Evolutionary Path and Innovative Development of Pharmaceutical Industrial Cluster—A Case Study of Shijiazhuang, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Method Selection
2.2. Case Selection and Data Collection
3. Evolution Path of the Traditional Pharmaceutical Industrial Clusters to Innovation Clusters
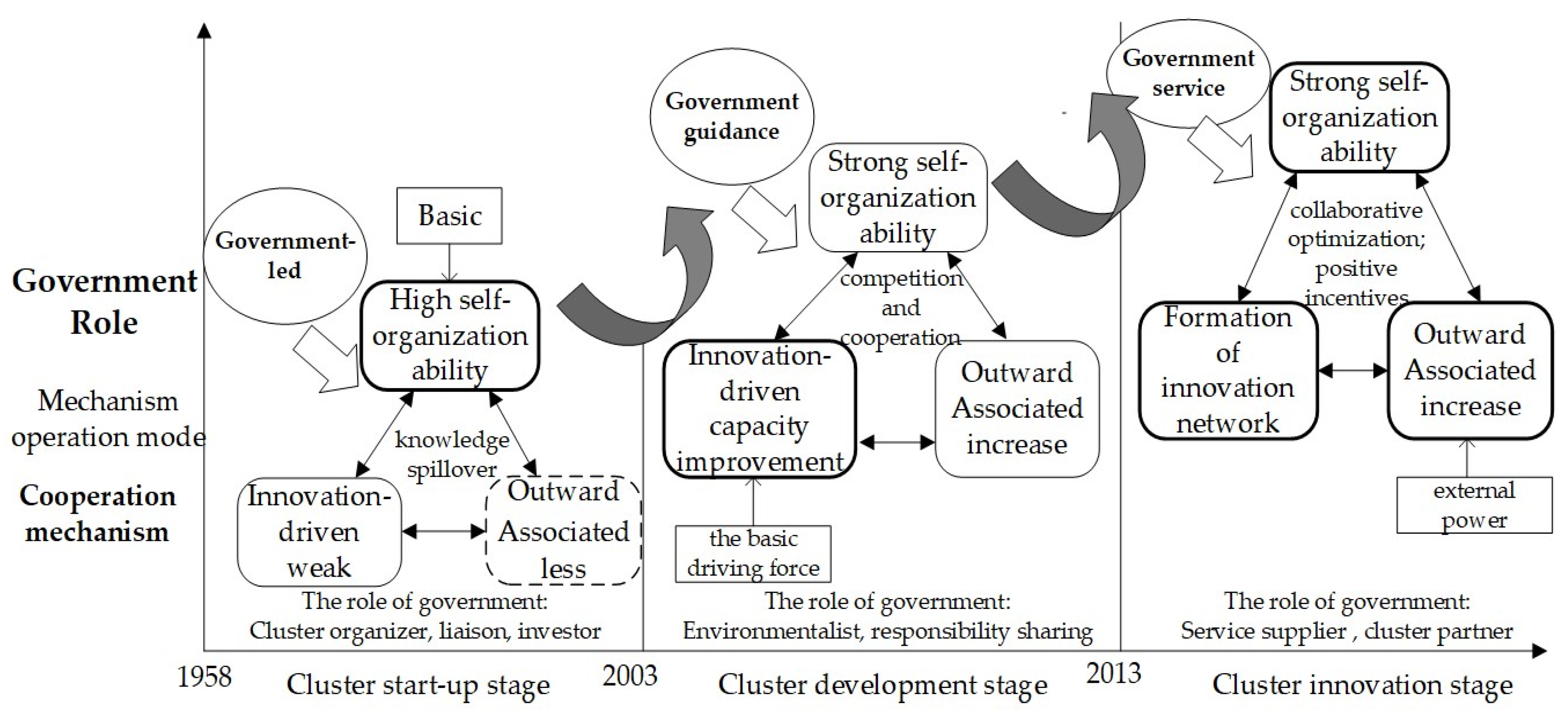
3.1. Initial Stage of Pharmaceutical Industrial Cluster (Before 2003)
3.2. Development Stages of Pharmaceutical Industrial Clusters (2003–2013)
3.3. Innovation Stages of Pharmaceutical Industrial Clusters (Since 2013)
4. Mechanism of Pharmaceutical Industrial Cluster Innovation Evolutionary
4.1. Self-Organizing Mechanism
4.2. Innovation-Driven Mechanism
4.3. Outward Associated Mechanism
4.4. Government Role Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautam, A.; Pan, X.G. The changing model of big pharma: Impact of key trends. Drug Discov. Today 2016, 21, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, I.C.; Oliveira, J.C.; Ring, D.T. Current Perspectives on the Development of Industry 4.0 in the Pharmaceutical Sector. J. Ind. Inf. Integr. 2020, 18, 100131. [Google Scholar] [CrossRef]
- Porter, M.E. Cluster and the New Economics of Competition. Harv. Bus. Rev. 1998, 76, 77–90. [Google Scholar] [PubMed]
- Barbieri, E.; Huang, M.L.; Pi, S.L.; Tassinari, M. Restructuring the Production of Medicines: An Investigation on the Pharmaceutical Sector in China and the Role of Mergers and Acquisitions. Int. J. Environ. Res. Public Health 2017, 14, 1179. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.E. Competitive Advantage: Creating and Sustaining Superior Performance: With a New Introduction, 1st Free Press ed.; Free Press: New York, NY, USA, 1998. [Google Scholar]
- Marshall, A. Principles of Economics; The Commercial Press: Beijing, China, 1977. [Google Scholar]
- Porter, M.E. The Competitive Advantage of Nations; Free Press: New York, NY, USA, 1990. [Google Scholar]
- Cooke, P.; Uranga, M.; Etxebarria, G. Regional innovation systems: Institutional and organisational dimensions. Res. Policy 1997, 26, 475–491. [Google Scholar] [CrossRef]
- Gereffi, G.; Lee, J. Economic and Social Upgrading in Global Value Chains and Industrial Clusters: Why Governance Matters. J. Bus. Ethics 2016, 133, 25–38. [Google Scholar] [CrossRef]
- Bettanti, A.; Lanati, A.; Missoni, A. Biopharmaceutical innovation ecosystems: A stakeholder model and the case of Lombardy. J. Technol. Transf. 2021. [Google Scholar] [CrossRef]
- Kong, M.Y.; Wang, X.Q.; Wu, Q.M. The development efficiency of China’s innovative industrial clusters-based on the DEA-Malmquist model. Arab. J. Geosci. 2021, 14, 638. [Google Scholar] [CrossRef]
- Li, J.Y.; Webster, D.; Cai, J.M.; Muller, L. Innovation Clusters Revisited: On Dimensions of Agglomeration, Institution, and Built-Environment. Sustainability 2019, 11, 3338. [Google Scholar] [CrossRef]
- Hartley, J.; Sorensen, E.; Torfing, J. Collaborative Innovation: A Viable Alternative to Market Competition and Organizational Entrepreneurship. Public Adm. Rev. 2013, 73, 821–830. [Google Scholar] [CrossRef]
