The Effect of Acetaminophen on Running Economy and Performance in Collegiate Distance Runners
Abstract
:1. Introduction
2. Materials and Methods
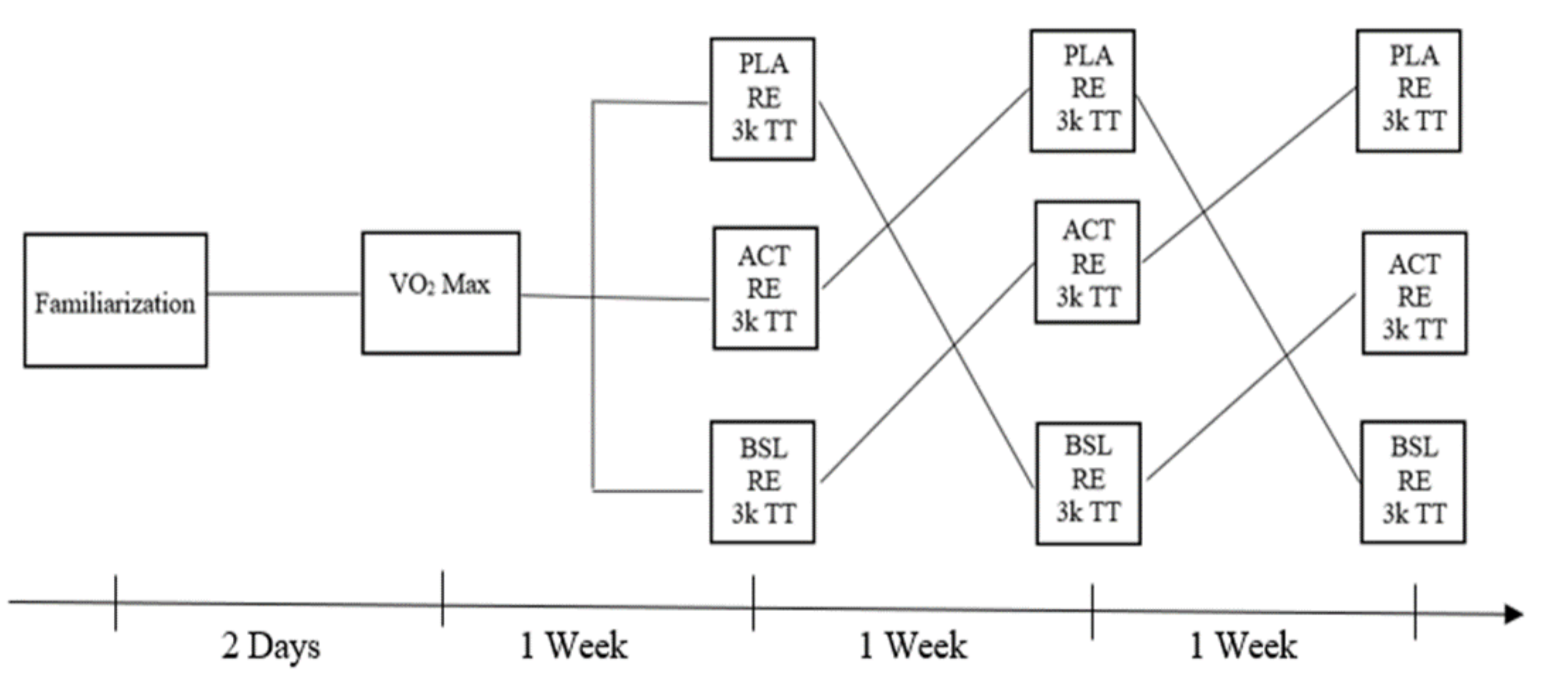
2.1. Experimental Approach
2.2. Subjects
2.3. Procedures
2.3.1. Familiarization and Lead-In
2.3.2. Anthropomorphic Measurements
2.3.3. Maximal Oxygen Consumption
2.3.4. Intervention Sessions
2.3.5. Running Economy
2.3.6. Track Time Trial
2.3.7. Statistical Analysis
3. Results
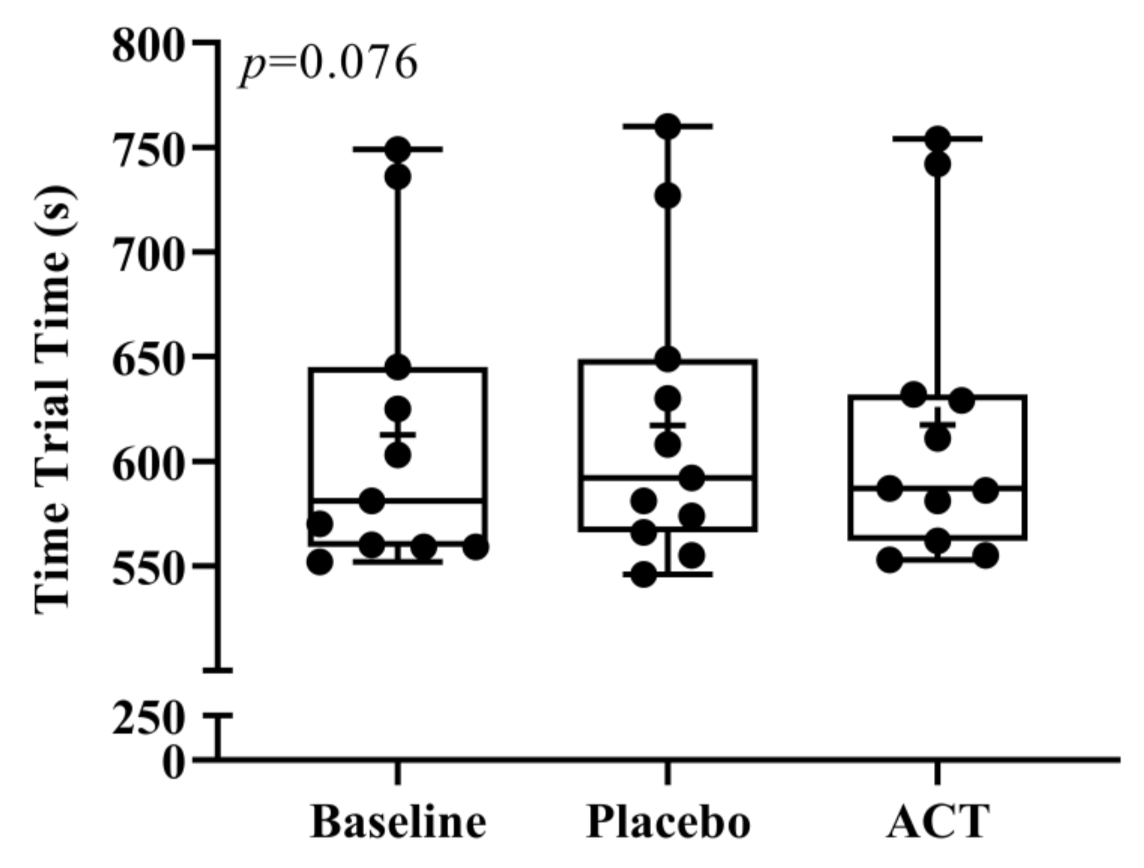
3.1. Time and Splits
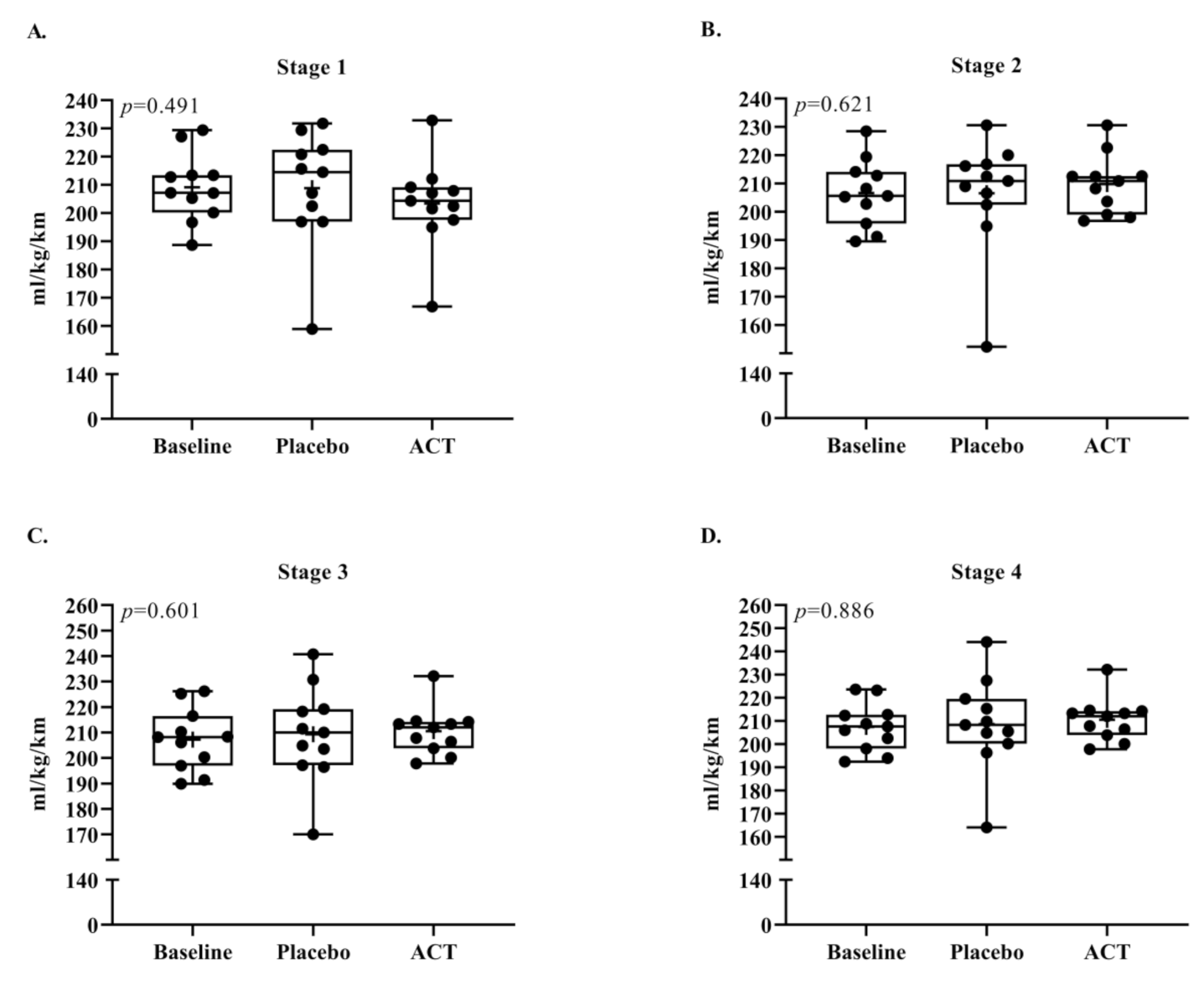
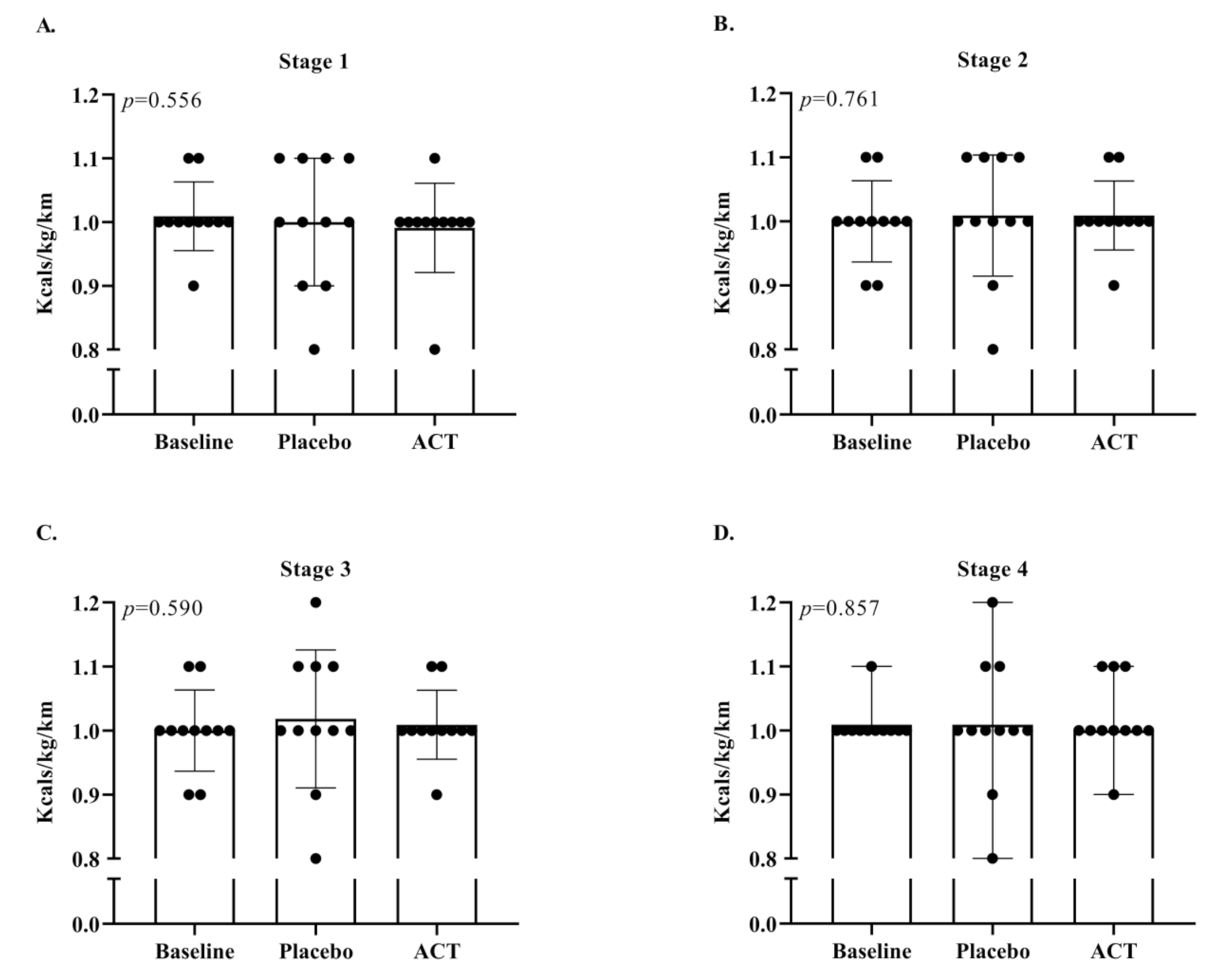
3.2. Running Economy
3.3. Heart Rate, Oxygen Consumption, and Saturation
3.4. RPE and Questionnaire Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meardon, S.A.; Hamill, J.; Derrick, T.R. Running Injury and Stride Time Variability over a Prolonged Run. Gait Posture 2011, 33, 36–40. [Google Scholar] [CrossRef]
- Mauger, A.R. Fatigue Is a Pain—the Use of Novel Neurophysiological Techniques to Understand the Fatigue-Pain Relationship. Front. Physiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagli, F.P.; Maridaki, M.; Paradisis, G.; Pilianidis, T.; Zacharogiannis, E. Effect of Acute Acetaminophen Injestion on Running Endurance Performance. Med. Sci. Sports Exerc. 2018, 49, 330–331. [Google Scholar] [CrossRef]
- Rosenbloom, C.J.; Morley, F.L.; Ahmed, I.; Cox, A.R. Oral Non-Steroidal Anti-Inflammatory Drug Use in Recreational Runners Participating in Parkrun UK: Prevalence of Use and Awareness of Risk. Int. J. Pharm. Pract. 2020, 28, 561–568. [Google Scholar] [CrossRef]
