
- Ikematsu, H.; Kawai, N. Laninamivir octanoate: A new long-acting neuraminidase inhibitor for the treatment of influenza. Expert Rev. Anti-Infect. Ther. 2011, 9, 851–857. [Google Scholar] [CrossRef]
- Inavir—Drug Information Sheet. Available online: http://k-tosaka.sakura.ne.jp/FLU/inavir-shiori-Eng.pdf (accessed on 13 October 2021).
- Ishizuka, H.; Yoshiba, S.; Okabe, H.; Yoshihara, K. Clinical Pharmacokinetics of Laninamivir, a Novel Long-Acting Neuraminidase Inhibitor, After Single and Multiple Inhaled Doses of Its Prodrug, CS-8958, in Healthy Male Volunteers. J. Clin. Pharmacol. 2010, 50, 1319–1329. [Google Scholar] [CrossRef]
- Toyama, K.; Furuie, H.; Ishizuka, H. Intrapulmonary Pharmacokinetics of Laninamivir, a Neuraminidase Inhibitor, after a Single Nebulized Administration of Laninamivir Octanoate in Healthy Japanese Subjects. Antimicrob. Agents Chemother. 2017, 62, 1. [Google Scholar] [CrossRef]
- Murasaka, T.; Ikemura, K.; Enokiya, T.; Muraki, Y.; Ikemura, M.; Terada, K.; Iwamoto, T.; Okuda, M. Impact of the number of repeated inhalations and patient characteristics on the residual amount of inhaled laninamivir octanoate hydrate dry powder in pediatric patients with influenza. J. Pharm. Health Care Sci. 2017, 3, 26. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wei, S.-K.; Lai, C.C.; Weng, T.-S.; Wang, H.-H. A Meta-Analysis Comparing the Efficacy and Safety of Peramivir with Other Neuraminidase Inhibitors for Influenza Treatment. Medicina 2020, 56, 63. [Google Scholar] [CrossRef]
- Ishiguro, N.; Koseki, N.; Kaiho, M.; Ariga, T.; Kikuta, H.; Oba, K.; Togashi, T.; Morita, K.; Inagawa, A.; Okamura, A.; et al. Clinical effectiveness of four neuraminidase inhibitors (oseltamivir, zanamivir, laninamivir, and peramivir) for children with influenza A and B in the 2014–2015 to 2016–2017 influenza seasons in Japan. J. Infect. Chemother. 2018, 24, 449–457. [Google Scholar] [CrossRef]
- Hata, A.; Akashi-Ueda, R.; Takamatsu, K.; Matsumura, T. Safety and efficacy of peramivir for influenza treatment. Drug Des. Dev. Ther. 2014, 8, 2017–2038. [Google Scholar] [CrossRef][Green Version]
- The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group. Randomised trial of efficacy and safety of zanamivir for oral inhalation in treatment of influenza A and B virus infections. Lancet 1998, 12, 1877–1881. [Google Scholar] [CrossRef]
- Walker, J.B.; Hussey, E.K.; Treanor, J.J.; Montalvo, A., Jr.; Hayden, F.G. Effects of the Neuraminidase Inhibitor Zanamivir on Otologic Manifestations of Experimental Human Influenza. J. Infect. Dis. 1997, 176, 1417–1422. [Google Scholar] [CrossRef]
- Monto, A.S.; Robinson, D.P.; Herlocher, M.L.; Hinson, J.M., Jr.; Elliott, M.J.; Crisp, A. Zanamivir in the Prevention of Influenza Among Healthy Adults. JAMA 1999, 282, 31. [Google Scholar] [CrossRef]
- Hayden, F.G.; Gubareva, L.V.; Monto, A.S.; Klein, T.C.; Elliott, M.J.; Hammond, J.M.; Sharp, S.J.; Ossi, M.J. Zanamivir for oral inhalation for the Prevention of Influenza in Families. N. Engl. J. Med. 2000, 343, 1282–1289. [Google Scholar] [CrossRef]