- Bush, S.R.; Oosterveer, P.; Bailey, M.; Mol, A.P.J. Sustainability governance of chains and networks: A review and future outlook. J. Clean Prod. 2015, 107, 8–19. [Google Scholar] [CrossRef]
- Okamuro, H.; Nishimura, J. Local Management of National Cluster Policies: Comparative Case Studies of Japanese, German, and French Biotechnology Clusters. Adm. Sci. 2015, 5, 213–239. [Google Scholar] [CrossRef]
- Hilliard, R.; Jacobson, D. Cluster versus Firm-Specific Factors in the Development of Dynamic Capabilities in the Pharmaceutical Industry in Ireland: A Study of Responses to Changes in Environmental Protection Regulations. Reg. Stud. 2011, 45, 1319–1328. [Google Scholar] [CrossRef]
- Zhong, Q.; Tang, T. Impact of Government Intervention on Industrial Cluster Innovation Network in Developing Countries. Emerg. Mark. Finance Trade 2018, 54, 3351–3365. [Google Scholar] [CrossRef]
- DuBois, F.L.; Mendes Primo, M.A. State Capitalism and Clusters: The Case of Brazilian Shipbuilding. Int. J. Emerg. Mark. 2016, 11, 214–231. [Google Scholar] [CrossRef]
- Gao, B.; Dunford, M.; Norcliffe, G.; Liu, W. Governance Capacity, State Policy and the Rise of the Chongqing Notebook Computer Cluster. Area Dev. Policy 2019, 4, 321–345. [Google Scholar] [CrossRef]
- Landesmann, M.A.; Stöllinger, R. Structural Change, Trade and Global Production Networks: An ‘Appropriate Industrial Policy’ for Peripheral and Catching-up Economies. Struct. Chang. Econ. Dyn. 2019, 48, 7–23. [Google Scholar] [CrossRef]
- Liu, R.; Weng, Q.; Mao, G.; Huang, T. Industrial Cluster, Government Agency and Entrepreneurial Development A Case Study of Wenzhou City, Zhejiang Province. Chin. Manag. Stud. 2013, 7, 253–280. [Google Scholar] [CrossRef]
- Yin, R.K. Case Study Research: Design and Methods, 3rd ed.; Sage Publications: London, UK, 2002. [Google Scholar]
- Siggelkow, N. Persuasion with Case Studies. Acad. Manage. J. 2007, 50, 20–24. [Google Scholar] [CrossRef]
- Eisenhardt, K.M.; Graebner, M.E. Theory Building From Cases: Opportunities and Challenges. Acad. Manag. J. 2007, 50, 25–32. [Google Scholar] [CrossRef]
- Eisenhardt, K.M. Building Theories from Case Study Research. Acad. Manag. Rev. 1989, 14, 532–550. [Google Scholar] [CrossRef]
- Patton, M.Q. Qualitative Research and Evaluation Methods, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2002. [Google Scholar]
- Krugman, P. Increasing Returns and Economic Geography. J. Polit. Econ. 1991, 99, 483–499. [Google Scholar] [CrossRef]
- Lecocq, C.; Van Looy, B. What differentiates top regions in the field of biotechnology? An empirical study of the texture characteristics of biotech regions in North America, Europe, and Asia-Pacific. Ind. Corp. Chang. 2016, 25, 671–688. [Google Scholar] [CrossRef][Green Version]
- Minniti, M. The role of government policy on entrepreneurial activity: Productive, unproductive, or destructive? Entrep. Theory Pract. 2008, 32, 779–790. [Google Scholar] [CrossRef]
- Lechner, C.; Leyronas, C. The competitive advantage of cluster firms: The priority of regional network position over extra-regional networks—A study of a French high-tech cluster. Entrep. Reg. Dev. 2012, 24, 457–473. [Google Scholar] [CrossRef]