- Tanner, T.; Aspley, S.; Munn, A.; Thomas, T. The Pharmacokinetic Profile of a Novel Fixed-Dose Combination Tablet of Ibuprofen and Paracetamol. BMC Clin. Pharmacol. 2010, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertolini, A.; Ferrari, A.; Ottani, A.; Guerzoni, S.; Tacchi, R.; Leone, S. Paracetamol: New Vistas of an Old Drug. CNS Drug Rev. 2006, 12, 250–275. [Google Scholar] [CrossRef] [PubMed]
- Van Wijck, K.; Lenaerts, K.; Van Bijnen, A.A.; Boonen, B.; Van Loon, L.J.C.; Dejong, C.H.C.; Buurman, W.A. Aggravation of Exercise-Induced Intestinal Injury by Ibuprofen in Athletes. Med. Sci. Sports Exerc. 2012, 44, 2257–2262. [Google Scholar] [CrossRef]
- Delextrat, A.; Ellis, M.O.; Baker, C.E.; Matthew, D.; Sum, A.; Hayes, L.D. Acetaminophen Ingestion Improves Repeated Sprint Cycling Performance in Females: A Randomized Crossover Trial. Kinesiology 2015, 47, 7. [Google Scholar]
- Foster, J.; Taylor, L.; Chrismas, B.C.R.; Watkins, S.L.; Mauger, A.R. The Influence of Acetaminophen on Repeated Sprint Cycling Performance. Eur. J. Appl. Physiol. 2014, 114, 41–48. [Google Scholar] [CrossRef]
- Mauger, A.R.; Jones, A.M.; Williams, C.A. Influence of Acetaminophen on Performance during Time Trial Cycling. J. Appl. Physiol. 2010, 108, 7. [Google Scholar] [CrossRef] [Green Version]