- Monto, A.S.; Pichichero, M.E.; Blanckenberg, S.J.; Ruuskanen, O.; Cooper, C.; Fleming, D.M.; Kerr, C. Zanamivir Prophylaxis: An Effective Strategy for the Prevention of Influenza Types A and B within Households. J. Infect. Dis. 2002, 186, 1582–1588. [Google Scholar] [CrossRef]
- Treanor, J.J.; Hayden, F.G.; Vrooman, P.S.; Barbarash, R.; Bettis, R.; Riff, D.; Singh, S.; Kinnersley, N.; Ward, P.; Mills, R.G. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: A randomized controlled trial. US oral neuraminidase study group. JAMA 2000, 283, 1016–1024. [Google Scholar] [CrossRef]
- Whitley, R.J.; Hayden, F.G.; Reisinger, K.S.; Young, N.; Dutkowski, R.; Ipe, D.; Mills, R.G.; Ward, P. Oral oseltamivir treatment of influenza in children. Pediatric Infect. Dis. J. 2001, 20, 127–133. [Google Scholar] [CrossRef]
- Hayden, F.G.; Atmar, R.L.; Schilling, M.; Johnson, C.; Poretz, D.; Paar, D.; Huson, L.; Ward, P.; Mills, R.G. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N. Engl. J. Med. 1999, 341, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Belshe, R.; Villanueva, C.; Lanno, R.; Hughes, C.; Small, I.; Dutkowski, R.; Ward, P.; Carr, J. Management of influenza in households: A prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J. Infect. Dis. 2004, 189, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Welliver, R.; Monto, A.S.; Carewicz, O.; Schatteman, E.; Hassman, M.; Hedrick, J.; Jackson, H.C.; Huson, L.; Ward, P.; Oxford, J.S. Effectiveness of oseltamivir in preventing influenza in household contacts: A randomized controlled trial. JAMA 2001, 285, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Duval, X.; van der Werf, S.; Blanchon, T.; Mosnier, A.; Bouscambert-Duchamp, M.; Tibi, A.; Enouf, V.; Charlois-Ou, C.; Vincent, C.; Andreoletti, L.; et al. Efficacy of Oseltamivir-Zanamivir Combination Compared to Each Monotherapy for Seasonal Influenza: A Randomized Placebo-Controlled Trial. PLoS Med. 2010, 7, e1000362. [Google Scholar] [CrossRef]
- Escuret, V.; Cornu, C.; Boutitie, F.; Enouf, V.; Mosnier, A.; Bouscambert-Duchamp, M.; Gaillard, S.; Duval, X.; Blanchon, T.; Leport, C. Oseltamivir-zanamivir bitherapy compared to oseltamivir monotherapy in the treatment of pandemic 2009 influenza A(H1N1) virus infections. Antivir. Res. 2012, 96, 130–137. [Google Scholar] [CrossRef]
- Kohno, S.; Kida, H.; Mizuguchi, M.; Shimada, J. Efficacy and Safety of Intravenous Peramivir for Treatment of Seasonal Influenza Virus Infection. Antimicrob. Agents Chemother. 2010, 54, 4568–4574. [Google Scholar] [CrossRef]
- Kohno, S.; Yen, M.-Y.; Cheong, H.-J.; Hirotsu, N.; Ishida, T.; Kadota, J.; Mizuguchi, M.; Kida, H.; Shimada, J. Phase III Randomized, Double-Blind Study Comparing Single-Dose Intravenous Peramivir with Oral Oseltamivir in Patients with Seasonal Influenza Virus Infection. Antimicrob. Agents Chemother. 2011, 55, 5267–5276. [Google Scholar] [CrossRef]
- Ison, M.G.; Hui, D.S.; Clezy, K.; O’Neil, B.J.; Flynt, A.; Collis, P.J.; Simon, T.J.; Alexander, W.J. A clinical trial of intravenous peramivir compared with oral oseltamivir for the treatment of seasonal influenza in hospitalized adults. Antivir. Ther. 2012, 18, 651–661. [Google Scholar] [CrossRef]