- Bouncken, R.; Gast, J.; Kraus, S.; Bogers, M. Coopetition: A systematic review, synthesis, and future research directions. Rev. Manag. Sci. 2015, 9, 577–601. [Google Scholar] [CrossRef]
- Chesbrough, H.; Schwartz, K. Innovating business models with co-development partnerships. Res.-Technol. Manag. 2007, 50, 55–59. [Google Scholar] [CrossRef]
- Turkina, E.; Van Assche, A.; Doloreux, D. How do firms in co-located clusters interact? Evidence from Greater Montreal. J. Econ. Geogr. 2020, 21, 761–782. [Google Scholar] [CrossRef]
- Schumpeter, J.A.; Clemence, R.V.; Swedberg, R. Essays: On Entrepreneurs, Innovations, Business Cycles, and the Evolution of Capitalism; Routledge: New York, NY, USA, 2017; ISBN 978-1-351-31148-9. [Google Scholar]
- Rydvalova, P.; Skala, M. Innovation and Innovation Partnership. In Innovation and Performance Drivers of Business Clusters: An Empirical Study; Zizka, M., Rydvalova, P., Eds.; Science, Technology and Innovation Studies; Springer International Publishing: Cham, Switzerland, 2021; pp. 47–57. ISBN 978-3-030-79907-6. [Google Scholar]
- Belso-Martinez, J.A.; Diez-Vial, I.; Lopez-Sanchez, M.J.; Mateu-Garcia, R. The Brokerage Role of Supporting Organizations inside Clusters: How Does It Work? Eur. Plan. Stud. 2018, 26, 706–725. [Google Scholar] [CrossRef]
- Cooke, P. Life sciences clusters and regional science policy. Urban Stud. 2004, 41, 1113–1131. [Google Scholar] [CrossRef]
- Tamburis, O.; Bonacci, I. Clusters and Communities: Raising the Bar towards Open Innovation 2.0 Paradigms. Int. J. Pharm. Healthc. Mark. 2019, 13, 288–305. [Google Scholar] [CrossRef]
- Melnychuk, T.; Schultz, C.; Wirsich, A. The Effects of University–Industry Collaboration in Preclinical Research on Pharmaceutical Firms’ R&D Performance: Absorptive Capacity’s Role. J. Prod. Innov. Manag. 2021, 38, 355–378. [Google Scholar] [CrossRef]
- Belso-Martínez, J.A.; Díez-Vial, I.; López-Sánchez, M.J.; Sánchez, M.D. How Can R&D Programs Induce Unplanned R&D Collaborative Networks in Clusters? Z. Für Wirtsch. 2021, 65, 118–131. [Google Scholar] [CrossRef]
- Ferras-Hernandez, X.; Nylund, P.A. Clusters as Innovation Engines: The Accelerating Strengths of Proximity. Eur. Manag. Rev. 2019, 16, 37–53. [Google Scholar] [CrossRef]
- Ltd, I.-I.B. Clusterele inovative din domeniul farmaceutic—Motor al dezvoltării regionale durabile. Rev. Univers Strateg. 2019, X, 241–248. [Google Scholar]
- Turkina, E.; Van Assche, A. Global Connectedness and Local Innovation in Industrial Clusters. J. Int. Bus. Stud. 2018, 49, 706–728. [Google Scholar] [CrossRef]
- Ai, C.-H.; Wu, H.-C.; Huang, T.-H.; Wang, R. How Does Knowledge Flow in Industrial Clusters? The Comparison between Both Naturally and Intentionally Formed Industrial Clusters in China. Asian, J. Technol. Innov. 2020, 1, 1–31. [Google Scholar] [CrossRef]
- Gugler, P.; Keller, M.; Tinguely, X. The Role of Clusters in the Global Innovation Strategy of MNEs: Theoretical Foundations and Evidence from the Basel Pharmaceutical Cluster. Compet. Rev. 2015, 25, 324–340. [Google Scholar] [CrossRef]
- Terstriep, J.; Lüthje, C. Innovation, Knowledge and Relations—On the Role of Clusters for Firms’ Innovativeness. Eur. Plan. Stud. 2018, 26, 2167–2199. [Google Scholar] [CrossRef]