- Mauger, A.R.; Taylor, L.; Harding, C.; Wright, B.; Foster, J.; Castle, P.C. Acute Acetaminophen (Paracetamol) Ingestion Improves Time to Exhaustion during Exercise in the Heat: Acetaminophen Improves Exercise Capacity in the Heat. Exp. Physiol. 2014, 99, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Morgan, P.T.; Vanhatalo, A.; Bowtell, J.L.; Jones, A.M.; Bailey, S.J. Acetaminophen Ingestion Improves Muscle Activation and Performance during a 3-Min All-out Cycling Test. Appl. Physiol. Nutr. Metab. 2019, 44, 434–442. [Google Scholar] [CrossRef]
- Park, L.L.; Baker, C.E.; Sum, A.; Hayes, L.D. The Influence of Acetaminophen on Sprint Interval Treadmill Running: A Randomized Crossover Trial. Kinesiology 2016, 48, 5. [Google Scholar] [CrossRef]
- Burtscher, M.; Gatterer, H.; Philipp, M.; Krusmann, P.; Kernbeiss, S.; Frontull, V.; Kofler, P. Effects of a Single Low-Dose Acetaminophen on Body Temperature and Running Performance in the Heat: A Pilot Project. Int. J. Physiol. Pathophysiol. Pharmacol. 2013, 5, 4. [Google Scholar]
- Jaeschke, H. Acetaminophen: Dose-Dependent Drug Hepatotoxicity and Acute Liver Failure in Patients. Dig. Dis. 2015, 33, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Conley, D.L.; Krahenbuhl, G.S. Running Economy and Distance Running Performance of Highly Trained Athletes. Med. Sci. Sports Exerc. 1980, 12, 357–360. [Google Scholar] [CrossRef]
- Barnes, K.R.; Kilding, A.E. Running Economy: Measurement, Norms, and Determining Factors. Sports Med.—Open 2015, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Austin, C.; Hokanson, J.; McGinnis, P.; Patrick, S. The Relationship between Running Power and Running Economy in Well-Trained Distance Runners. Sports 2018, 6, 142. [Google Scholar] [CrossRef] [Green Version]