- Shobugawa, Y.; Saito, R.; Sato, I.; Kawashima, T.; Dapat, C.; Dapat, I.C.; Kondo, H.; Saito, K.; Kondo, H.; Suzuki, Y.; et al. Clinical effectiveness of neuraminidase inhibitors—Oseltamivir, zanamivir, laninamivir, and peramivir—For treatment of influenza A(H3N2) and A(H1N1)pdm09 infection: An observational study in the 2010–2011 influenza season in Japan. J. Infect. Chemother. 2012, 18, 858–864. [Google Scholar] [CrossRef]
- Hikita, T.; Hikita, H.; Hikita, F.; Hikita, N.; Hikita, S. Clinical Effectiveness of Peramivir in Comparison with Other Neuraminidase Inhibitors in Pediatric Influenza Patients. Int. J. Pediatrics 2012, 2012, 834181. [Google Scholar] [CrossRef]
- Watanabe, A.; Chang, S.; Kim, M.J.; Chu, D.W.; Ohashi, Y. Long-Acting Neuraminidase Inhibitor Laninamivir Octanoate versus Oseltamivir for Treatment of Influenza: A Double-Blind, Randomized, Noninferiority Clinical Trial. Clin. Infect. Dis. 2010, 51, 1167–1175. [Google Scholar] [CrossRef]
- Mawatari, M.; Saito, R.; Hibino, A.; Kondo, H.; Yagami, R.; Odagiri, T.; Tanabe, I.; Shobugawa, Y. Effectiveness of four types of neuraminidase inhibitors approved in Japan for the treatment of influenza. PLoS ONE 2019, 14, e0224683. [Google Scholar] [CrossRef]
- Koseki, N.; Kaiho, M.; Kikuta, H.; Oba, K.; Togashi, T.; Ariga, T.; Ishiguro, N. Comparison of the clinical effectiveness of zanamivir and laninamivir octanoate for children with influenza A (H3N2) and B in the 2011–2012 season. Influenza Other Resp. Viruses 2014, 8, 151–158. [Google Scholar] [CrossRef]
- Kashiwagi, S.; Watanabe, A.; Ikematsu, H.; Uemori, M.; Awamura, S. Long-acting Neuraminidase Inhibitor Laninamivir Octanoate as Post-exposure Prophylaxis for Influenza. Clin. Infect. Dis. 2016, 63, 330–337. [Google Scholar] [CrossRef]
- EMA; Xofluza. Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210854s001lbl.pdf (accessed on 13 October 2021).
- EMA; Xofluza. Authorisation Details. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xofluza#assessment-history-section (accessed on 13 October 2021).
- Hayden, F.G.; Sugaya, N.; Hirotsu, N.; Lee, N.; de Jong, M.D.; Hurt, A.C.; Ishida, T.; Sekino, H.; Yamada, K.; Portsmouth, S. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N. Engl. J. Med. 2018, 379, 913–923. [Google Scholar] [CrossRef]
- Fang, Q.; Wang, D. Advanced researches on the inhibition of influenza virus by Favipiravir and Baloxavir. Biosaf. Health. 2020, 2, 64–70. [Google Scholar] [CrossRef]
- Omoto, S.; Speranzini, V.; Hashimoto, T.; Noshi, T.; Yamaguchi, H.; Kawai, M.; Uehara, T.; Shishido, T.; Naito, A.; Cusak, S. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci. Rep. 2018, 8, 9633. [Google Scholar] [CrossRef]
- Koshimichi, H.; Ishibashi, T.; Wajima, T. Population Pharmacokinetics of Baloxavir Marboxil in Japanese Pediatric Influenza Patients. J. Pharm. Sci. 2019, 108, P3112–P3117. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Kim, Y.; Jang, I.-J.; Lee, S.H. Pharmacokinetics and safety of a novel influenza treatment (baloxavir marboxil) in Korean subjects compared with Japanese subjects. Am. Soc. Clin. Pharmacol. Theraputics. 2021, 15, 422–432. [Google Scholar] [CrossRef]
- EMA; Xofluza. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/xofluza-epar-product-information_en.pdf (accessed on 13 October 2021).