- Eraydin, A.; Armatli-Köroğlu, B. Innovation, Networking and the New Industrial Clusters: The Characteristics of Networks and Local Innovation Capabilities in the Turkish Industrial Clusters. Entrep. Reg. Dev. 2005, 17, 237–266. [Google Scholar] [CrossRef]
- Lee, K.; Szapiro, M.; Mao, Z. From Global Value Chains (GVC) to Innovation Systems for Local Value Chains and Knowledge Creation. Eur. J. Dev. Res. 2018, 30, 424–441. [Google Scholar] [CrossRef]
- Humphrey, J.; Schmitz, H. How Does Insertion in Global Value Chains Affect Upgrading in Industrial Clusters? Reg. Stud. 2002, 36, 1017–1027. [Google Scholar] [CrossRef]
- Nguyen, A.T.N.; Cieślik, A. Determinants of Foreign Direct Investment from Europe to Asia. World Econ. 2021, 44, 1842–1858. [Google Scholar] [CrossRef]
- Gancarczyk, M. Enterprise- and Industry-Level Drivers of Cluster Evolution and Their Outcomes for Clusters from Developed and Less-Developed Countries. Eur. Plan. Stud. 2015, 23, 1932–1952. [Google Scholar] [CrossRef]
- Achilladelis, B.; Antonakis, N. The Dynamics of Technological Innovation: The Case of the Pharmaceutical Industry. Res. Policy 2001, 30, 535–588. [Google Scholar] [CrossRef]
- Tanzi, V.; Zee, H.H. Fiscal Policy and Long-Run Growth. Staff Pap. 1997, 44, 179–209. [Google Scholar] [CrossRef]
- Yeh, A.G.; Yang, F.F.; Wang, J. Economic Transition and Urban Transformation of China: The Interplay of the State and the Market. Urban Stud. 2015, 52, 2822–2848. [Google Scholar] [CrossRef]
- Vernay, A.-L.; D’Ippolito, B.; Pinkse, J. Can the Government Create a Vibrant Cluster? Understanding the Impact of Cluster Policy on the Development of a Cluster. Entrep. Reg. Dev. 2018, 30, 901–919. [Google Scholar] [CrossRef]
- Bogers, M.; Chesbrough, H.; Moedas, C. Open Innovation: RESEARCH, PRACTICES, AND POLICIES. Calif. Manag. Rev. 2018, 60, 5–16. [Google Scholar] [CrossRef]
- Vlaisavljevic, V.; Medina, C.C.; Van Looy, B. The role of policies and the contribution of cluster agency in the development of biotech open innovation ecosystem. Technol. Forecast. Soc. Chang. 2020, 155, 119987. [Google Scholar] [CrossRef]
- Galvao, A.; Mascarenhas, C.; Marques, C.; Ferreira, J.; Ratten, V. Triple helix and its evolution: A systematic literature review. J. Sci. Technol. Policy Manag. 2019, 10, 812–833. [Google Scholar] [CrossRef]

| Data Type | Description | Collection Methods |
|---|---|---|
| Access to Online Information | Websites, news, newspapers, etc. | Online and offline multi-channel collection |
| Interviews and Field Observations | Cluster management committee, leading enterprises | Multiple rounds of interviews and phone calls |
| File Information | Internal brochures, meeting minutes, official documents, etc. | Management committee and company |
| Database Retrieval Information | Domestic and foreign Journal Databases | Multi-person multi-dimensional search |
| Main Categories | Level of Coding | Secondary Coding | Initial Data Citation |
|---|---|---|---|
| Self-Organization | Resource Endowment | Labor Resources | Wartime production (e.g., gauze, cotton, some alcohol); the production processes are very simple; there is simple technology and sufficient manpower. |