- Abe, D.; Yanagawa, K.; Yamanobe, K.; Tamura, K. Assessment of Middle-Distance Running Performance in Sub-Elite Young Runners Using Energy Cost of Running. Eur. J. Appl. Physiol. 1998, 77, 320–325. [Google Scholar] [CrossRef]
- Pescatello, L.S. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2014. [Google Scholar]
- Warburton, D.E.R.; Jamnik, V.; Bredin, S.S.D.; Shephard, R.J.; Gledhill, N. The 2018 Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination. Health Fit. J. Can. 2018, 11, 31–34. [Google Scholar]
- Jones, A.; Doust, J. A 1% Treadmill Grade Most Accurately Reflects the Energetic Cost of Outdoor Running. J. Sports Sci. 1996, 14, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Waldman, H.S.; Heatherly, A.J.; Waddell, A.F.; Krings, B.M.; O’Neal, E.K. Five-Kilometer Time Trial Reliability of a Nonmotorized Treadmill and Comparison of Physiological and Perceptual Responses vs. a Motorized Treadmill. J. Strength Cond. Res. 2018, 32, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Binger, M.; Evans, C.; Trachte, T.; Van Guilder, G.P. No Influence of Ischemic Preconditioning on Running Economy. Eur. J. Appl. Physiol. 2017, 117, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.R.; Esau, S.P.; MacIntosh, B.R. Economy of Running: Beyond the Measurement of Oxygen Uptake. J Appl Physiol 2009, 107, 5. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.J. Paracetamol (Acetaminophen): Mechanisms of Action. Pediatric Anesth. 