- Baker, J.; Block, S.L.; Matharu, B.; Burleigh Macutkiewicz, L.; Wildum, S.; Dimonaco, S.; Collinson, N.; Clinch, B.; Piedra, P.A. Baloxavir Marboxil Single-dose Treatment in Influenza-infected Children. Pediatric Infect. Dis. J. 2020, 39, 700–705. [Google Scholar] [CrossRef]
- Watanabe, A.; Ishida, T.; Hirotsu, N.; Kawaguchi, K.; Ishibashi, T.; Shishido, T.; Uehara, T. Baloxavir marboxil in Japanese patients with seasonal influenza: Dose response and virus type/subtype outcomes from a randomized phase 2 study. Antivir. Res. 2019, 163, 75–81. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study of S-033188 (Baloxavir Marboxil) Compared with Placebo or Oseltamivir in Otherwise Healthy Patients With Influenza (CAPSTONE 1). Available online: https://clinicaltrials.gov/ct2/show/NCT02954354 (accessed on 13 October 2021).
- ClinicalTrials.gov. Study of S-033188 (Baloxavir Marboxil) Compared With Placebo or Oseltamivir in Patients with Influenza at High Risk of Influenza Complications (CAPSTONE 2). Available online: https://clinicaltrials.gov/ct2/show/results/NCT02949011 (accessed on 13 October 2021).
- ClinicalTrials.gov. Study to Assess the Safety, Pharmacokinetics, and Efficacy of Baloxavir Marboxil in Healthy Pediatric Participants with Influenza-like Symptoms. Available online: https://clinicaltrials.gov/ct2/show/NCT03629184?term=baloxavir+Marboxil&cond=Influenza&draw=2&rank=3 (accessed on 13 October 2021).
- Ison, M.G.; Portsmouth, S.; Yoshida, Y.; Shishido, T.; Mitchener, M.; Tsuchiya, K.; Uehara, T.; Hayden, F.G. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): A randomised, placebo-controlled, phase 3 trial. Lancet Infect. Dis. 2020, 20, 1204–1214. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study to Assess the Safety, Pharmacokinetics, and Efficacy of Baloxavir Marboxil in Healthy Pediatric Participants from Birth to <1 Year with Influenza-like Symptoms. Available online: https://clinicaltrials.gov/ct2/show/NCT03653364?term=baloxavir+Marboxil&cond=Influenza&draw=2&rank=1 (accessed on 13 October 2021).
- Ikematsu, H.; Hayden, F.G.; Kawaguchi, K.; Kinoshita, M.; Jong, D.; Lee, N.; Takashima, S.; Noshi, T.; Tsuchiya, K.; Uehara, T. Baloxavir marboxil for prophylaxis against influenza in household contacts. N. Engl. J. Medicine. 2020, 383, 309–320. [Google Scholar] [CrossRef]
- Umemura, T.; Mutoh, Y.; Kawamura, T.; Saito, M.; Mizuno, T.; Ota, A.; Kozaki, K.; Yamada, T.; Ikeda, Y.; Ichihara, T. Efficacy of baloxavir marboxil on household transmission of influenza infection. J. Pharm. Health Care Sci. 2020, 6, 1–6. [Google Scholar] [CrossRef]
- Liu, T.; Liu, M.; Chen, F.; Chen, F.; Tian, Y.; Huang, Q.; Liu, S.; Yang, J. A small-molecule compound has anti-influenza A virus activity by acting as a ‘‘PB2 inhibitor”. Mol. Pharm. 2018, 15, 4110–4120. [Google Scholar] [CrossRef]