| Abundant Land Resources | When the national development zone was first established, as there was a historical foundation and abundant land resources here. | ||
| Social Elements | Location Advantage | Shijiazhuang is a location straddling semi-arid and semi-humid zones, offering unique conditions for the cultivation of medicinal herbs, production of raw materials, and research and development of pharmaceutical products. | |
| Political Elements | The predecessor of CSPC (ShiYao) was a pharmaceutical factory established in the base area of North China at that time. After the founding of the country, the major national projects of the First Five-Year Plan period included North China Pharmaceutical, and then, gradually concentrated on the construction of the factory. | ||
| Mutation factors | Historical path dependence | The operation began in 1955. It was desirable to build a pharmaceutical factory in China, in order to have their own production at the earliest point possible. This matter was considered during the founding of new China, so it was built here. | |
| Innovation-Driven | Experiential Learning | Professionalism | The drugs produced included a class of penicillin, a class of cephalosporin antibiotics. Such drugs are allergenic, so the production plant can only carry out the production of a single product. Thus, considering the cost problem, general pharmaceutical companies will produce the same class of drugs. |
| Heterogeneity | Western medicine technology in developed countries is more advanced, but China also needs to retain its own advantages. YiLing Pharmaceutical and Shineway Pharmaceutical are part of the development of the heritage of traditional Chinese medicine. | ||
| Innovation Policy | Development Zone Construction | In 1991, the State Council issued a document approving the establishment of Shijiazhuang National Hi-Tech Industrial Development Zone. In 1992, the People’s Government of Hebei Province approved the establishment of Shijiazhuang Economic and Technological Development Zone. | |
| Investment Promotion | The phase was started by spending a large amount of money to attract investment, encouraging companies to settle, bringing new innovations, and allowing companies to cluster. | ||
| Outward Associated | Cluster Interaction | Technology Introduction and Collaboration | At the beginning of the start-up, it was all about the production and processing of API, and the introduction of other people’s technologies; these are, of course, general production technologies and not core technologies. |
| Geographical Proximity | The gap between the fast-developing clusters in Beijing and Tianjin (which are closer) is larger, but interaction between clusters can be encouraged to give full play to the positive externalities of quality resources. |
| Main Categories | Level of Coding | Secondary Coding | Initial Data Citation |
|---|---|---|---|
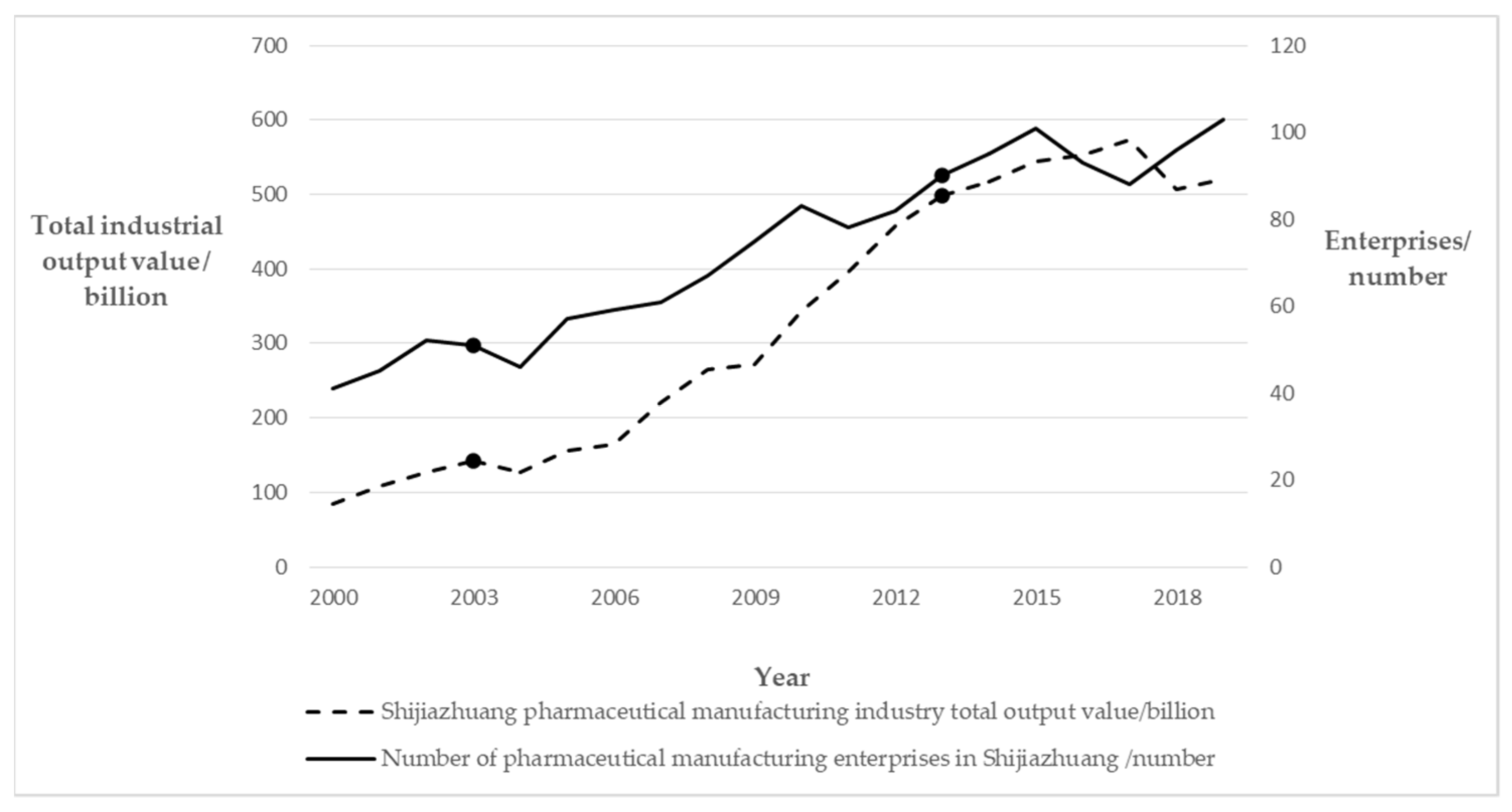
| Self-Organization | Corporate Diversity | Technology-based SMEs | In this period, there were 70 to 80 large enterprises, and the total business comprised more than 500 small enterprises. Many science- and technology-based small- and medium-sized enterprises gradually emerged. |