2008, 18, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.U.; Pyne, D.B.; Telford, R.D.; Hawley, J.A. Factors Affecting Running Economy in Trained Distance Runners. Sports Med. 2004, 34, 465–485. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and Supraspinal Factors in Human Muscle Fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Gao, Z.; Mei, Q.; Fekete, G.; Baker, J.S.; Gu, Y. The Effect of Prolonged Running on the Symmetry of Biomechanical Variables of the Lower Limb Joints. Symmetry 2020, 12, 720. [Google Scholar] [CrossRef]
- Pickering, G.; Loriot, M.; Libert, F.; Eschalier, A.; Beaune, P.; Dubray, C. Analgesic Effect of Acetaminophen in Humans: First Evidence of a Central Serotonergic Mechanism. Clin. Pharmacol. Ther. 2006, 79, 371–378. [Google Scholar] [CrossRef]
- Tomlin, D.L.; Wenger, H.A. The Relationship between Aerobic Fitness and Recovery from High Intensity Intermittent Exercise. Sports Med. 2001, 31, 1–11. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; Scalzo, P.; D’Agostino, J.; Moore, Z.A.; Diaz-Ruiz, A.; Fabbri, E.; Zane, A.; Chen, B.; Becker, K.G.; Lehrmann, E.; et al. Skeletal Muscle Ex Vivo Mitochondrial Respiration Parallels Decline in Vivo Oxidative Capacity, Cardiorespiratory Fitness, and Muscle Strength: The Baltimore Longitudinal Study of Aging. Aging Cell 2018, 17, e12725. [Google Scholar] [CrossRef] [Green Version]
| Characteristic (Units) | All Participants (n = 11) | Men (n = 9) | Women (n = 2) |
|---|---|---|---|
| Age (years) | 18.8 ± 0.6 | 18.7 ± 0.5 | 19.5 ± 0.7 |
| Height (cm) | 171.1 ± 6.9 | 173.7 ± 4.2 | 159.5 ± 2.1 |
| Weight (kg) | 58.6 ± 5.3 | 59.7 ± 5.1 | 53.8 ± 2.9 |