- Yang, F.; Pang, B.; Lai, K.K.; Cheung, N.N.; Dai, J.; Zhang, W.; Zhang, J.; Chan, K.H.; Chen, H.; Sze, K.-H.; et al. Discovery of a Novel Specific Inhibitor Targeting Influenza A Virus Nucleoprotein with Pleiotropic Inhibitory Effects on Various Steps of the Viral Life Cycle. J. Virol. 2021, 95, e01432-20. [Google Scholar] [CrossRef]
- Watanabe, T.; Hayashi, K.; Kan, T.; Ohwaki, M.; Kawahara, T. Anti-Influenza virus effects of Enterococcus faecalis KH2 and Lactobacillus plantarum SNK12 RNA. Biosci. Microbiota Food Health 2021, 40, 43–49. [Google Scholar] [CrossRef]
- You, H.L.; Chen, C.J.; Eng, H.L.; Liao, P.L.; Huang, S.T. The Effectiveness and Mechanism of Toona sinensis Extract Inhibit Attachment of Pandemic Influenza A (H1N1) Virus. Evid. Based Complement Alternat. Med. 2013, 2013, 479718. [Google Scholar] [CrossRef] [PubMed]
- You, H.L.; Huang, C.C.; Chen, C.J.; Chang, C.C.; Liao, P.L.; Huang, S.T. Anti-pandemic influenza A (H1N1) virus potential of catechin and gallic acid. Journal of the Chinese Medical Association. JCMA 2018, 81, 458–468. [Google Scholar] [CrossRef] [PubMed]
| Drug Class | Active Substance | CID | Trade Name |
|---|---|---|---|
| NAI | 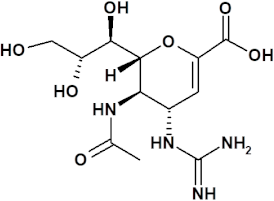 zanamivir | 60855 | Relenza® registered in EU, USA and AsiaDectova® registered in EU |
| NAI | 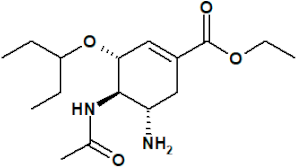 oseltamivir | 65028 | Tamiflu® registered in EU, USA and Asia |
| NAI | 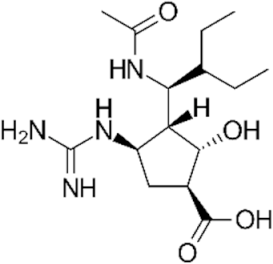 peramivir | 154234 | Rapivap® registered in USA and Asia |
| NAI | 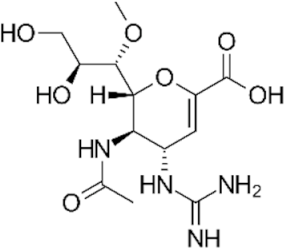 laninamivir | 502272 | Inavir® registered in Asia |
| CENI | 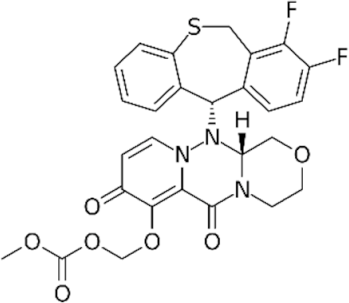 baloxavir marboxil | 124081896 | Xofluza® registered in EU, USA and Asia |
| Drug | Frequency | Adverse Effects |
|---|---|---|
| Zanamivir for oral inhalation | 1% to 10% | Skin reaction, such as rash |
| 0.1% to 1% | Disordered respiratory function, bronchospasm, throat tightness or constriction, vasovagal-like reactions, and allergic-type reactions, including oropharyngeal edema and urticaria. | |
| <0.01% | Anaphylactic reaction, facial edema, toxic epidermal necrolysis, erythema multiforme, and Stevens–Johnson syndrome | |
| Underestimated | Neuropsychiatric adverse effects (NPAEs), seizures, delirium, hallucination, abnormal behavior, and depressed level of consciousness | |
| Intravenous zanamivir | 1% to 10% | Diarrhea, rash, hepatocellular injury, increased levels of transaminases (ALT and AST), neutropenia, and renal failure |
| 0.1% to 10% | Urticaria and increased alkaline phosphatase | |
| Underestimated | Anaphylactic reaction, facial and oropharyngeal edema, NPAEs, renal impairment, paralytic ileus, and hypotension | |
| Oseltamivir | >10% | Headache and nausea |
| 1% to 10% | Vomiting, bronchitis, sore, throat, nasopharyngitis, sinusitis, pain, and dizziness | |
| 0.1% to 1% | Hypersensitivity reaction, rash, urticaria, dermatitis, cardiac arrhythmia, and convulsions | |