| Leading Enterprises | The core of the development area consisted of three large plants—CSPC (ShiYao), NCPC (HuaYao), and Shijiazhuang No.4 pharmaceutical—which were pillar biopharmaceutical enterprises driving the development of the entire cluster. | ||
| Social Elements | Group Elements | Geographical proximity has led to the formation of simple small groups, or simple partnerships, between some companies. It is up to the senior companies to allow some low-end enterprises to produce APIs (Active Pharmaceutical Ingredients), followed by their own processing into finished drugs. | |
| Political Elements | The provincial government has proposed four development goals for the development zone, in addition to an overall goal for a development zone. | ||
| Innovation-Driven | The prototype of government–industry–academia | Cooperation with Universities | Some new products or new technologies are to be developed in cooperation with universities. |
| Government Support | For pharmaceutical companies, national policies have a great impact. | ||
| Corporate Connections | Relatively speaking, there is a division of labor in which large companies relegate some technologies that they think may be less profitable to other companies. | ||
| Innovation Network | Platform Construction | Establishing cluster platforms for financing and cooperation, supporting and leading the development of enterprises within the cluster, and managing large and small affairs at the cluster level. | |
| Technology Breakthrough | There are two aspects of technology: one is the tangible technology itself, and the other is its value. Depending on the quality of the product, the technology, value, and intellectual property will be more valuable and more meaningful. | ||
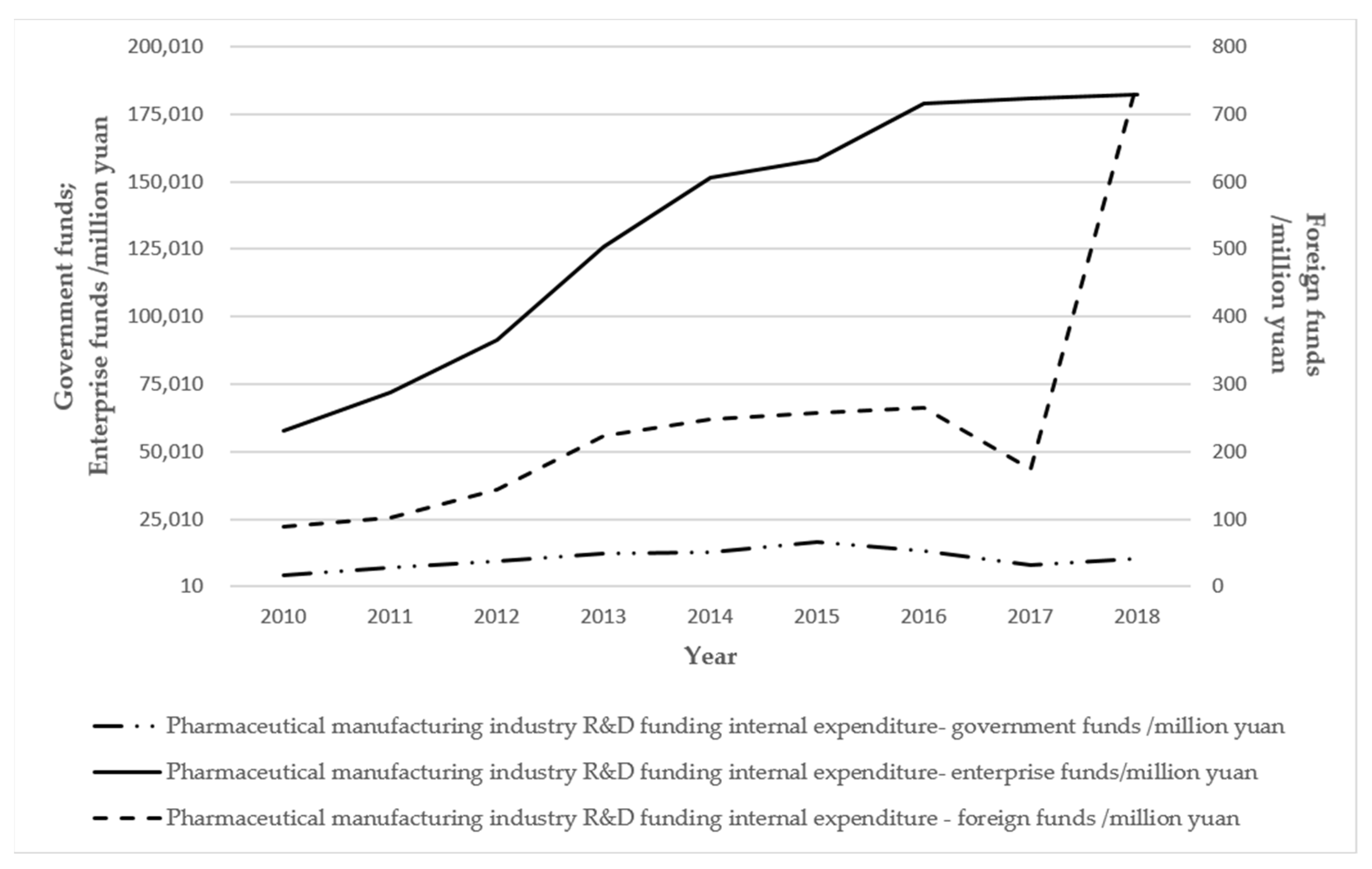
| R & D investment | Material input | The main investments, such as those into research and development and advertising, are essential. The general investment costs account for about five percent of their production value, and two or three percent of their R & D funds. Advertising is essential, and the growth of the intangible assets of a company is dependent on advertising. | |
| Human Resources | The development zone to introduce incentives for the introduction of high-tech talent, for these technology-based demonstration enterprises, the introduction of support for scientific and technological innovation, and technology-based small- and medium-sized enterprises all require support policies. Every year, five percent of financial expenditures are used for incentives or support for scientific and technological innovation. | ||
| Outward Associated | Investment channels | Financial Support | With the continuous development of the company itself, the source of capital is varied. Listed companies have more diversified sources of capital. |
| Supply Chain | International Market Development | Some companies export more, whereas some export less. CSPC (ShiYao) and NCPC (HuaYao) both have exports. Further business investment is also in the scope; that is to say, an enterprise requires some downstream enterprises in order to lead other teams, as well as investing in building factories. | |