| BMI (kg/m2) | 20.0 ± 1.1 | 19.8 ± 0.8 | 21.2 ± 1.7 |
| BF% | 9.7 ± 5.1 | 7.6 ± 1.0 | 19.5 ± 4.2 |
| ABS VO2 max (L/min) | 3.6 ± 0.6 | 3.8 ± 0.4 | 2.6 ± 0.3 |
| REL VO2 max (mL/kg/min) | 60.6 ± 7.7 | 63.3 ± 5.3 | 48.4 ± 2.2 |
| Weekly training distance (km/wk) | 98.1 ± 16.7 | 103.0 ± 9.9 | 76.0 ± 28.3 |
| Stage | Speed (m/min) (Male, Female) | BSL (M ± SD) | PLA (M ± SD) | ACT (M ± SD) | p-Value |
|---|---|---|---|---|---|
| Oxygen Consumption (mL/kg/min) | |||||
| 1 | 174.4, 160.9 | 36.0 ± 2.6 | 35.9 ± 3.6 | 35.0 ± 2.8 | 0.529 |
| 2 | 187.8, 174.4 | 38.3 ± 2.8 | 38.3 ± 3.7 | 38.9 ± 2.4 | 0.148 |
| 3 | 201.2, 187.8 | 41.2 ± 3.5 | 41.6 ± 3.5 | 41.8 ± 2.2 | 0.234 |
| 4 | 214.6, 201.2 | 44.0 ± 2.7 | 44.2 ± 3.9 | 44.2 ± 2.5 | 0.159 |
| Heart Rate (bpm) | |||||
| 1 | 174.4, 160.9 | 134 ± 11 | 139 ± 8 | 136 ± 9 | 0.518 |
| 2 | 187.8, 174.4 | 143 ± 10 | 150 ± 9 | 147 ± 9 | 0.135 |
| 3 | 201.2, 187.8 | 149 ± 8 | 155 ± 8 | 154 ± 8 | 0.071 |
| 4 | 214.6, 201.2 | 158 ± 8 | 162 ± 9 | 160 ± 8 | 0.176 |
| Post TT Oxygen Saturation (%) | |||||
| N/A | (Post 3 km TT) | 87.6 ± 2.8 | 87.6 ± 3.0 | 87.4 ± 3.1 | 0.913 |
| Post TT Heart Rate (bpm) | |||||
| N/A | (Post 3 km TT) | 176.7 ± 6.4 | 174.1 ± 11.3 | 175.7 ± 6.2 | 0.846 |
| Stage/Question | Speed (m/min) (Male, Female) | BSL Med (25th, 75th) | PLA Med (25th, 75th) | ACT Med (25th, 75th) | p-Value |
|---|---|---|---|---|---|
| RPE during RE Stages | |||||
| 1 | 174.4, 160.9 | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 0.273 |
| 2 | 187.8, 174.4 | 2 (1, 2.5) | 2 (2, 2.5) | 2 (1, 3) | 0.957 |