| 00.1% to 0.1% | Thrombocytopenia, anaphylactic reactions, toxic epidermal necrolysis, hepatic failure, hepatitis, evaluated liver enzymes, gastrointestinal bleeding, visual disturbances, and NPAEs | |
| Peramivir | >10% | Diarrhea |
| 1% to 10% | Neutropenia, nausea, vomiting, injection site rash, and increased AST and ALT | |
| Underestimated | Insomnia, fever, proteinuria, tympanic membrane erythema, anaphylactic reactions, severe dermatological reactions such as Stevens–Johnson syndrome and exfoliative dermatitis, and NPAEs | |
| Laninamivir | 1% to 10% | Cough, diarrhea, and headache |
| 0.1% to 1% | Gastritis, abnormal behavior, and nervous system disorders |
| Drug | Therapeutic Indication | Age Interval | Pharmaceutical Form | Dose |
|---|---|---|---|---|
| Zanamivir | Treatment of acute uncomplicated influenza A and B | ≥5 years and older (≥7 years in USA and Canada) | Powder for oral inhalation | 10 mg twice daily for 5 days |
| Post-exposure prophylaxis | ≥5 years and older | 10 mg once daily for 10 days | ||
| Seasonal prophylaxis | (≥7 years in Canada) | 10 mg once daily for 28 days | ||
| Zanamivir | Treatment of hospitalized seriously ill patients | ≥6 months and older | Solution for infusion | Weight-based dose 6 months < 6 years 14 mg per kg 2xd ≥ 6 < 18 years 12 mg per kg 2xd Adults > 50 kg 600 md 2xd for 5–10 days |
| Oseltamivir | Treatment of acute uncomplicated influenza A and B | No age limits (EU) ≥2 weeks and older (USA) | Capsules 30 mg, 45 mg, 75 mg Oral suspension | ≥13 years: 75 mg 2xd for 5 days <13 years old—weight-based dose |
| Post-exposure prophylaxis | ≥1 year old | ≥13 years: 75 mg 1xd for 10 days at least | ||
| Seasonal prophylaxis | No age limits (EU) | 75 mg 1xd for up to 6 weeks | ||
| Peramivir | Treatment of acute uncomplicated influenza A and B | ≥18 years old | Solution for infusion | 2 years ≤ 12 years single 12 mg/kg |
| Adults: single 600 mg i.v. | ||||
| Laninamivir | Treatment of acute uncomplicated influenza A and B Post-exposure prophylaxis | No age limits | Oral inhalation powder | <10 years 20 mg single dose ≥10 years 40 mg single <10 years 20 mg single dose ≥10 years 40 mg single dose |
| Baloxavir marboxil | Treatment of acute uncomplicated influenza A and B (healthy and high risk of complications in USA) | ≥12 years old | Tablets for oral use | Weight-based dose 40 > 80 kg 40 mg single dose ≥80 kg 80 mg single dose |
| Post-exposure prophylaxis (in EU) |
| Type of Treatment | Primary End Point | Effect | Study |
|---|---|---|---|
| Zanamivir vs. placebo given up to 30 h of symptom onset | TTAS * | 1 day shorter with zanamivir | Hayden et al. [41] |
| Zanamivir vs. placebo (patients with fever) given up to 30 h of symptom onset | TTAS | 3 days shorter with zanamivir | Hayden et al. [41] |
| Zanamivir vs. placebo | TTAS | 0.6 day (14.4 h) shorter with zanamivir | Heneghan et al. [53] |
| Zanamivir vs. placebo (patients without fever) | TTAS | 0 (no significant differences) | The MIST Study Group [111] |