| Upstream and downstream bidding | Internationalization is in line with the international situation, but also needs to be in line with the domestic situation. Companies also bid domestically, including their upstream and downstream products. There is a procurement and collection center, which has two bids per year to determine downstream suppliers, based on cost, quality, and supply capability. |
| Main Categories | Level of Coding | Secondary Coding | Initial Data Citation |
|---|---|---|---|
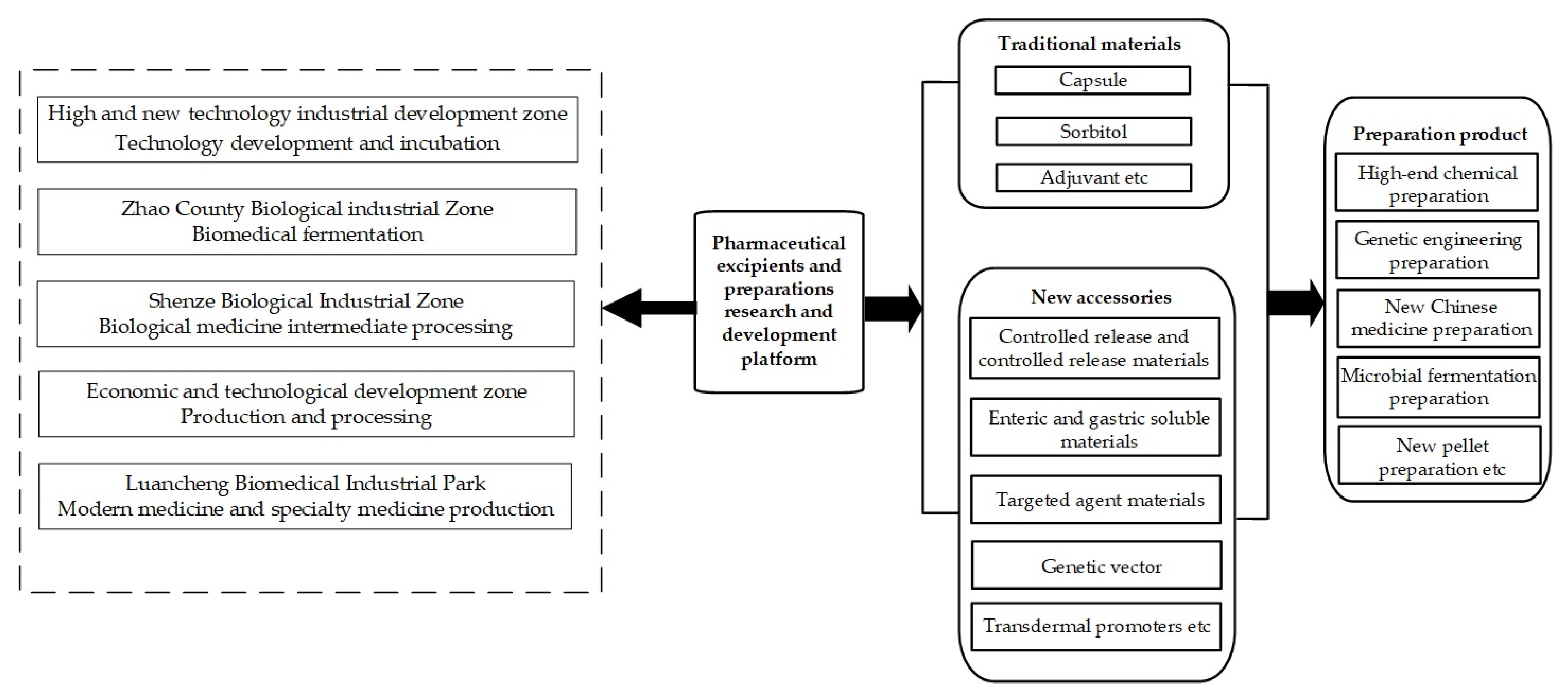
| Self-Organization | Industry Chain | Cluster Learning | The clustering of enterprises facilitates centralized and unified management, and is also conducive to the transfer of knowledge, and the formation of a complete industrial chain. |
| Leading Enterprises | The leading company cooperates with chemical plants in Shijiazhuang and Zhao Counties for the supply of raw materials, due to the close distance and lower transportation costs. There is close cooperation in this regard. | ||
| Competing and Coexisting | Unified Cluster Development | The Shijiazhuang pharmaceutical excipients and preparations industrial cluster mainly comprises five different functional clusters; that is, five functional areas with their own advantages and scientific and technological resources, where the functional differences make each area more specialized. | |
| Price and Resource competition | There is some competition, in terms of price and resources, among the small pharmaceutical companies in the cluster. However, due to the severe environmental problems occurring in the past two years, the degree of cooperation among the Chinese medicine factories has deepened, especially in terms of breakthroughs addressing some technical difficulties. | ||
| Innovation-Driven | Subject interaction | Cooperation with Universities | Some new products or new technologies are to be developed in cooperation with universities in Beijing and Tianjin. |
| Industrial Associations | There are industrial associations and industrial alliances, and agreements have been signed between the management committee and these parties to jointly work on related projects, and build a platform for the enterprises to communicate and cooperate with each other. | ||
| Engineering Lab | A gradual increase in the number of science and technology R & D institutions in the cluster is witnessed. There is a national testing center, a provincial technical center, a post-doctoral station, and several engineering laboratories. | ||
| Technology Innovation | Intellectual Property | Each of the cluster’s pharmaceutical companies has hundreds of patents. They attach great importance to intellectual property rights, and include research institutes and research laboratories. | |
| R & D Team | A strategic cooperation alliance with Hebei University has been previously established, and now there is some cooperation with the China University of Medicine, Hebei University of Economics and Business, and Hebei University of Engineering. | ||