| 3 | 201.2, 187.8 | 2.5 (1.5, 3) | 3 (2, 3) | 2 (2, 4) | 0.519 |
| 4 | 214.6, 201.2 | 3 (1.5, 5) | 3 (2, 5) | 2 (2, 5) | 0.922 |
| Responses to Questionnaire | |||||
| Q1 | Effort level | 9 (8, 9) | 9 (8, 9.5) | 9 (9, 9) | 0.091 |
| Q2 | Exercise difficulty | 8.5 (8, 9) | 9 (8, 10) | 9 (8, 9) | 0.656 |
| Q3 | Performance level | 8 (8, 9) | 8 (7, 9) | 8 (7.5, 9) | 0.368 |
| Q4 | Perceived exertion | 8 (7, 9) | 8 (8, 9) | 9 (8, 9) | 0.140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huffman, R.P.; Van Guilder, G.P. The Effect of Acetaminophen on Running Economy and Performance in Collegiate Distance Runners. Int. J. Environ. Res. Public Health 2022, 19, 2927. https://doi.org/10.3390/ijerph19052927
Huffman RP, Van Guilder GP. The Effect of Acetaminophen on Running Economy and Performance in Collegiate Distance Runners. International Journal of Environmental Research and Public Health. 2022; 19(5):2927. https://doi.org/10.3390/ijerph19052927
Chicago/Turabian StyleHuffman, Riley P., and Gary P. Van Guilder. 2022. "The Effect of Acetaminophen on Running Economy and Performance in Collegiate Distance Runners" International Journal of Environmental Research and Public Health 19, no. 5: 2927. https://doi.org/10.3390/ijerph19052927
APA StyleHuffman, R. P., & Van Guilder, G. P. (2022). The Effect of Acetaminophen on Running Economy and Performance in Collegiate Distance Runners. International Journal of Environmental Research and Public Health, 19(5), 2927. https://doi.org/10.3390/ijerph19052927