| Zanamivir vs. placebo (patients with fever) | TTAS | 2 days shorter with zanamivir | The MIST Study Group [111] |
| Zanamivir vs. placebo (high-risk patients) | TTAS | 2.5 days shorter with zanamivir | The MIST Study Group [111] |
| Dectova vs. oseltamivir (patients hospitalized in serious condition and ICU ** patients) | TTAS | Similar effect | Marty et al. [84] |
| Oseltamivir vs. placebo (adult patients with fever) | TTAS | 1.3 days shorter with oseltamivir | Tamiflu summary of product characteristics [67] |
| Oseltamivir vs. placebo (pediatric patients) | TTAS | 1.5 days shorter with oseltamivir | Tamiflu summary of product characteristics [67] |
| Peramivir 200 mg or 400 mg vs. oseltamivir (hospitalized patients) | Time to clinical stability | P200mg—31.0 h P400mg—24.3 h Oseltamivir—35.5 h | Ison et al. [125] |
| Peramivir 300 mg or 600 mg vs. oseltamivir | TTAS | P300mg—78.0 h P600mg—81.0 h Placebo—81.8 h | Kohno et al. [124] |
| Peramivir vs. oseltamivir (pediatric patients) | Fever duration | A significant advantage of peramivir | Shobugawa et al. [126] |
| Peramivir vs. zanamivir inhalation (pediatric patients) | Fever duration | Peramivir 1 day shorter than zanamivir | Hikita et al. [127] |
| Peramivir vs. laninamivir | Fever duration | Peramivir 1 day shorter than laninamivir | Hikita et al. [127] |
| Laninamivir 20 mg or 40 mg vs. oseltamivir | TTAS | L20mg—85.8 h L40—73.0 h Os—73.6 h | Watanabe et al. [128] |
| Baloxavir vs. placebo (adolescents) | TTAS | Baloxavir—53.7 h Placebo—80.2 h | CAPSTONE-1 [142] |
| Baloxavir vs. oseltamivir vs. placebo (adult patients) | TTAS | Baloxavir—53.7 h Oseltamivir—53.8 h Placebo—80.0 h | CAPSTONE-1 [142] |
| Baloxavir vs. placebo | Fever duration | Baloxavir—24.5 h Placebo—42 h | CAPSTONE-1 [142] |
| Baloxavir vs. oseltamivir vs. placebo | TTAS | Baloxavir—73.2 h Oseltamivir—81.0 h Placebo—102.3 h | CAPSTONE-2 [143] |
| Baloxavir vs. oseltamivir (pediatric patients) | TTAS | Baloxavir—138.1 h Oseltamivir—150.0 h | MiniSTONE [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świerczyńska, M.; Mirowska-Guzel, D.M.; Pindelska, E. Antiviral Drugs in Influenza. Int. J. Environ. Res. Public Health 2022, 19, 3018. https://doi.org/10.3390/ijerph19053018
Świerczyńska M, Mirowska-Guzel DM, Pindelska E. Antiviral Drugs in Influenza. International Journal of Environmental Research and Public Health. 2022; 19(5):3018. https://doi.org/10.3390/ijerph19053018
Chicago/Turabian StyleŚwierczyńska, Magdalena, Dagmara M. Mirowska-Guzel, and Edyta Pindelska. 2022. "Antiviral Drugs in Influenza" International Journal of Environmental Research and Public Health 19, no. 5: 3018. https://doi.org/10.3390/ijerph19053018
APA StyleŚwierczyńska, M., Mirowska-Guzel, D. M., & Pindelska, E. (2022). Antiviral Drugs in Influenza. International Journal of Environmental Research and Public Health, 19(5), 3018. https://doi.org/10.3390/ijerph19053018