| Innovation Environment | Talent Introduction | A number of academic workstations and post-doctoral stations in the cluster have attracted high-level talent, creating a permanent “think tank” for the cluster. Based on a high-level R & D team, in-depth research continues, and the development and transformation of innovative results is accelerated. The cluster includes many famous scholars and technical backbones from home and abroad. | |
| “Emptying the cage, changing the birds” | Enterprises with backward production capacity are to be gradually eliminated, with the additional aim to incubate innovative subjects for enterprises that can be transformed and guided, as well as for those that will be newly settled in. | ||
| Outward Associated | Global Value Chain | International Business | Talent is very important—thus, there are preparations to facilitate the introduction of returnees, as well as to set up country industrial parks and a returnee business park. In addition, some international business development is carried out. |
| International Certification | International certification is in progress. Some medicines have passed the EU certification, and some have passed the Japanese certification; furthermore, several powder injections have passed the EU certification. | ||
| Enhance international competitiveness | Open door to the outside world | In recent years, the level of openness has continued to improve. Shijiazhuang Economic and Technological Development Zone foreign-invested enterprises and foreign trade export enterprises are increasing, and the amount of foreign investment and export earnings are also growing. | |
| Overseas study | In the field of scientific and technological innovation, the key laboratories established are in cooperation with research institutes, doctoral and academic workstations, national monitoring centers, and so on (i.e., R & D centers). CSPC (ShiYao) has established global R & D centers in the U.S. and other locations. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Wu, F.; Zhang, S. Evolutionary Path and Innovative Development of Pharmaceutical Industrial Cluster—A Case Study of Shijiazhuang, China. Int. J. Environ. Res. Public Health 2022, 19, 2928. https://doi.org/10.3390/ijerph19052928
Fu L, Wu F, Zhang S. Evolutionary Path and Innovative Development of Pharmaceutical Industrial Cluster—A Case Study of Shijiazhuang, China. International Journal of Environmental Research and Public Health. 2022; 19(5):2928. https://doi.org/10.3390/ijerph19052928
Chicago/Turabian StyleFu, Liping, Fan Wu, and Shan Zhang. 2022. "Evolutionary Path and Innovative Development of Pharmaceutical Industrial Cluster—A Case Study of Shijiazhuang, China" International Journal of Environmental Research and Public Health 19, no. 5: 2928. https://doi.org/10.3390/ijerph19052928
APA StyleFu, L., Wu, F., & Zhang, S. (2022). Evolutionary Path and Innovative Development of Pharmaceutical Industrial Cluster—A Case Study of Shijiazhuang, China. International Journal of Environmental Research and Public Health, 19(5), 2928. https://doi.org/10.3390/ijerph19052928






